Visuo-Cognitive Phenotypes in Early Multiple Sclerosis: A Multisystem Model of Visual Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Afferent Visual Processing Measures
2.3.1. Retinal Nerve Fibre Layer Thickness (RNFL)
2.3.2. Visual Acuity
2.4. Cognitive Visual Processing Measures
2.4.1. Ocular Motor Apparatus
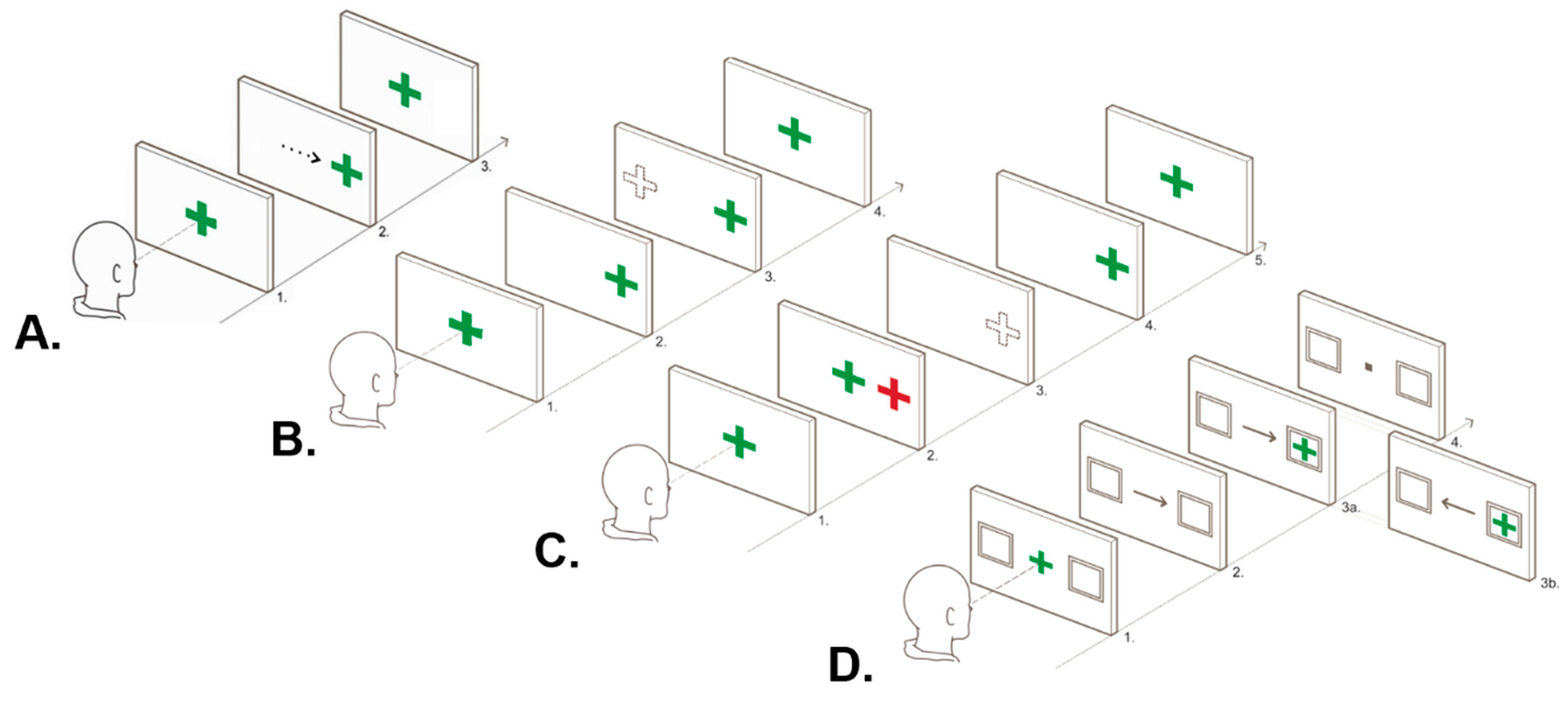
2.4.2. Ocular Motor Tasks
2.4.3. Ocular Motor Data Processing
2.5. Efferent Visual Processing Measures
2.5.1. Diagnosis of Eye Movement Disorder
2.5.2. Versional Dysconjugacy Index
2.6. Clinical Characteristics
2.6.1. Neuropsychological Assessment
2.6.2. Assessment of Disease and Disability Severity
2.7. Data Preparation
2.8. Data Analysis
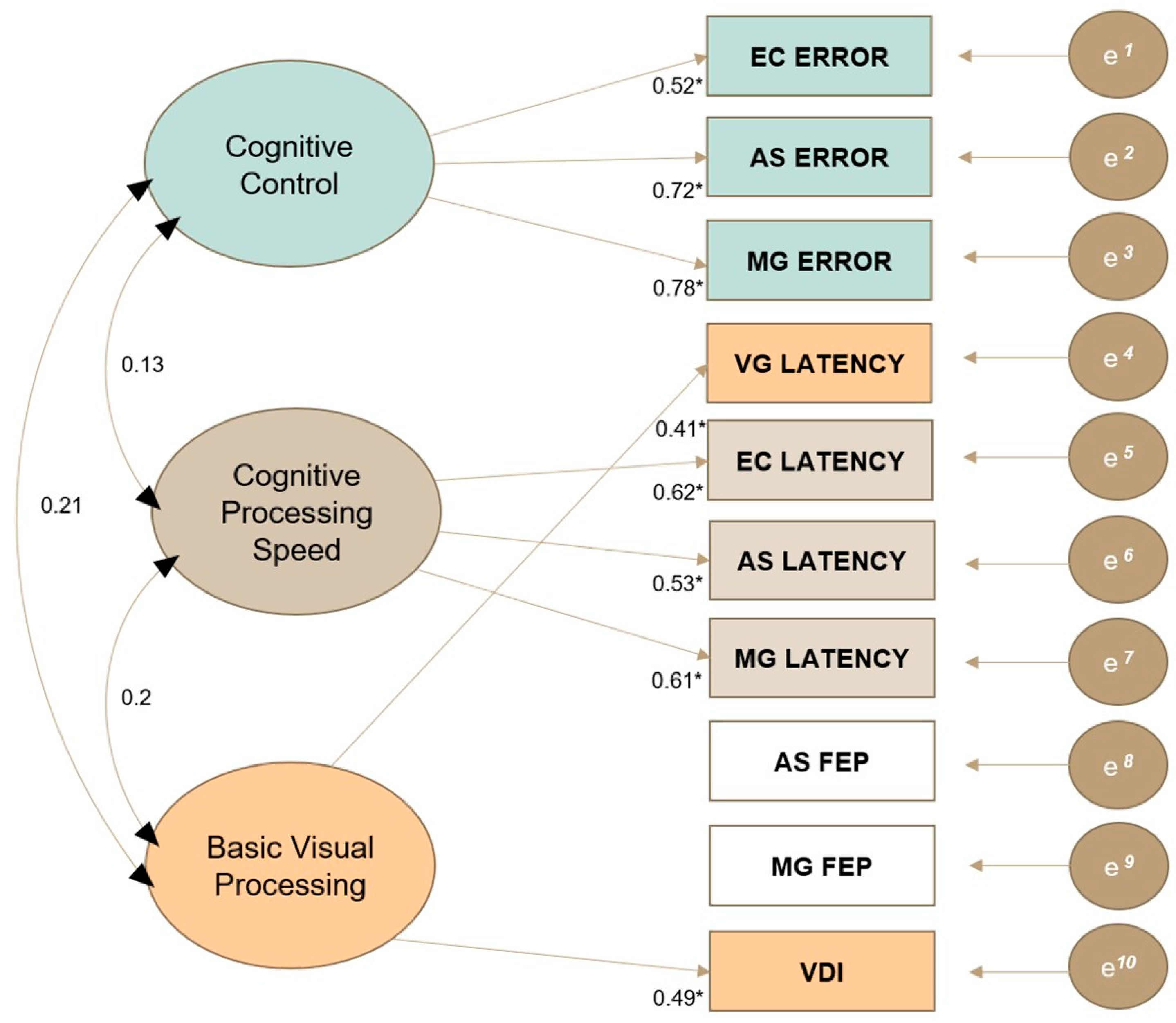
2.8.1. Exploratory Factor Analysis
2.8.2. Latent Profile Analysis
- Selection of variables. The variables of RNFL, VG latency, EC latency, EC error, AS latency, AS FEP, AS error, MG latency, MG FEP, MG error, VDI_R and VDI_L were included in the LPA models. Visual acuity was initially included in models following LogMAR conversion. However, the inclusion of visual acuity negatively impacted the sample size and interpretability of generated phenotypes, despite the comparable visual acuity estimates between profiles. Consequently, visual acuity was removed from the LPA model and treated as a demographic variable for post-hoc analyses.
- Model specification and selection. Given the non-normal distribution of most visual processing variables, maximum likelihood with robust standard errors (MLR) was applied to latent profile models [38]. There was no a priori assumption regarding how many phenotypes exist due to the lack of previous research on visuo-cognitive phenotypes in MS. Therefore, models with 1 to 6 profiles were sequentially run and model fit indices were examined to determine the best fitting model, including the Bayesian information criterion and the bootstrap likelihood ratio test [39]. The theoretical and clinical interpretability of each model was also considered in the selection process. Please see Supplementary Materials, Section S4.1, for further details regarding model selection. To avoid local maxima, 250 sets of starting values were used for the initial maximisation stage and the 50 best solutions were retained for final-stage optimisation in each model [38]. The highest log-likelihood value was successfully replicated in our selected model, indicating that a global solution was found.
- Model interpretation. Results of the EFA were used to inform our interpretation of phenotypes, with qualitative descriptors assigned to profiles based on the unique visual processing deficits that differentiated each phenotype. As patient data were standardised against those of healthy controls, a value of zero represented the healthy control mean. Thus, z-scores between 1 and 2 represented a mild impairment, z-scores between 2 and 3 represented a moderate impairment and z-scores greater than 3 represented a severe impairment for OM variables. RNFL was inversely interpreted, with z-scores below −1 denoting afferent visual processing impairment.
- Sensitivity analysis. Given the asymmetrical distribution of imputed values for some missing datapoints, a sensitivity analysis was conducted by running another series of LPA models with pairwise missingness to compare against imputed LPA models. Please see Section S4.2 of the Supplementary Materials for further details.
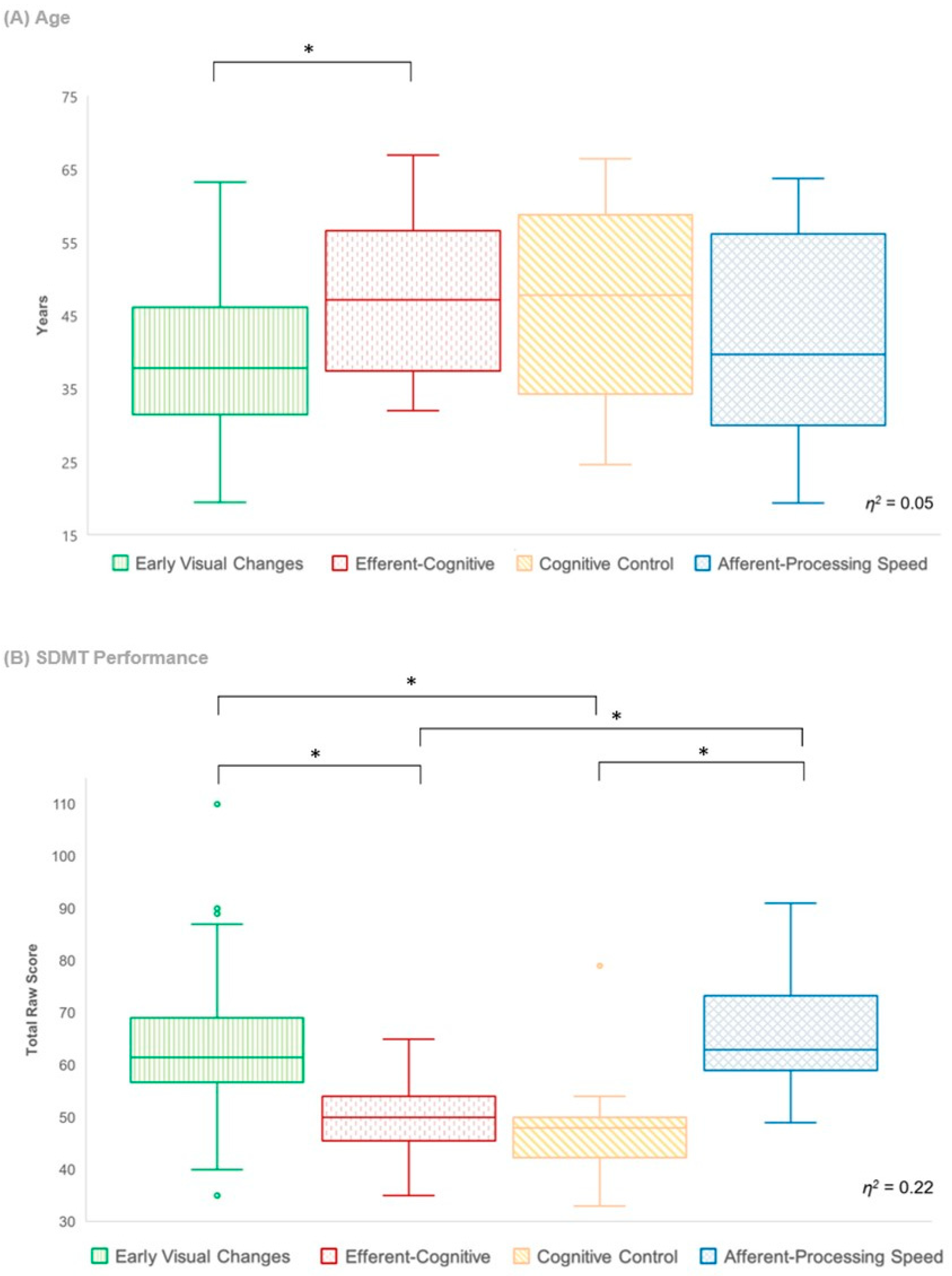
- Phenotype differences. For the selected LPA model, the entropy was 0.94 and all average latent class probabilities were greater than 0.9, suggesting that the generated profiles could be treated as discrete categories for post hoc analyses [39]. Thus, inter-phenotype differences across various clinical characteristics were examined using a Kruskal–Wallis test with Dunn’s multiple comparison to gain further insight into the clinical relevance of each phenotype.
3. Results
3.1. Descriptive Statistics
3.2. Exploratory Factor Analysis
3.3. Latent Profile Analysis
4. Discussion
4.1. Early Visual Changes Phenotype
4.2. Efferent-Cognitive Phenotype
4.3. Cognitive Control Phenotype
4.4. Afferent-Processing Speed Phenotype
4.5. Clinical Characteristics of Phenotypes
4.6. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemens, L.; Langdon, D. How does cognition relate to employment in multiple sclerosis? A systematic review. Mult. Scler. Relat. Disord. 2018, 26, 183–191. [Google Scholar] [CrossRef]
- Cattaneo, D.; Lamers, I.; Bertoni, R.; Feys, P.; Jonsdottir, J. Participation restriction in people with multiple sclerosis: Prevalence and correlations with cognitive, walking, balance, and upper limb impairments. Arch. Phys. Med. Rehabil. 2017, 98, 1308–1315. [Google Scholar] [CrossRef]
- Hynčicová, E.; Vyhnálek, M.; Kalina, A.; Martinkovič, L.; Nikolai, T.; Lisý, J.; Hort, J.; Meluzínová, E.; Laczó, J. Cognitive impairment and structural brain changes in patients with clinically isolated syndrome at high risk for multiple sclerosis. J. Neurol. 2017, 264, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Pitteri, M.; Romualdi, C.; Magliozzi, R.; Monaco, S.; Calabrese, M. Cognitive impairment predicts disability progression and cortical thinning in MS: An 8-year study. Mult. Scler. J. 2017, 23, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Mitolo, M.; Venneri, A.; Wilkinson, I.D.; Sharrack, B. Cognitive rehabilitation in multiple sclerosis: A systematic review. J. Neurol. Sci. 2015, 354, 1–9. [Google Scholar] [CrossRef]
- De Meo, E.; Portaccio, E. It is time to define cognitive phenotypes in multiple sclerosis. Mult. Scler. 2023, 29, 489–491. [Google Scholar] [CrossRef] [PubMed]
- De Meo, E.; Portaccio, E.; Giorgio, A.; Ruano, L.; Goretti, B.; Niccolai, C.; Patti, F.; Chisari, C.G.; Gallo, P.; Grossi, P. Identifying the Distinct Cognitive Phenotypes in Multiple Sclerosis. JAMA Neurol. 2020, 78, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, V.M.; Tosto, G.; Riley, C.S. Cognitive phenotypes in multiple sclerosis. J. Neurol. 2018, 265, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Podda, J.; Ponzio, M.; Pedulla, L.; Bragadin, M.M.; Battaglia, M.A.; Zaratin, P.; Brichetto, G.; Tacchino, A. Predominant cognitive phenotypes in multiple sclerosis: Insights from patient-centered outcomes. Mult. Scler. Relat. Disord. 2021, 51, 102919. [Google Scholar] [CrossRef] [PubMed]
- Hancock, L.M.; Galioto, R.; Samsonov, A.; Busch, R.M.; Hermann, B.; Matias-Guiu, J.A. A proposed new taxonomy of cognitive phenotypes in multiple sclerosis: The International Classification of Cognitive Disorders in MS (IC-CoDiMS). Mult. Scler. J. 2023, 29, 615–627. [Google Scholar] [CrossRef]
- Chen, M.H.; Chiaravalloti, N.D.; Genova, H.M.; Costa, S.L. Visual and motor confounds on the symbol digit modalities test. Mult. Scler. Relat. Disord. 2020, 45, 102436. [Google Scholar] [CrossRef]
- Jakimovski, D.; Benedict, R.H.; Weinstock-Guttman, B.; Ozel, O.; Fuchs, T.A.; Lincoff, N.; Bergsland, N.; Dwyer, M.G.; Zivadinov, R. Visual deficits and cognitive assessment of multiple sclerosis: Confounder, correlate, or both? J. Neurol. 2021, 268, 2578–2588. [Google Scholar] [CrossRef]
- Jasse, L.; Vukusic, S.; Durand-Dubief, F.; Vartin, C.; Piras, C.; Bernard, M.; Pélisson, D.; Confavreux, C.; Vighetto, A.; Tilikete, C. Persistent visual impairment in multiple sclerosis: Prevalence, mechanisms and resulting disability. Mult. Scler. J. 2013, 19, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Heesen, C.; Böhm, J.; Reich, C.; Kasper, J.; Goebel, M.; Gold, S. Patient perception of bodily functions in multiple sclerosis: Gait and visual function are the most valuable. Mult. Scler. J. 2008, 14, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Kale, N. Optic neuritis as an early sign of multiple sclerosis. Eye Brain 2016, 8, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, E.; Ara, J.R.; Martin, J.; Almarcegui, C.; Dolz, I.; Vilades, E.; Gil-Arribas, L.; Fernandez, F.J.; Polo, V.; Larrosa, J.M.; et al. Retinal and Optic Nerve Degeneration in Patients with Multiple Sclerosis Followed up for 5 Years. Ophthalmology 2017, 124, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Clough, M.; Dobbing, J.; Stankovich, J.; Ternes, A.; Kolbe, S.; White, O.; Fielding, J. Cognitive processing speed deficits in multiple sclerosis: Dissociating sensorial and motor processing changes from cognitive processing speed. Mult. Scler. Relat. Disord. 2020, 38, 101522. [Google Scholar] [CrossRef] [PubMed]
- Clough, M.; Mitchell, L.; Millist, L.; Lizak, N.; Beh, S.; Frohman, T.C.; Frohman, E.M.; White, O.B.; Fielding, J. Ocular motor measures of cognitive dysfunction in multiple sclerosis II: Working memory. J. Neurol. 2015, 262, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Ternes, A.-M.; Clough, M.; Foletta, P.; White, O.; Fielding, J. Characterization of inhibitory failure in Multiple Sclerosis: Evidence of impaired conflict resolution. J. Clin. Exp. Neuropsychol. 2019, 41, 320–329. [Google Scholar] [CrossRef]
- Mirmosayyeb, O.; Zivadinov, R.; Weinstock-Guttman, B.; Benedict, R.H.; Jakimovski, D. Optical coherence tomography (OCT) measurements and cognitive performance in multiple sclerosis: A systematic review and meta-analysis. J. Neurol. 2023, 270, 1266–1285. [Google Scholar] [CrossRef]
- Cooray, G.K.; Sundgren, M.; Brismar, T. Mechanism of visual network dysfunction in relapsing-remitting multiple sclerosis and its relation to cognition. Clin. Neurophysiol. 2020, 131, 361–367. [Google Scholar] [CrossRef]
- Costa, S.L.; Gonçalves, O.F.; DeLuca, J.; Chiaravalloti, N.; Chakravarthi, R.; Almeida, J. The temporal dynamics of visual processing in multiple sclerosis. Appl. Neuropsychol. Adult 2016, 23, 133–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arnett, P.A.; Cadden, M.; Roman, C.A.; Guty, E.; Riegler, K.; Thomas, G. Sensory-motor and affective-fatigue factors are associated with symbol digit performance in multiple sclerosis. J. Int. Neuropsychol. Soc. 2022, 28, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Arnett, P.A.; Smith, M.M.; Barwick, F.H.; Benedict, R.H.; Ahlstrom, B.P. Oralmotor slowing in multiple sclerosis: Relationship to neuropsychological tasks requiring an oral response. J. Int. Neuropsychol. Soc. 2008, 14, 454–462. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, N.; Hutchinson, M.; McGuigan, C.; Chataway, J. 2017 McDonald diagnostic criteria: A review of the evidence. Mult. Scler. Relat. Disord. 2018, 24, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Clough, M.; Millist, L.; Lizak, N.; Beh, S.; Frohman, T.C.; Frohman, E.M.; White, O.B.; Fielding, J. Ocular motor measures of cognitive dysfunction in multiple sclerosis I: Inhibitory control. J. Neurol. 2015, 262, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Tiew, S.; Lim, C.; Sivagnanasithiyar, T. Using an excel spreadsheet to convert Snellen visual acuity to LogMAR visual acuity. Eye 2020, 34, 2148–2149. [Google Scholar] [CrossRef]
- Clough, M. The Utility of Ocular Motor Assessment in Patients with a Clinically Isolated Syndrome Suggestive of Multiple Sclerosis. Ph.D. Thesis, Monash University, Melbourne, Australia, 2014. [Google Scholar]
- Bijvank, J.N.; Van Rijn, L.; Balk, L.; Tan, H.; Uitdehaag, B.; Petzold, A. Diagnosing and quantifying a common deficit in multiple sclerosis: Internuclear ophthalmoplegia. Neurology 2019, 92, e2299–e2308. [Google Scholar] [CrossRef]
- Smith, A. Symbol Digit Modalities Test Manual (Revised); Western Psychological Services: Los Angeles, CA, USA, 1982. [Google Scholar]
- Nelson, H.E.; Willison, J. National Adult Reading Test (NART); Nfer-Nelson: Windsor, UK, 1991. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II; American Psychological Association: Washington, DC, USA, 1996. [Google Scholar] [CrossRef]
- Budenz, D.L.; Anderson, D.R.; Varma, R.; Schuman, J.; Cantor, L.; Savell, J.; Greenfield, D.S.; Patella, V.M.; Quigley, H.A.; Tielsch, J. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology 2007, 114, 1046–1052. [Google Scholar] [CrossRef]
- Muthén, L.; Muthén, B. Mplus User’s Guide, 6th ed.; Muthén & Muthén: Los Angeles, CA, USA, 2011; Volume 10. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 1 June 2022).
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 1 June 2022).
- Rosseel, Y.; Oberski, D.; Byrnes, J.; Vanbrabant, L.; Savalei, V.; Merkle, E.; Hallquist, M.; Rhemtulla, M.; Katsikatsou, M.; Barendse, M. lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2017, 48, 1–36. Available online: https://lavaan.ugent.be (accessed on 1 June 2022).
- Spurk, D.; Hirschi, A.; Wang, M.; Valero, D.; Kauffeld, S. Latent profile analysis: A review and “how to” guide of its application within vocational behavior research. J. Vocat. Behav. 2020, 120, 103445. [Google Scholar] [CrossRef]
- Ferguson, S.L.; Moore, E.W.G.; Hull, D.M. Finding latent groups in observed data: A primer on latent profile analysis in Mplus for applied researchers. Int. J. Behav. Dev. 2020, 44, 458–468. [Google Scholar] [CrossRef]
- Strober, L.B.; Rao, S.M.; Lee, J.-C.; Fischer, E.; Rudick, R. Cognitive impairment in multiple sclerosis: An 18 year follow-up study. Mult. Scler. Relat. Disord. 2014, 3, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Talman, L.S.; Bisker, E.R.; Sackel, D.J.; Long, D.A., Jr.; Galetta, K.M.; Ratchford, J.N.; Lile, D.J.; Farrell, S.K.; Loguidice, M.J.; Remington, G. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann. Neurol. 2010, 67, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Clough, M.; Bartholomew, J.; White, O.B.; Fielding, J. Working memory phenotypes in early multiple sclerosis: Appraisal of phenotype frequency, progression and test sensitivity. J. Clin. Med. 2022, 11, 2936. [Google Scholar] [CrossRef] [PubMed]
- Sandry, J.; Simonet, D.V.; Brandstadter, R.; Krieger, S.; Sand, I.K.; Graney, R.A.; Buchanan, A.V.; Lall, S.; Sumowski, J.F. The Symbol Digit Modalities Test (SDMT) is sensitive but non-specific in MS: Lexical access speed, memory, and information processing speed independently contribute to SDMT performance. Mult. Scler. Relat. Disord. 2021, 51, 102950. [Google Scholar] [CrossRef] [PubMed]
- Sandry, J.; Levy, S.; Sumowski, J.F. Psychometrically valid interpretation of cognitive assessments is a prerequisite for classification of cognitive phenotypes in multiple sclerosis. Mult. Scler. 2023, 29, 1039–1040. [Google Scholar] [CrossRef] [PubMed]
- Talanow, T.; Kasparbauer, A.-M.; Steffens, M.; Meyhöfer, I.; Weber, B.; Smyrnis, N.; Ettinger, U. Facing competition: Neural mechanisms underlying parallel programming of antisaccades and prosaccades. Brain Cogn. 2016, 107, 37–47. [Google Scholar] [CrossRef]
- Bedi, H.; Goltz, H.C.; Wong, A.M.; Chandrakumar, M.; Niechwiej-Szwedo, E. Error correcting mechanisms during antisaccades: Contribution of online control during primary saccades and offline control via secondary saccades. PLoS ONE 2013, 8, e68613. [Google Scholar] [CrossRef][Green Version]
- Herweg, N.A.; Weber, B.; Kasparbauer, A.; Meyhöfer, I.; Steffens, M.; Smyrnis, N.; Ettinger, U. Functional magnetic resonance imaging of sensorimotor transformations in saccades and antisaccades. Neuroimage 2014, 102, 848–860. [Google Scholar] [CrossRef]
- Jaun-Frutiger, K.; Cazzoli, D.; Müri, R.M.; Bassetti, C.L.; Nyffeler, T. The frontal eye field is involved in visual vector inversion in humans—A theta burst stimulation study. PLoS ONE 2013, 8, e83297. [Google Scholar] [CrossRef][Green Version]
- Mesaros, S.; Rocca, M.A.; Riccitelli, G.; Pagani, E.; Rovaris, M.; Caputo, D.; Ghezzi, A.; Capra, R.; Bertolotto, A.; Comi, G. Corpus callosum damage and cognitive dysfunction in benign MS. Hum. Brain Mapp. 2009, 30, 2656–2666. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.; Coleman, K.; Jesso, S.; Jodoin, V.D.; Smolewska, K.; Warriner, E.; Finger, E.; Pasternak, S.H. Depressive symptoms negatively impact Montreal Cognitive Assessment performance: A memory clinic experience. Can. J. Neurol. Sci. 2016, 43, 513–517. [Google Scholar] [CrossRef]
- Wurpts, I.C.; Geiser, C. Is adding more indicators to a latent class analysis beneficial or detrimental? Results of a Monte-Carlo study. Front. Psychol. 2014, 5, 920. [Google Scholar] [CrossRef]
- Azzam, D.; Ronquillo, Y. Snellen Chart; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lee, K.J.; Carlin, J.B. Multiple imputation in the presence of non-normal data. Stat. Med. 2017, 36, 606–617. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr. Hmisc: Harrell Miscellaneous. 2019. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 1 June 2022).
- Matta, T.H.; Flournoy, J.C.; Byrne, M.L. Making an unknown unknown a known unknown: Missing data in longitudinal neuroimaging studies. Dev. Cogn. Neurosci. 2018, 33, 83–98. [Google Scholar] [CrossRef] [PubMed]
| Measure | Description | Variable(s) of Interest |
|---|---|---|
| Afferent Visual Processing | ||
| OCT a | Structural measure of afferent visual pathway | Global RNFL thickness (μm) |
| Snellen Chart a | Functional measure of afferent visual pathway | Visual acuity score |
| Cognitive Visual Processing | ||
| Visually Guided Task | Basic prosaccade task primarily measuring simple processing speed | Latency |
| Endogenously Cued Task | Cognitive OM task primarily measuring processing speed and attentional control | Latency of correct trials and error rate |
| Antisaccade and Memory Guided Tasks | Cognitive OM tasks primarily measuring processing speed, spatial accuracy, inhibitory control and spatial working memory | Latency of correct trials, error rate and final eye position |
| Efferent Visual Processing | ||
| VDI | Sub-clinical measure of efferent pathway | Dysconjugacy ratio of abducting and adducting eye on a basic prosaccade task |
| Ophthalmic Assessment a | Clinical measure of efferent pathway | Diagnosis of INO, nystagmus or oscillopsia |
| Clinical Characteristics | ||
| Symptom Duration a | Subjective measure of symptom duration | Months since first reported symptom |
| EDSS a | Measure of disability severity | Total score |
| Oral SDMT | Screening measure of cognitive impairment | Total raw score |
| BDI | Screening measure of depressive symptoms | Total score |
| NART | Estimated premorbid intellectual functioning | Standard score |
| HC (n = 25) | CIS (n = 50) | CDMS (n = 90) | Group Differences | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (F:M) | 16:9 | 39:11 | 77:13 | HC- CIS | HC-CDMS | CIS-CDMS | ||||||
| n | Mean (SD) | Range | n | Mean (SD) | Range | n | Mean (SD) | Range | p | |||
| Age | 25 | 34.24 (13.13) | 21–65 | 50 | 35.67 (10.01) | 19–59 | 90 | 43.18 (10.81) | 19–66 | .86 | .001 | <.001 |
| NART Std. Score | 18 | 118.22 (3.54) | 110–124 | 49 | 113.24 (6.4) | 96–124 | 87 | 115.37 (5.32) | 100–126 | .004 | .12 | .08 |
| BDI Total Score | 25 | 3.08 (2.25) | 0–8 | 50 | 7 (6.15) | 0–24 | 88 | 7.27 (7.56) | 0–38 | .04 | .02 | .97 |
| SDMT Total Score | 25 | 65.66 (12.13) | 46.5–97 | 49 | 63.76 (13.09) | 35–110 | 85 | 60.64 (12.53) | 33–91 | .81 | .19 | .36 |
| n | Median (IQR) | Range | n | Median (IQR) | Range | n | Median (IQR) | Range | p | |||
| Symptom Duration (mo.) | - | - | - | 32 | 5.5 (19.75) | 0–216 | 64 | 78.5 (123.25) | 4–524 | - | - | <.001 |
| EDSS Total Score | - | - | - | 43 | 0 (0) | 0–4 | 76 | 0 (1) | 0–6 | - | - | .006 |
| Visual Acuity (LogMAR) | - | - | - | 46 | 0 (0) | −0.12–0.6 | 80 | 0 (0) | −0.12–0.3 | - | - | .71 |
| Variable | Healthy Control Group | Patient Group (CIS and CDMS) | ||||
|---|---|---|---|---|---|---|
| n | Median (IQR) a | Range | n | Median (IQR) a | Range | |
| RNFL | - | - | - | 124 | 91.5 (19) | 48–121 |
| VG_LATENCY | 25 | 170.06 * (35.16) | 134.97–219.89 | 140 | 187.86 * (31.73) | 133.08–271.26 |
| EC_LATENCY | 25 | 244.81 (61.09) | 80.36–380.57 | 138 | 247.08 (123.44) | 44.07–652.67 |
| EC_ERROR | 25 | 4.17 * (4.17) | 0–36.17 | 140 | 11.81 * (18.72) | 0–50 |
| AS_LATENCY | 25 | 282.38 (161.08) | 108.31–497.21 | 139 | 300.74 (152.25) | 121.79–694.44 |
| AS_FEP | 17 | 21.51 (6.26) | 11.87–28.51 | 138 | 22.79 (13.35) | 5.72–66.23 |
| AS_ERROR | 25 | 6.67 * (4.17) | 0–33.33 | 138 | 12.5 * (20.53) | 0–77.1 |
| MG_LATENCY | 24 | 330.04 (143.23) | 155.77–659.24 | 112 | 323.37 (134.84) | 104.09–844.84 |
| MG_FEP | 23 | 13.32 (7.72) | 5.67–28.75 | 112 | 12.557 (5.34) | 5.28–38.15 |
| MG_ERROR | 24 | 10.42 * (8.1) | 2.08–36.17 | 112 | 16.7 * (20.9) | 0–75 |
| VDI_R | 19 | 1.03 (0.08) | 0.86–1.12 | 136 | 1.03 (0.12) | 0.81–1.57 |
| VDI_L | 19 | 1.04 (0.09) | 0.88–1.17 | 134 | 1.03 (0.14) | 0.65–1.62 |
| Qualitative Phenotype Descriptor | Demographic Characteristics | Profile Estimates a | Afferent | Cognitive | Efferent | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | F:M | CDMS (%) | Age (m) | Basic Processing Speed | Cognitive Processing Speed | Cognitive Control | Spatial Accuracy | ||||||||||
| RNFL (μm) | VG_LAT | EC_LAT | AS_LAT | MG_LAT | EC_ERR | AS_ERR | MG_ERR | AS_FEP | MG_FEP | VDI_R | VDI_L | ||||||
| Early Visual Changes | 100 | 82:18 | 56 | 38.72 | Mean Est. | −0.809 ** | 0.676 ** | −0.097 | −0.144 | −0.387 ** | 0.997 ** | 0.739 ** | 0.723 ** | 0.83 ** | −0.241 ** | −0.234 | 0.175 |
| Median | - | 0.489 | - | −0.183 | - | 0.513 | 0.405 | 0.083 | 0.152 | −0.345 | - | - | |||||
| S.E. | 0.123 | 0.115 | 0.138 | 0.098 | 0.073 | 0.189 | 0.167 | 0.188 | 0.265 | 0.092 | 0.147 | 0.111 | |||||
| Efferent-Cognitive | 12 | 10:2 | 92 | 47.78 | Mean Est. | −0.921 * | 1.347 ** | 0.089 | 0.290 | −0.273 | 1.362 ** | 3.681 ** | 1.953 ** | 2.941 ** | 0.496 | 6.519 ** | 0.999 |
| Median | - | - | - | 0.031 | −0.347 | - | - | - | - | 0.241 | - | - | |||||
| S.E. | 0.400 | 0.268 | 0.305 | 0.234 | 0.167 | 0.331 | 0.819 | 0.555 | 1.031 | 0.259 | 0.588 | 1.004 | |||||
| Cognitive Control | 12 | 10:2 | 83 | 46.73 | Mean Est. | −0.921 * | 0.818* | 0.261 | 0.650 | 0.681* | 2.770 ** | 7.415 ** | 3.826 ** | 3.231 ** | −0.116 | −0.797 | −0.122 |
| Median | - | - | - | - | 0.436 | 3.43 | - | - | - | - | - | - | |||||
| S.E. | 0.441 | 0.375 | 0.470 | 0.498 | 0.267 | 0.810 | 1.049 | 0.744 | 1.056 | 0.256 | 0.537 | 0.405 | |||||
| Afferent- Processing Speed | 16 | 14:2 | 81 | 41.48 | Mean Est. | −1.085 ** | 1.440 ** | 2.609 ** | 1.156 ** | 1.116 ** | 0.670 | 0.393 | 0.717 | 2.286 * | 0.239 | 0.261 | −0.096 |
| Median | - | - | - | - | - | 0.046 | −0.201 | - | 0.984 | - | - | −0.417 | |||||
| S.E. | 0.357 | 0.430 | 0.494 | 0.289 | 0.351 | 0.360 | 0.309 | 0.368 | 1.069 | 0.206 | 0.402 | 0.376 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagias, H.; Byrne, M.L.; Millist, L.; White, O.; Clough, M.; Fielding, J. Visuo-Cognitive Phenotypes in Early Multiple Sclerosis: A Multisystem Model of Visual Processing. J. Clin. Med. 2024, 13, 649. https://doi.org/10.3390/jcm13030649
Vagias H, Byrne ML, Millist L, White O, Clough M, Fielding J. Visuo-Cognitive Phenotypes in Early Multiple Sclerosis: A Multisystem Model of Visual Processing. Journal of Clinical Medicine. 2024; 13(3):649. https://doi.org/10.3390/jcm13030649
Chicago/Turabian StyleVagias, Hariklia, Michelle L. Byrne, Lyn Millist, Owen White, Meaghan Clough, and Joanne Fielding. 2024. "Visuo-Cognitive Phenotypes in Early Multiple Sclerosis: A Multisystem Model of Visual Processing" Journal of Clinical Medicine 13, no. 3: 649. https://doi.org/10.3390/jcm13030649
APA StyleVagias, H., Byrne, M. L., Millist, L., White, O., Clough, M., & Fielding, J. (2024). Visuo-Cognitive Phenotypes in Early Multiple Sclerosis: A Multisystem Model of Visual Processing. Journal of Clinical Medicine, 13(3), 649. https://doi.org/10.3390/jcm13030649







