Machine Learning and Deep Learning in Spinal Injury: A Narrative Review of Algorithms in Diagnosis and Prognosis
Abstract
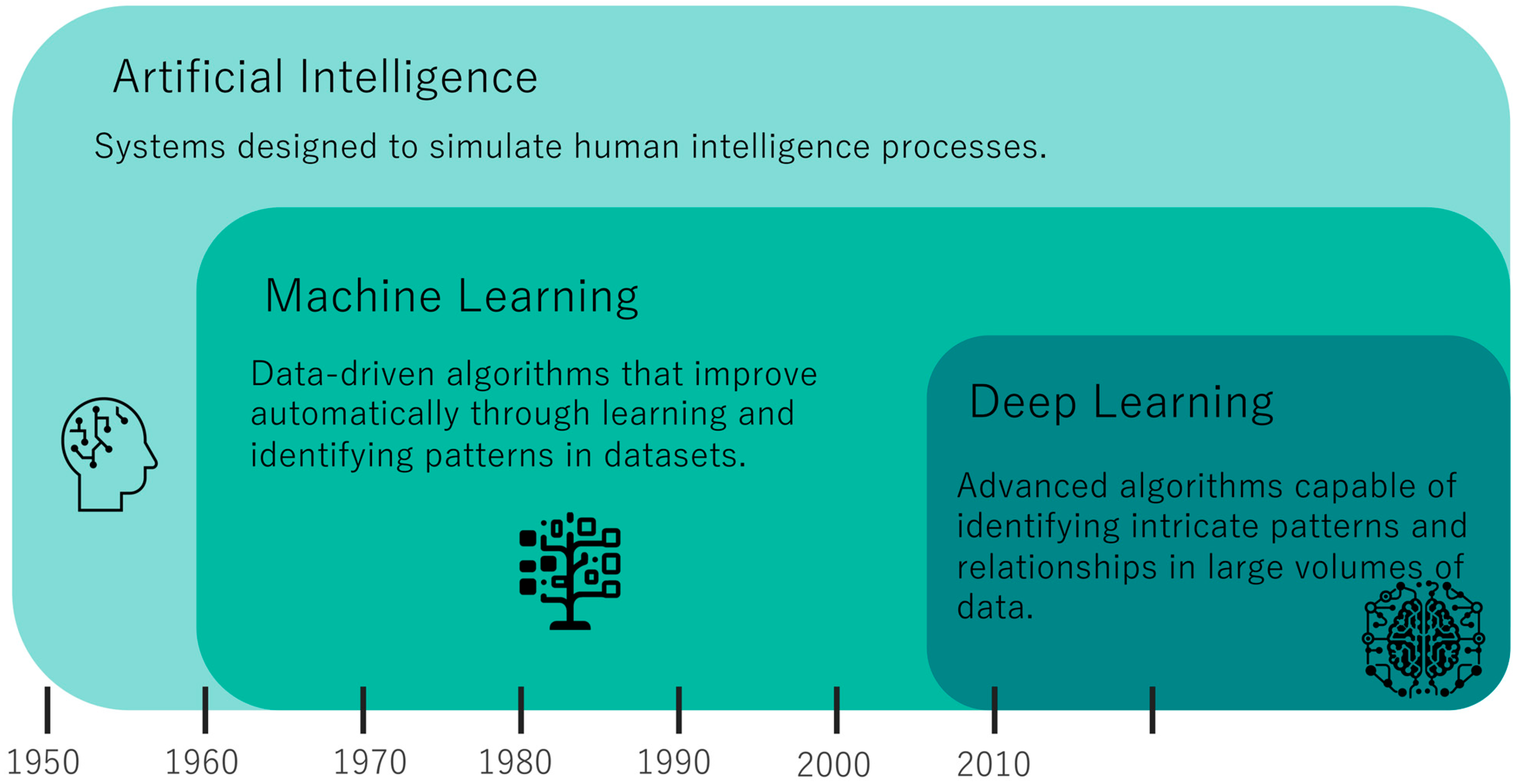
1. Introduction
2. Materials and Methods
2.1. Search Criteria
2.2. Eligibility Criteria
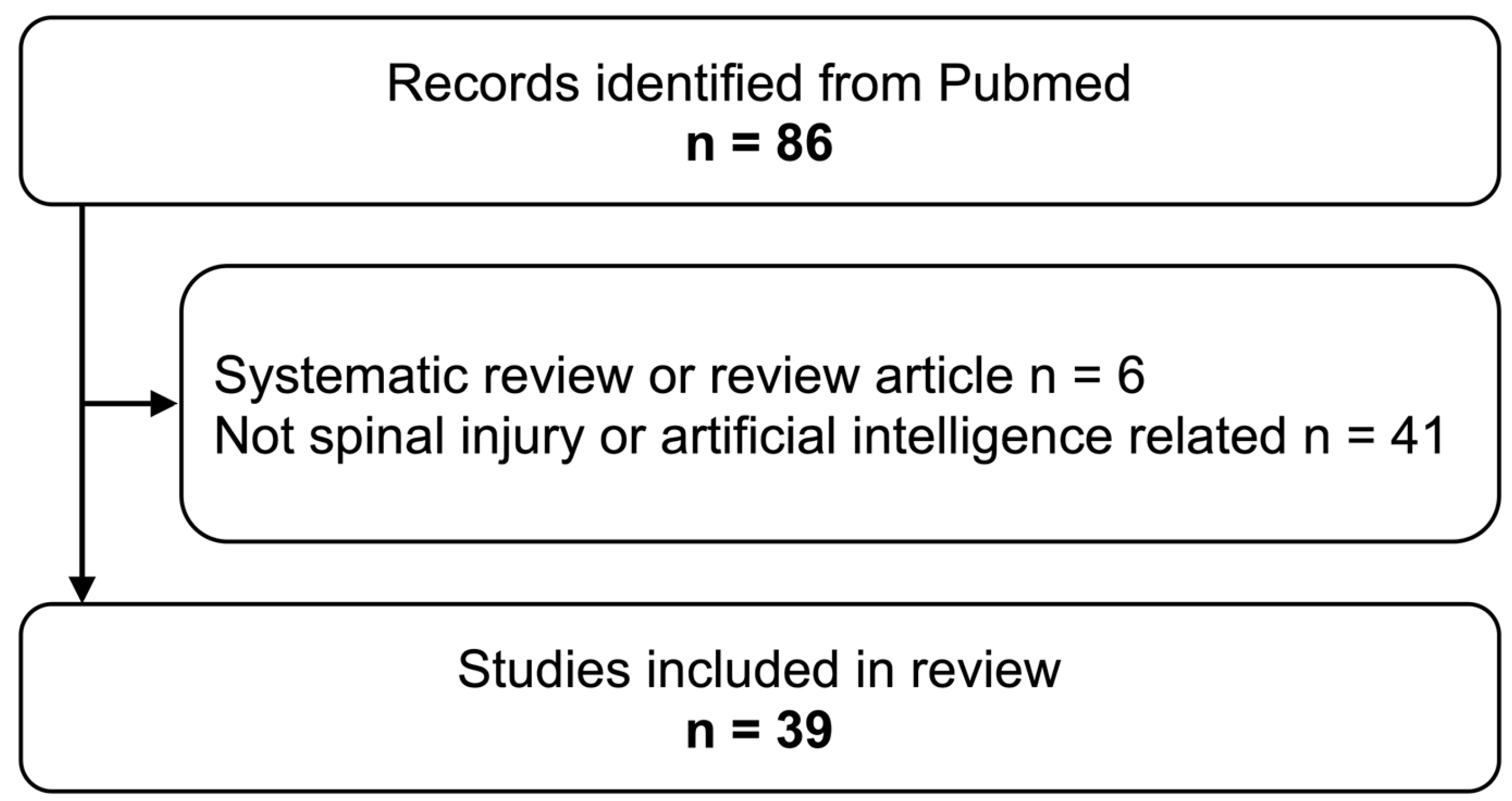
2.3. Study Selection
2.4. Review Framework and Components
- Diagnosis/Prognosis: The role the algorithms played in diagnosing or predicting the outcome of the spinal injuries.
- Target Pathology: The specific type of spinal injury being investigated in each study.
- Patients Studied: Number of the patients who participated in the studies.
- Images Studied: Number of images analyzed in the studies.
- Type of Data: Categories of data, such as imaging or tabulated clinical data, used in the study.
- Computational Task: The specific computational goal for which machine learning or deep learning algorithms were employed, such as object detection, segmentation, or classification.
- Machine Learning Models: Types of machine learning and deep learning algorithms used, such as decision trees, random forests, etc.
- Summaries: Summaries of the key findings and essential takeaways from each individual study.
3. Results
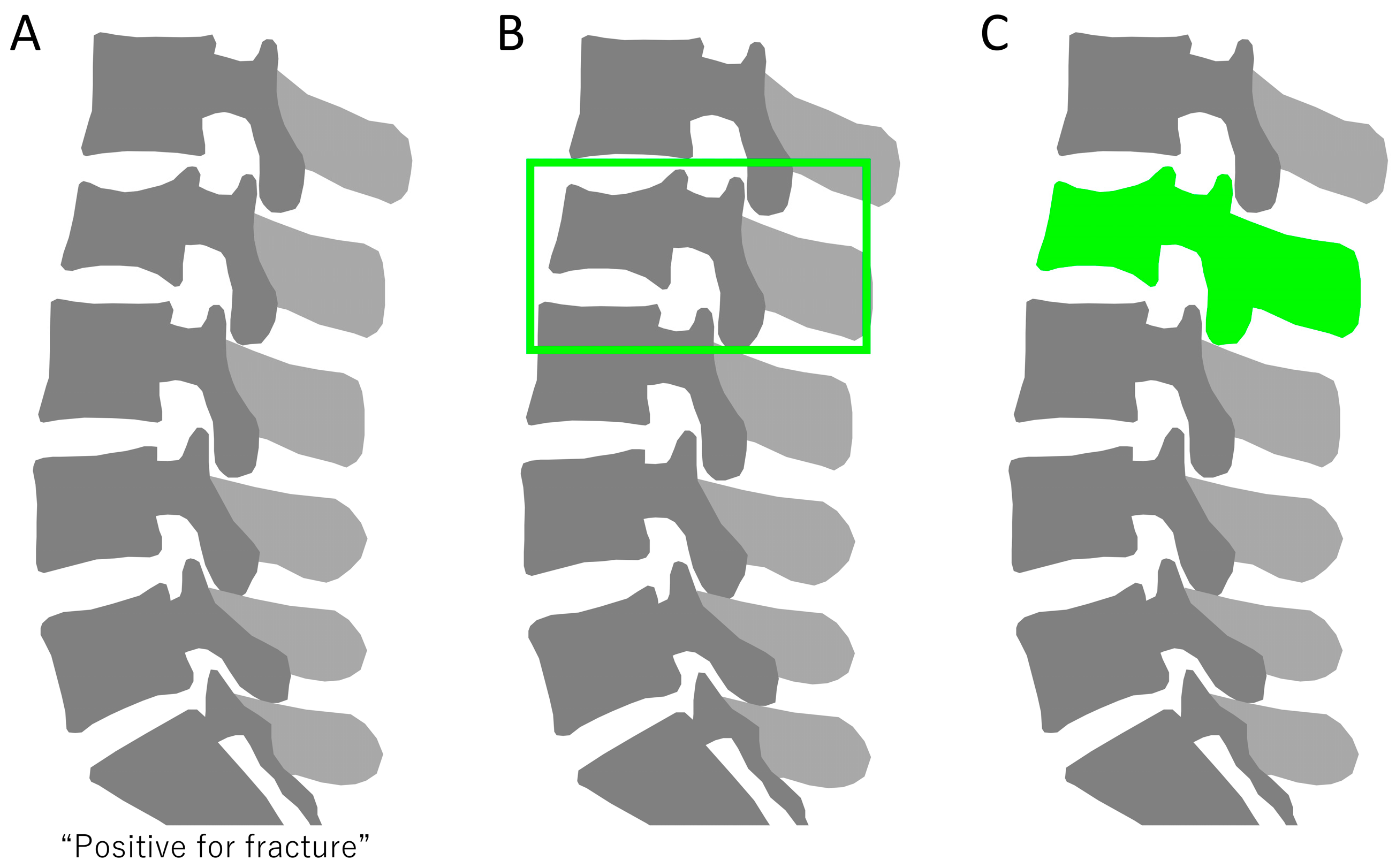
3.1. Algorithms for Diagnosing Spinal Injuries
3.2. Algorithms for Predicting Spinal Injury Outcomes
3.3. Types of Data and Study Design
4. Discussion
4.1. Overview of Machine Learning Applications in Spinal Care
4.2. Diagnostic Approaches
4.2.1. X-ray
Diagnostic Accuracy Using Entire X-rays
Evaluation of Manually Cropped Regions
Object Detection and Ensemble Approaches
Cervical Spine
Age-Specific Algorithm Performance
4.2.2. DEXA
4.2.3. CT
Automated Detection Algorithms
Fracture Classification
Opportunistic Screening and Fracture Liaison
3D-Based Algorithms
Distinguishing Benign from Malignant Vertebral Fractures
Cervical Spine
4.2.4. MRI
Diagnosing Fresh Osteoporotic Vertebral Fracture
Distinguishing Benign from Malignant Vertebral Fractures
4.3. Prognostic Approaches
4.4. Advantages and Disadvantages of Each Model
4.5. Future Direction
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Utheim, N.C.; Helseth, E.; Stroem, M.; Rydning, P.; Mejlænder-Evjensvold, M.; Glott, T.; Hoestmaelingen, C.T.; Aarhus, M.; Roenning, P.A.; Linnerud, H. Epidemiology of Traumatic Cervical Spinal Fractures in a General Norwegian Population. Inj. Epidemiol. 2022, 9, 10. [Google Scholar] [CrossRef]
- Katsuura, Y.; Osborn, J.M.; Cason, G.W. The Epidemiology of Thoracolumbar Trauma: A Meta-Analysis. J. Orthop. 2016, 13, 383–388. [Google Scholar] [CrossRef]
- Zileli, M.; Sharif, S.; Fornari, M. Incidence and Epidemiology of Thoracolumbar Spine Fractures: WFNS Spine Committee Recommendations. Neurospine 2021, 18, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Davenport, T.; Kalakota, R. The Potential for Artificial Intelligence in Healthcare. Future Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef]
- McDonnell, J.M.; Evans, S.R.; McCarthy, L.; Temperley, H.; Waters, C.; Ahern, D.; Cunniffe, G.; Morris, S.; Synnott, K.; Birch, N.; et al. The Diagnostic and Prognostic Value of Artificial Intelligence and Artificial Neural Networks in Spinal Surgery: A Narrative Review. Bone Jt. J. 2021, 103, 1442–1448. [Google Scholar] [CrossRef]
- Dietz, N.; Jaganathan, V.; Alkin, V.; Mettille, J.; Boakye, M.; Drazin, D. Machine Learning in Clinical Diagnosis, Prognostication, and Management of Acute Traumatic Spinal Cord Injury (SCI): A Systematic Review. J. Clin. Orthop. Trauma 2022, 35, 102046. [Google Scholar] [CrossRef] [PubMed]
- Hornung, A.L.; Hornung, C.M.; Mallow, G.M.; Barajas, J.N.; Rush, A.; Sayari, A.J.; Galbusera, F.; Wilke, H.-J.; Colman, M.; Phillips, F.M.; et al. Artificial Intelligence in Spine Care: Current Applications and Future Utility. Eur. Spine J. 2022, 31, 2057–2081. [Google Scholar] [CrossRef]
- Khan, O.; Badhiwala, J.H.; Grasso, G.; Fehlings, M.G. Use of Machine Learning and Artificial Intelligence to Drive Personalized Medicine Approaches for Spine Care. World Neurosurg. 2020, 140, 512–518. [Google Scholar] [CrossRef]
- Galbusera, F.; Casaroli, G.; Bassani, T. Artificial Intelligence and Machine Learning in Spine Research. JOR Spine 2019, 2, e1044. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.F.; Crabtree, N.J.; Bromiley, P.A.; Cootes, T.; Broadley, P.; Lang, I.; Offiah, A.C. Diagnostic Performance of Morphometric Vertebral Fracture Analysis (MXA) in Children Using a 33-Point Software Program. Bone 2020, 133, 115249. [Google Scholar] [CrossRef]
- Alqahtani, F.F.; Messina, F.; Offiah, A.C. Are Semi-Automated Software Program Designed for Adults Accurate for the Identification of Vertebral Fractures in Children? Eur. Radiol. 2019, 29, 6780–6789. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.F.; Messina, F.; Kruger, E.; Gill, H.; Ellis, M.; Lang, I.; Broadley, P.; Offiah, A.C. Evaluation of a Semi-Automated Software Program for the Identification of Vertebral Fractures in Children. Clin. Radiol. 2017, 72, 904.e11–904.e20. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.E.; Yao, J.; Muñoz, H.; Summers, R.M. Automated Detection, Localization, and Classification of Traumatic Vertebral Body Fractures in the Thoracic and Lumbar Spine at CT. Radiology 2016, 278, 64–73. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y. A Classification Method for Thoracolumbar Vertebral Fractures Due to Basketball Sports Injury Based on Deep Learning. Comput. Math. Methods Med. 2022, 2022, 8747487. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Hsu, B.W.-Y.; Yin, Y.-K.; Lin, F.-H.; Yang, T.-H.; Yang, R.-S.; Lee, C.-K.; Tseng, V.S. Application of Deep Learning Algorithm to Detect and Visualize Vertebral Fractures on Plain Frontal Radiographs. PLoS ONE 2021, 16, e0245992. [Google Scholar] [CrossRef]
- Chou, P.-H.; Jou, T.H.-T.; Wu, H.-T.H.; Yao, Y.-C.; Lin, H.-H.; Chang, M.-C.; Wang, S.-T.; Lu, H.H.-S.; Chen, H.-H. Ground Truth Generalizability Affects Performance of the Artificial Intelligence Model in Automated Vertebral Fracture Detection on Plain Lateral Radiographs of the Spine. Spine J. 2022, 22, 511–523. [Google Scholar] [CrossRef]
- Derkatch, S.; Kirby, C.; Kimelman, D.; Jozani, M.J.; Davidson, J.M.; Leslie, W.D. Identification of Vertebral Fractures by Convolutional Neural Networks to Predict Nonvertebral and Hip Fractures: A Registry-Based Cohort Study of Dual X-Ray Absorptiometry. Radiology 2019, 293, 405–411. [Google Scholar] [CrossRef]
- Naguib, S.M.; Hamza, H.M.; Hosny, K.M.; Saleh, M.K.; Kassem, M.A. Classification of Cervical Spine Fracture and Dislocation Using Refined Pre-Trained Deep Model and Saliency Map. Diagnostics 2023, 13, 1273. [Google Scholar] [CrossRef]
- Nicolaes, J.; Liu, Y.; Zhao, Y.; Huang, P.; Wang, L.; Yu, A.; Dunkel, J.; Libanati, C.; Cheng, X. External Validation of a Convolutional Neural Network Algorithm for Opportunistically Detecting Vertebral Fractures in Routine CT Scans. Osteoporos. Int. 2023, 35, 143–152. [Google Scholar] [CrossRef]
- Ong, T.; Copeland, R.; Thiam, C.N.; Cerda Mas, G.; Marshall, L.; Sahota, O. Integration of a Vertebral Fracture Identification Service into a Fracture Liaison Service: A Quality Improvement Project. Osteoporos. Int. 2021, 32, 921–926. [Google Scholar] [CrossRef]
- Park, T.; Yoon, M.A.; Cho, Y.C.; Ham, S.J.; Ko, Y.; Kim, S.; Jeong, H.; Lee, J. Automated Segmentation of the Fractured Vertebrae on CT and Its Applicability in a Radiomics Model to Predict Fracture Malignancy. Sci. Rep. 2022, 12, 6735. [Google Scholar] [CrossRef]
- Rosenberg, G.S.; Cina, A.; Schiró, G.R.; Giorgi, P.D.; Gueorguiev, B.; Alini, M.; Varga, P.; Galbusera, F.; Gallazzi, E. Artificial Intelligence Accurately Detects Traumatic Thoracolumbar Fractures on Sagittal Radiographs. Medicina 2022, 58, 998. [Google Scholar] [CrossRef]
- Roux, C.; Rozes, A.; Reizine, D.; Hajage, D.; Daniel, C.; Maire, A.; Bréant, S.; Taright, N.; Gordon, R.; Fechtenbaum, J.; et al. Fully Automated Opportunistic Screening of Vertebral Fractures and Osteoporosis on More than 150 000 Routine Computed Tomography Scans. Rheumatology 2022, 61, 3269–3278. [Google Scholar] [CrossRef]
- Rueckel, J.; Sperl, J.I.; Kaestle, S.; Hoppe, B.F.; Fink, N.; Rudolph, J.; Schwarze, V.; Geyer, T.; Strobl, F.F.; Ricke, J.; et al. Reduction of Missed Thoracic Findings in Emergency Whole-Body Computed Tomography Using Artificial Intelligence Assistance. Quant. Imaging Med. Surg. 2021, 11, 2486–2498. [Google Scholar] [CrossRef]
- Shen, L.; Gao, C.; Hu, S.; Kang, D.; Zhang, Z.; Xia, D.; Xu, Y.; Xiang, S.; Zhu, Q.; Xu, G.; et al. Using Artificial Intelligence to Diagnose Osteoporotic Vertebral Fractures on Plain Radiographs. J. Bone Miner. Res. 2023, 38, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Small, J.E.; Osler, P.; Paul, A.B.; Kunst, M. CT Cervical Spine Fracture Detection Using a Convolutional Neural Network. Am. J. Neuroradiol. 2021, 42, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Cheung, Y.Y.; Hassanpour, S. Deep Neural Networks for Automatic Detection of Osteoporotic Vertebral Fractures on CT Scans. Comput. Biol. Med. 2018, 98, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Valentinitsch, A.; Trebeschi, S.; Kaesmacher, J.; Lorenz, C.; Löffler, M.T.; Zimmer, C.; Baum, T.; Kirschke, J.S. Opportunistic Osteoporosis Screening in Multi-Detector CT Images via Local Classification of Textures. Osteoporos. Int. 2019, 30, 1275–1285. [Google Scholar] [CrossRef]
- Voter, A.F.; Larson, M.E.; Garrett, J.W.; Yu, J.-P.J. Diagnostic Accuracy and Failure Mode Analysis of a Deep Learning Algorithm for the Detection of Cervical Spine Fractures. Am. J. Neuroradiol. 2021, 42, 1550–1556. [Google Scholar] [CrossRef]
- Yabu, A.; Hoshino, M.; Tabuchi, H.; Takahashi, S.; Masumoto, H.; Akada, M.; Morita, S.; Maeno, T.; Iwamae, M.; Inose, H.; et al. Using Artificial Intelligence to Diagnose Fresh Osteoporotic Vertebral Fractures on Magnetic Resonance Images. Spine J. 2021, 21, 1652–1658. [Google Scholar] [CrossRef]
- Yeh, L.-R.; Zhang, Y.; Chen, J.-H.; Liu, Y.-L.; Wang, A.-C.; Yang, J.-Y.; Yeh, W.-C.; Cheng, C.-S.; Chen, L.-K.; Su, M.-Y. A Deep Learning-Based Method for the Diagnosis of Vertebral Fractures on Spine MRI: Retrospective Training and Validation of ResNet. Eur. Spine J. 2022, 31, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T.; Maki, S.; Furuya, T.; Yokota, H.; Matsumoto, K.; Takaoka, H.; Miyamoto, T.; Okimatsu, S.; Shiga, Y.; Inage, K.; et al. Automated Differentiation Between Osteoporotic Vertebral Fracture and Malignant Vertebral Fracture on MRI Using a Deep Convolutional Neural Network. Spine 2022, 47, E347–E352. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, A.; Pisov, M.; Bukharaev, A.; Petraikin, A.; Morozov, S.; Gombolevskiy, V.; Belyaev, M. Interpretable Vertebral Fracture Quantification via Anchor-Free Landmarks Localization. Med. Image Anal. 2023, 83, 102646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, F.; Xu, J.; Zhao, Q.; Huang, C.; Yu, Y.; Yuan, H. Automated Detection and Classification of Acute Vertebral Body Fractures Using a Convolutional Neural Network on Computed Tomography. Front. Endocrinol. 2023, 14, 1132725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, Z.; Qiu, L.; Liang, D.; Wang, K.; Xu, J.; Zhao, J.; Sun, J. Automatic Vertebral Fracture and Three-Column Injury Diagnosis with Fracture Visualization by a Multi-Scale Attention-Guided Network. Med. Biol. Eng. Comput. 2023, 61, 1661–1674. [Google Scholar] [CrossRef]
- Cho, S.T.; Shin, D.-E.; Kim, J.-W.; Yoon, S.; Il Lee, H.; Lee, S. Prediction of Progressive Collapse in Osteoporotic Vertebral Fractures Using Conventional Statistics and Machine Learning. Spine 2023, 48, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cai, J.; Zeng, Y.; Ye, H.; Yang, T.; Liu, Z.; Liu, Q. Development and Validation of a Machine Learning Model to Predict Imminent New Vertebral Fractures after Vertebral Augmentation. BMC Musculoskelet. Disord. 2023, 24, 472. [Google Scholar] [CrossRef]
- Kong, S.H.; Lee, J.-W.; Bae, B.U.; Sung, J.K.; Jung, K.H.; Kim, J.H.; Shin, C.S. Development of a Spine X-Ray-Based Fracture Prediction Model Using a Deep Learning Algorithm. Endocrinol. Metab. 2022, 37, 674–683. [Google Scholar] [CrossRef]
- Leister, I.; Haider, T.; Vogel, M.; Vastmans, J.; Langthaler, P.; Mattiassich, G.; Christ, A.; Etschmaier, M.; Eijkenboom, A.; Burghuber, J.; et al. A Predictive Model to Identify Treatment-Related Risk Factors for Odontoid Fracture Nonunion Using Machine Learning. Spine 2023, 48, 164–171. [Google Scholar] [CrossRef]
- Takahashi, S.; Terai, H.; Hoshino, M.; Tsujio, T.; Kato, M.; Toyoda, H.; Suzuki, A.; Tamai, K.; Yabu, A.; Nakamura, H. Machine-Learning-Based Approach for Nonunion Prediction Following Osteoporotic Vertebral Fractures. Eur. Spine J. 2022, 32, 3788–3796. [Google Scholar] [CrossRef]
- Murata, K.; Endo, K.; Aihara, T.; Suzuki, H.; Sawaji, Y.; Matsuoka, Y.; Nishimura, H.; Takamatsu, T.; Konishi, T.; Maekawa, A.; et al. Artificial Intelligence for the Detection of Vertebral Fractures on Plain Spinal Radiography. Sci. Rep. 2020, 10, 20031. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Chen, H.-H.; Horng-Shing Lu, H.; Hondar Wu, H.-T.; Chang, M.-C.; Chou, P.-H. Can a Deep-Learning Model for the Automated Detection of Vertebral Fractures Approach the Performance Level of Human Subspecialists? Clin. Orthop. Relat. Res. 2021, 479, 1598–1612. [Google Scholar] [CrossRef]
- Mehta, S.D.; Sebro, R. Computer-Aided Detection of Incidental Lumbar Spine Fractures from Routine Dual-Energy X-Ray Absorptiometry (DEXA) Studies Using a Support Vector Machine (SVM) Classifier. J. Digit. Imaging 2020, 33, 204–210. [Google Scholar] [CrossRef]
- Monchka, B.A.; Schousboe, J.T.; Davidson, M.J.; Kimelman, D.; Hans, D.; Raina, P.; Leslie, W.D. Development of a Manufacturer-Independent Convolutional Neural Network for the Automated Identification of Vertebral Compression Fractures in Vertebral Fracture Assessment Images Using Active Learning. Bone 2022, 161, 116427. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Maki, S.; Furuya, T.; Mikami, Y.; Mizutani, M.; Takada, I.; Okimatsu, S.; Yunde, A.; Miura, M.; Shiratani, Y.; et al. Automated Fracture Screening Using an Object Detection Algorithm on Whole-Body Trauma Computed Tomography. Sci. Rep. 2022, 12, 16549. [Google Scholar] [CrossRef]
- Doerr, S.A.; Weber-Levine, C.; Hersh, A.M.; Awosika, T.; Judy, B.; Jin, Y.; Raj, D.; Liu, A.; Lubelski, D.; Jones, C.K.; et al. Automated Prediction of the Thoracolumbar Injury Classification and Severity Score from CT Using a Novel Deep Learning Algorithm. Neurosurg. Focus 2022, 52, E5. [Google Scholar] [CrossRef]
- Goller, S.S.; Foreman, S.C.; Rischewski, J.F.; Weißinger, J.; Dietrich, A.-S.; Schinz, D.; Stahl, R.; Luitjens, J.; Siller, S.; Schmidt, V.F.; et al. Differentiation of Benign and Malignant Vertebral Fractures Using a Convolutional Neural Network to Extract CT-Based Texture Features. Eur. Spine J. 2023, 32, 4314–4320. [Google Scholar] [CrossRef]
- Golla, A.-K.; Lorenz, C.; Buerger, C.; Lossau, T.; Klinder, T.; Mutze, S.; Arndt, H.; Spohn, F.; Mittmann, M.; Goelz, L. Cervical Spine Fracture Detection in Computed Tomography Using Convolutional Neural Networks. Phys. Med. Biol. 2023, 68, 115010. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, J.; Duan, Z.; Liao, Z.; Wang, S.; Liu, W. Artificial Intelligence in Spinal Imaging: Current Status and Future Directions. Int. J. Environ. Res. Public Health 2022, 19, 11708. [Google Scholar] [CrossRef] [PubMed]
- Baur, D.; Kroboth, K.; Heyde, C.-E.; Voelker, A. Convolutional Neural Networks in Spinal Magnetic Resonance Imaging: A Systematic Review. World Neurosurg. 2022, 166, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Yu, K.; Xie, Z.; Wang, P.; Zhang, W.; Huang, Y.; Wang, Y.; Wu, X. Current Applications of Machine Learning in Spine: From Clinical View. Global Spine J. 2022, 12, 1827–1840. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-C.; Pareek, A.; Seyyedi, S.; Banerjee, I.; Lungren, M.P. Fusion of Medical Imaging and Electronic Health Records Using Deep Learning: A Systematic Review and Implementation Guidelines. NPJ Digit. Med. 2020, 3, 136. [Google Scholar] [CrossRef]
- Pagano, S.; Holzapfel, S.; Kappenschneider, T.; Meyer, M.; Maderbacher, G.; Grifka, J.; Holzapfel, D.E. Arthrosis Diagnosis and Treatment Recommendations in Clinical Practice: An Exploratory Investigation with the Generative AI Model GPT-4. J. Orthop. Traumatol. 2023, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Meskó, B. The Impact of Multimodal Large Language Models on Health Care’s Future. J. Med. Internet Res. 2023, 25, e52865. [Google Scholar] [CrossRef] [PubMed]
| Author(s) | Year | Target Pathology | Patients Studied | Images Studied | Type of Data | Task | Machine Learning Models | Summary |
|---|---|---|---|---|---|---|---|---|
| Alqahtani et al. [10] | 2020 | Vertebral fractures in children | 420 | 420 | DXA | Point Detection | AVERT | The vertebral fracture analysis technique for adults is not reliable for fractures in children, suggesting the need for a pediatric standard. |
| Alqahtani et al. [11] | 2019 | Vertebral fractures in children | 100 | 100 | X-ray, DXA | Point Detection | AVERT and SpineAnalyzer | The study found that neither AVERT nor SpineAnalyzer is reliably satisfactory for vertebral fracture diagnosis in children. |
| Alqahtani et al. [12] | 2017 | Vertebral fractures in children | 137 | 1781 (vertebrae) | X-ray | Point Detection | SpineAnalyzer | The current software is inadequate for diagnosing vertebral fractures in children and needs retraining to include child-specific factors. |
| Burns et al. [13] | 2016 | Thoracolumbar vertebral fractures | 104 | 3D volume data | CT | Object Detection, Segmentation | Support vector machine | A fully automated computer system detects and anatomically localizes vertebral body fractures on CT images. |
| Chen et al. [14] | 2022 | Thoracolumbar vertebral fractures (AO classification) | 1130 | 1130 | CT | Object Detection | Faster R-CNN | A deep-learning-based classification method achieves high accuracy in classifying thoracolumbar vertebral fractures. |
| Chen et al. [15] | 2021 | Osteoporotic vertebral fractures | 1306 | 1306 | X-ray | Classification | ResNeXt-50 | A deep learning algorithm was developed to detect and visualize vertebral fractures on plain frontal radiographs. |
| Chou et al. [16] | 2022 | Thoracolumbar vertebral fractures | 1305 | 1305 | X-ray, | Object Detection, Classification, Segmentation | Ensemble model, YOLOv3 | The AI model trained on older adult data effectively identified vertebral fractures in older patients and intact vertebrae in younger adults. |
| Derkatch et al. [17] | 2019 | Osteoporotic vertebral fractures | 12,742 | 12,742 | DXA | Classification | Inception-ResNet V2 and DenseNet | CNNs can identify vertebral fractures on vertebral fracture assessment images with high accuracy and predict clinical fracture outcomes. |
| Naguib et al. [18] | 2023 | Cervical spine fracture | NA | 2009 | X-ray | Classification | AlexNet and GoogLeNet | A computer-aided-diagnosis system based on deep learning for classifying cervical spine injuries as fractures or dislocations. |
| Nicolaes et al. [19] | 2023 | Osteoporotic vertebral fractures | 4810 | 3D volume data | CT | Object Detection, Classification | 3D CNN (model not specified) | The CNN algorithm achieved 94% sensitivity and 93% specificity in identifying vertebral fractures in routine CT scans. |
| Ong et al. [20] | 2021 | Osteoporotic vertebral fractures | 850 | 4461 | CT | Point Detection | Model developed by Optasia Medical | Integration of a vertebral fracture identification service into a Fracture Liaison Service is possible and increases workload. |
| Park et al. [21] | 2022 | Benign and malignant vertebral fractures | 276 | 529 | CT | Segmentation, Classification | U-Net, AsanFEx in MATLAB for radiomics | An algorithm for segmenting fractured vertebrae on CT scans was created, performing on par with experts in predicting fracture malignancy. |
| Rosenberg et al. [22] | 2022 | Thoracolumbar vertebral fractures | 151 | 630 (vertebrae) | X-ray | Classification | ResNet18, VGG16 | A deep learning model was developed to accurately detect traumatic thoracolumbar fractures on sagittal radiographs. |
| Roux et al. [23] | 2022 | Osteoporotic vertebral fractures | 152,268 | NA | CT | Classification | Software (Zebra Medical Vision) | Opportunistic screening of vertebral fractures and osteoporosis on more than 150,000 routine CT scans. |
| Rueckel et al. [24] | 2021 | Missed thoracic findings in emergency CT | 105 | NA | CT | Segmentation, Point Detection | Software (Siemens) | AI assistance reduces missed thoracic findings in emergency whole-body CT scans. |
| Shen et al. [25] | 2023 | Osteoporotic vertebral fractures | 12,673 | 12,673 | X-ray | Object Detection, Segmentation | CNN (model not specified) | A deep-learning-based system for diagnosing and grading osteoporotic vertebral fractures on plain radiographs was developed and validated. |
| Small et al. [26] | 2021 | Cervical spine fractures | 665 | NA | CT | Segmentation, Classification | CNN developed by Aidoc | A CNN demonstrated 92% accuracy in detecting cervical spine fractures on CT, with potential for worklist prioritization and assisting radiologists. |
| Tomita et al. [27] | 2018 | Osteoporotic vertebral fractures | 1432 | NA | CT | Classification | ResNet34, LSTM | Automatic detection of osteoporotic vertebral fractures on CT scans using deep neural networks. |
| Valentinitsch et al. [28] | 2019 | Osteoporotic vertebral fractures | 154 | NA | CT | Classification | Random forest | An automatic screening tool using 3D texture and regional vBMD excels over global vBMD in detecting vertebral fractures. |
| Voter et al. [29] | 2021 | Cervical spine fractures | 1904 | NA | CT | Segmentation, Classification | Deep learning algorithm (Aidoc) | An AI system for detecting cervical spine fractures showed low accuracy, questioning its generalizability and practical deployment. |
| Yabu et al. [30] | 2021 | Osteoporotic vertebral fractures | 814 | 1624 | MRI | Classification | Ensemble model | The performance of the CNN in detecting fresh osteoporotic vertebral fractures using MR images was comparable to that of two spine surgeons. |
| Yeh et al. [31] | 2022 | Benign and malignant vertebral fractures | 190 | NA | MRI | Classification | ResNet50 | A ResNet50-based deep learning method enhanced less experienced clinicians’ ability to diagnose vertebral fractures from spine MRI. |
| Yoda et al. [32] | 2022 | Benign and malignant vertebral fractures | 97 | 697 | MRI | Classification | Xception | A CNN model distinguished osteoporotic and malignant vertebral fractures on MRI with accuracy comparable to spine surgeons. |
| Zakharov et al. [33] | 2023 | Osteoporotic vertebral fractures | 100 | 3565 | CT | Point Detection | CNN (model not specified) | A two-step algorithm localizes the vertebral column in 3D CT and detects and quantifies individual vertebral fractures in 2D. |
| Zhang et al. [34] | 2023 | Thoracolumbar vertebral fractures (AO classification) | 1217 | NA | CT | Object Detection, Classification | U-Net, GCN, 3D-ResNet | The AO system automatically detects and classifies acute thoracolumbar spine fractures on CT with high accuracy per AO standards. |
| Zhang et al. [35] | 2023 | Thoracolumbar vertebral fractures | 197 | 989 (vertebrae) | CT | Object Detection, Classification | Multi-scale attention-guided network | A novel network for diagnosing vertebral fractures and three-column injuries with fracture visualization at a vertebra level. |
| Author(s) | Year | Target Pathology | Patients Studied | Images Studied | Type of Data | Task | Machine Learning Models | Summary |
|---|---|---|---|---|---|---|---|---|
| Cho et al. [36] | 2023 | Osteoporotic vertebral fractures (progression of collapse) | 670 | NA | X-ray, MRI | Classification | Decision tree, random forest | The model predicts progressive collapse in osteoporotic vertebral fractures using machine learning and statistics. |
| Jiang et al. [37] | 2023 | Osteoporotic vertebral fractures (fracture prediction) | 235 | NA | MRI | Classification | Random survival forest, Cox hazards model | A machine learning model combining radiomics and clinical data predicts new vertebral fractures post-vertebral augmentation. |
| Kong et al. [38] | 2022 | Osteoporotic vertebral fractures (fracture prediction) | 1595 | 1595 | X-ray | Classification | CNN, DeepSurv | A spine X-ray-based fracture prediction model was developed using deep learning with longitudinal data. |
| Leister et al. [39] | 2023 | Odontoid fracture nonunion prediction | 415 | NA | X-ray, CT | Classification | XGBoost, binary logistic regression | The study aimed to identify treatment-related risk factors for odontoid fracture nonunion using machine learning models. |
| Takahashi et al. [40] | 2022 | Osteoporotic vertebral fractures (nonunion prediction) | 505 | NA | MRI | Classification | Decision tree, XGBoost, random forest | Machine learning models improve nonunion prediction following osteoporotic vertebral fractures. |
| Model | Definition | Advantage | Disadvantage |
|---|---|---|---|
| Support Vector Machines (SVMs) | Supervised learning models used for classification and regression analysis, known for their effectiveness in high-dimensional spaces. | Effective in classifying spinal trauma cases based on imaging features. Good performance with smaller datasets. | Struggle with very large datasets. Less accurate in noisy data environments, like mixed injury types. |
| Random Forests | An ensemble learning method for classification and regression, using multiple decision trees. | Used for predicting the prognosis of spinal injury. Strong in handling diverse clinical data types without overfitting. | Complexity of the model can hinder its interpretability. |
| XGBoost | A scalable and efficient version of gradient boosted decision trees. | Applied in spinal trauma for outcome prediction and risk assessment. Known for its quick processing of complex datasets. | Can overfit if not properly tuned. |
| Convolutional Neural Networks (CNNs) | Deep neural networks particularly effective in analyzing images, used for pattern recognition in images. | Widely used for detecting fractures and assessing injury severity in spinal trauma imaging. Effective in image-based analysis and interpretation. | Require large, diverse datasets for training. Demand high computational resources. |
| Recurrent Neural Networks (RNNs) | Neural networks designed to process sequential data, recognizing patterns in time-dependent data. | Utilized in tracking and predicting patient recovery progress and response to treatment in spinal trauma cases. | Difficult to train the model. Susceptible to problems like the vanishing gradient, affecting long-term data analysis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maki, S.; Furuya, T.; Inoue, M.; Shiga, Y.; Inage, K.; Eguchi, Y.; Orita, S.; Ohtori, S. Machine Learning and Deep Learning in Spinal Injury: A Narrative Review of Algorithms in Diagnosis and Prognosis. J. Clin. Med. 2024, 13, 705. https://doi.org/10.3390/jcm13030705
Maki S, Furuya T, Inoue M, Shiga Y, Inage K, Eguchi Y, Orita S, Ohtori S. Machine Learning and Deep Learning in Spinal Injury: A Narrative Review of Algorithms in Diagnosis and Prognosis. Journal of Clinical Medicine. 2024; 13(3):705. https://doi.org/10.3390/jcm13030705
Chicago/Turabian StyleMaki, Satoshi, Takeo Furuya, Masahiro Inoue, Yasuhiro Shiga, Kazuhide Inage, Yawara Eguchi, Sumihisa Orita, and Seiji Ohtori. 2024. "Machine Learning and Deep Learning in Spinal Injury: A Narrative Review of Algorithms in Diagnosis and Prognosis" Journal of Clinical Medicine 13, no. 3: 705. https://doi.org/10.3390/jcm13030705
APA StyleMaki, S., Furuya, T., Inoue, M., Shiga, Y., Inage, K., Eguchi, Y., Orita, S., & Ohtori, S. (2024). Machine Learning and Deep Learning in Spinal Injury: A Narrative Review of Algorithms in Diagnosis and Prognosis. Journal of Clinical Medicine, 13(3), 705. https://doi.org/10.3390/jcm13030705






