COVID-19 Vaccination and Disease Course in People with Multiple Sclerosis in Greece
Abstract
:1. Introduction
2. Materials and Methods
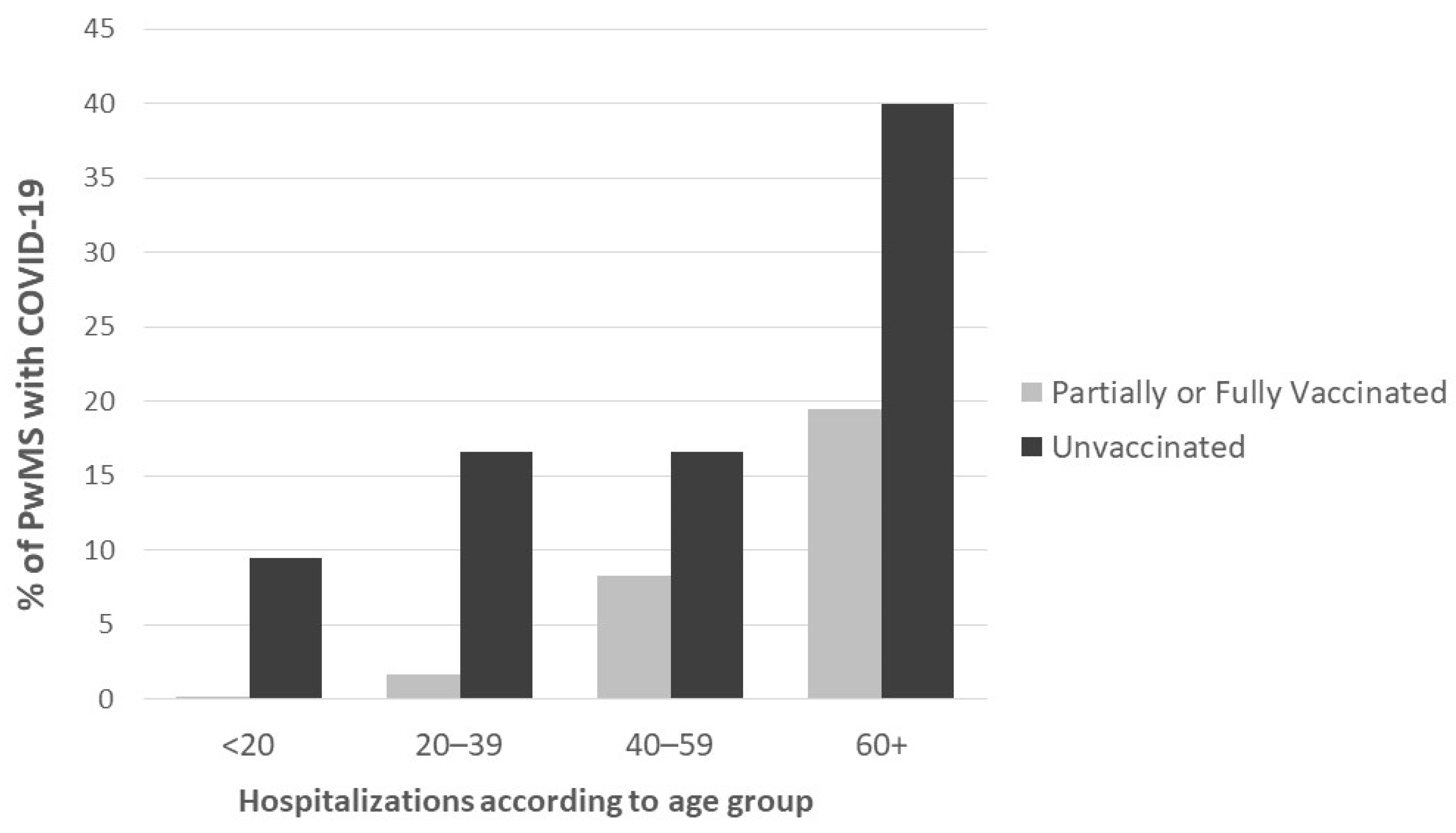
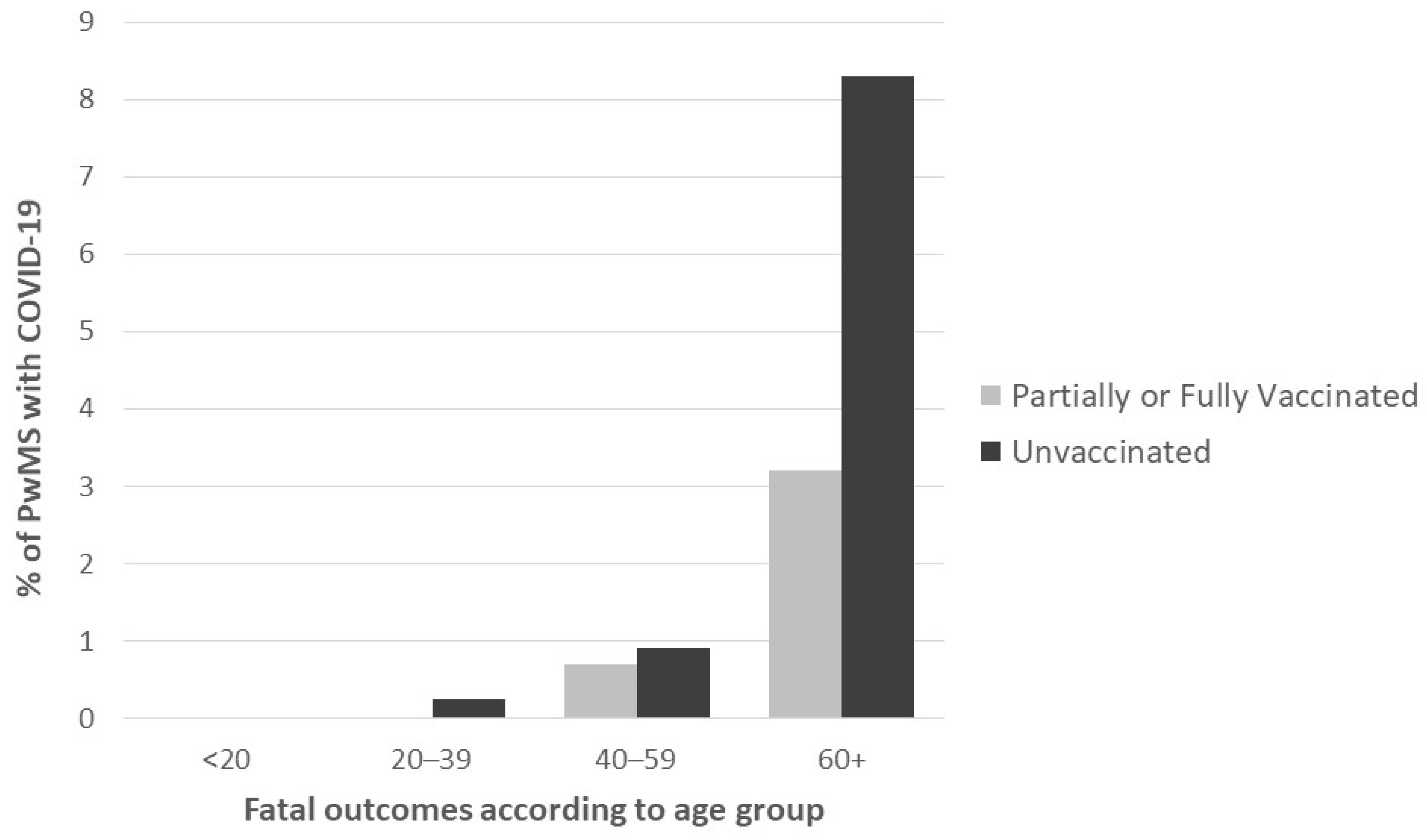
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 27 January 2023).
- Montgomery, S.; Hillert, J.; Bahmanyar, S. Hospital admission due to infections in multiple sclerosis patients. Eur. J. Neurol. 2013, 20, 1153–1160. [Google Scholar] [CrossRef]
- Sormani, M.P.; Schiavetti, I.; Carmisciano, L.; Cordioli, C.; Filippi, M.; Radaelli, M.; Immovilli, P.; Capobianco, M.; De Rossi, N.; Brichetto, G.; et al. COVID-19 Severity in Multiple Sclerosis: Putting Data into Context. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1105. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Yadaw, A.S.; Li, Y.C.; Bose, S.; Iyengar, R.; Bunyavanich, S.; Pandey, G. Clinical features of COVID-19 mortality: Development and validation of a clinical prediction model. Lancet Digit. Health 2020, 2, e516–e525. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Qiu, W.; Bu, B.; Xu, Y.; Yang, H.; Huang, D.; Lau, A.Y.; Guo, J.; Zhang, M.N.; Zhang, X.; et al. Risk of COVID-19 infection in MS and neuromyelitis optica spectrum disorders. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e787. [Google Scholar] [CrossRef] [PubMed]
- Castillo Álvarez, F.; López Pérez, M.; Marzo Sola, M.E. Risk of SARS-CoV-2 infection and clinical outcomes in multiple sclerosis patients in La Rioja (Spain): Riesgo de infección por SARS-CoV-2 y resultados clínicos en pacientes con esclerosis múltiple en la Rioja (España). Med. Clin. 2020, 155, 362–363. [Google Scholar] [CrossRef] [PubMed]
- Louapre, C.; Collongues, N.; Stankoff, B.; Giannesini, C.; Papeix, C.; Bensa, C.; Deschamps, R.; Créange, A.; Wahab, A.; Pelletier, J.; et al. Clinical Characteristics and Outcomes in Patients with Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurol. 2020, 77, 1079–1088. [Google Scholar] [CrossRef]
- Salter, A.; Fox, R.J.; Newsome, S.D.; Halper, J.; Li, D.K.B.; Kanellis, P.; Costello, K.; Bebo, B.; Rammohan, K.; Cutter, G.R.; et al. Outcomes and Risk Factors Associated With SARS-CoV-2 Infection in a North American Registry of Patients With Multiple Sclerosis. JAMA Neurol. 2021, 78, 699–708. [Google Scholar] [CrossRef]
- Barzegar, M.; Mirmosayyeb, O.; Gajarzadeh, M.; Afshari-Safavi, A.; Nehzat, N.; Vaheb, S.; Shaygannejad, V.; Maghzi, A.H. COVID-19 Among Patients with Multiple Sclerosis: A Systematic Review. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1001. [Google Scholar] [CrossRef]
- Bsteh, G.; Bitschnau, C.; Hegen, H.; Auer, M.; Di Pauli, F.; Rommer, P.; Deisenhammer, F.; Berger, T. Multiple sclerosis and COVID-19: How many are at risk? Eur. J. Neurol. 2021, 28, 3369–3374. [Google Scholar] [CrossRef]
- Chaudhry, F.; Jageka, C.; Levy, P.D.; Cerghet, M.; Lisak, R.P. Review of the COVID-19 Risk in Multiple Sclerosis. J. Cell. Immunol. 2021, 3, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Etemadifar, M.; Nouri, H.; Maracy, M.R.; Akhavan Sigari, A.; Salari, M.; Blanco, Y.; Sepúlveda, M.; Zabalza, A.; Mahdavi, S.; Baratian, M.; et al. Risk factors of severe COVID-19 in people with multiple sclerosis: A systematic review and meta-analysis. Rev. Neurol. 2022, 178, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.J.; D’Silva, K.M.; Hsu, T.Y.; DiIorio, M.; Fu, X.; Cook, C.; Prisco, L.; Martin, L.; Vanni, K.M.M.; Zaccardelli, A.; et al. Coronavirus Disease 2019 Outcomes Among Recipients of Anti-CD20 Monoclonal Antibodies for Immune-Mediated Diseases: A Comparative Cohort Study. ACR Open Rheumatol. 2022, 4, 238–246. [Google Scholar] [CrossRef]
- Grebenciucova, E.; Pruitt, A. Infections in Patients Receiving Multiple Sclerosis Disease-Modifying Therapies. Curr. Neurol. Neurosci. Rep. 2017, 17, 88. [Google Scholar] [CrossRef]
- Baker, D.; Amor, S.; Kang, A.S.; Schmierer, K.; Giovannoni, G. The underpinning biology relating to multiple sclerosis disease modifying treatments during the COVID-19 pandemic. Mult. Scler. Relat. Disord. 2020, 43, 102174. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Sun, Q.; Knopf, J.; Herrmann, M.; Lin, L.; Jiang, J.; Shao, C.; Li, P.; He, X.; et al. Immune response in COVID-19: What is next? Cell Death Differ. 2022, 29, 1107–1122. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Qiao, L.; Wang, W.; Chen, D. Inflammatory Response Cells During Acute Respiratory Distress Syndrome in Patients with Coronavirus Disease 2019 (COVID-19). Ann. Intern. Med. 2020, 173, 402–404. [Google Scholar] [CrossRef]
- Berger, J.R.; Brandstadter, R.; Bar-Or, A. COVID-19 and MS disease-modifying therapies. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e761. [Google Scholar] [CrossRef]
- Sormani, M.P.; Salvetti, M.; Labauge, P.; Schiavetti, I.; Zephir, H.; Carmisciano, L.; Bensa, C.; De Rossi, N.; Pelletier, J.; Cordioli, C.; et al. DMTs and COVID-19 severity in MS: A pooled analysis from Italy and France. Ann. Clin. Transl. Neurol. 2021, 8, 1738–1744. [Google Scholar] [CrossRef]
- Tasat, D.R.; Yakisich, J.S. Rationale for the use of sphingosine analogues in COVID-19 patients. Clin. Med. 2021, 21, e84–e87. [Google Scholar] [CrossRef]
- Teymouri, S.; Pourbayram Kaleybar, S.; Hejazian, S.S.; Hejazian, S.M.; Ansarin, K.; Ardalan, M.; Zununi Vahed, S. The effect of Fingolimod on patients with moderate to severe COVID-19. Pharmacol. Res. Perspect. 2023, 11, e01039. [Google Scholar] [CrossRef] [PubMed]
- MS International Federation. COVID-19 Vaccines and MS. Available online: https://www.msif.org/news/2020/02/10/the-coronavirus-and-ms-what-you-need-to-know/ (accessed on 27 January 2023).
- Korsukewitz, C.; Reddel, S.W.; Bar-Or, A.; Wiendl, H. Neurological immunotherapy in the era of COVID-19—Looking for consensus in the literature. Nat. Rev. Neurol. 2020, 16, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Reyes, S.; Cunningham, A.L.; Kalincik, T.; Havrdová, E.K.; Isobe, N.; Pakpoor, J.; Airas, L.; Bunyan, R.F.; van der Walt, A.; Oh, J.; et al. Update on the management of multiple sclerosis during the COVID-19 pandemic and post pandemic: An international consensus statement. J. Neuroimmunol. 2021, 357, 577627. [Google Scholar] [CrossRef] [PubMed]
- National Multiple Sclerosis Society. Disease Modifying Therapy Guidelines during COVID-19. Available online: https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/ms-treatment-guidelines-during-coronavirus (accessed on 27 January 2023).
- Simpson-Yap, S.; De Brouwer, E.; Kalincik, T.; Rijke, N.; Hillert, J.A.; Walton, C.; Edan, G.; Moreau, Y.; Spelman, T.; Geys, L.; et al. Associations of Disease-Modifying Therapies with COVID-19 Severity in Multiple Sclerosis. Neurology 2021, 97, e1870–e1885. [Google Scholar] [CrossRef]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Magalashvili, D.; Sonis, P.; Dolev, M.; Menascu, S.; Flechter, S.; Falb, R.; et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211012835. [Google Scholar] [CrossRef]
- Ciampi, E.; Uribe-San-Martin, R.; Soler, B.; García, L.; Guzman, J.; Pelayo, C.; Jürgensen, L.; Guzman, I.; Vera, F.; Galleguillos, L.; et al. Safety and humoral response rate of inactivated and mRNA vaccines against SARS-CoV-2 in patients with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2022, 59, 103690. [Google Scholar] [CrossRef]
- Sormani, M.P.; Inglese, M.; Schiavetti, I.; Carmisciano, L.; Laroni, A.; Lapucci, C.; Da Rin, G.; Serrati, C.; Gandoglia, I.; Tassinari, T.; et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. eBioMedicine 2021, 72, 103581. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef]
- Gadani, S.P.; Reyes-Mantilla, M.; Jank, L.; Harris, S.; Douglas, M.; Smith, M.D.; Calabresi, P.A.; Mowry, E.M.; Fitzgerald, K.C.; Bhargava, P. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. eBioMedicine 2021, 73, 103636. [Google Scholar] [CrossRef]
- Yuzefpolskiy, Y.; Morawski, P.; Fahning, M.; Speake, C.; Lord, S.; Chaudhary, A.; Morishima, C.; Wener, M.H.; Kita, M.; McCarthy, L.; et al. Cutting Edge: Effect of Disease-Modifying Therapies on SARS-CoV-2 Vaccine-Induced Immune Responses in Multiple Sclerosis Patients. J. Immunol. 2022, 208, 1519–1524. [Google Scholar] [CrossRef]
- Pugliatti, M.; Hartung, H.P.; Oreja-Guevara, C.; Pozzilli, C.; Airas, L.; Alkhawajah, M.; Grigoriadis, N.; Magyari, M.; Van Wijmeersch, B.; Zakaria, M.; et al. Anti-SARS-CoV-2 vaccination in people with multiple sclerosis: Lessons learnt a year in. Front. Immunol. 2022, 13, 1045101. [Google Scholar] [CrossRef]
- Achiron, A.; Dolev, M.; Menascu, S.; Zohar, D.N.; Dreyer-Alster, S.; Miron, S.; Shirbint, E.; Magalashvili, D.; Flechter, S.; Givon, U.; et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult. Scler. 2021, 27, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Briggs, F.B.S.; Mateen, F.J.; Schmidt, H.; Currie, K.M.; Siefers, H.M.; Crouthamel, S.; Bebo, B.F.; Fiol, J.; Racke, M.K.; O’Connor, K.C.; et al. COVID-19 Vaccination Reactogenicity in Persons with Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1104. [Google Scholar] [CrossRef] [PubMed]
- Ehde, D.M.; Roberts, M.K.; Herring, T.E.; Alschuler, K.N. Willingness to obtain COVID-19 vaccination in adults with multiple sclerosis in the United States. Mult. Scler. Relat. Disord. 2021, 49, 102788. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.M.; Hollen, C.; Yang, Q.; Brumbach, B.H.; Spain, R.I.; Wooliscroft, L. COVID-19 vaccination willingness among people with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2021, 7, 20552173211017159. [Google Scholar] [CrossRef]
- Fragoso, Y.D.; Gomes, S.; Gonçalves, M.V.M.; Mendes Junior, E.; Oliveira, B.E.S.; Rocha, C.F.; Santos, G.; Tauil, C.B.; Araujo, R.V.; Peron, J.P.S. New relapse of multiple sclerosis and neuromyelitis optica as a potential adverse event of AstraZeneca AZD1222 vaccination for COVID-19. Mult. Scler. Relat. Disord. 2022, 57, 103321. [Google Scholar] [CrossRef]
- Khayat-Khoei, M.; Bhattacharyya, S.; Katz, J.; Harrison, D.; Tauhid, S.; Bruso, P.; Houtchens, M.K.; Edwards, K.R.; Bakshi, R. COVID-19 mRNA vaccination leading to CNS inflammation: A case series. J. Neurol. 2022, 269, 1093–1106. [Google Scholar] [CrossRef]
- Maniscalco, G.T.; Manzo, V.; Di Battista, M.E.; Salvatore, S.; Moreggia, O.; Scavone, C.; Capuano, A. Severe Multiple Sclerosis Relapse After COVID-19 Vaccination: A Case Report. Front. Neurol. 2021, 12, 721502. [Google Scholar] [CrossRef]
- Nistri, R.; Barbuti, E.; Rinaldi, V.; Tufano, L.; Pozzilli, V.; Ianniello, A.; Marinelli, F.; De Luca, G.; Prosperini, L.; Tomassini, V.; et al. Case Report: Multiple Sclerosis Relapses After Vaccination Against SARS-CoV2: A Series of Clinical Cases. Front. Neurol. 2021, 12, 765954. [Google Scholar] [CrossRef]
- EODY-NPHO. Daily Epidemiological Surveillance Report COVID-19. Available online: https://eody.gov.gr/imerisia-ekthesi-epitirisis-covid-19-01-12-2021/ (accessed on 1 December 2021).
- Hellenic Government. Tracking COVID-19 Greece. Available online: https://covid19.gov.gr/covid-map-en/ (accessed on 27 January 2023).
- Hellenic Government. COVID-19 Vaccination Statistics (Weekly). Available online: https://www.data.gov.gr/datasets/mdg_emvolio_weekly/ (accessed on 27 January 2023).
- Vitiello, A.; Ferrara, F.; Auti, A.M.; Di Domenico, M.; Boccellino, M. Advances in the Omicron variant development. J. Intern. Med. 2022, 292, 81–90. [Google Scholar] [CrossRef]
- Bakirtzis, C.; Grigoriadou, E.; Boziki, M.K.; Kesidou, E.; Siafis, S.; Moysiadis, T.; Tsakona, D.; Thireos, E.; Nikolaidis, I.; Pourzitaki, C.; et al. The Administrative Prevalence of Multiple Sclerosis in Greece on the Basis of a Nationwide Prescription Database. Front. Neurol. 2020, 11, 1012. [Google Scholar] [CrossRef] [PubMed]
- Simpson-Yap, S.; Pirmani, A.; Kalincik, T.; De Brouwer, E.; Geys, L.; Parciak, T.; Helme, A.; Rijke, N.; Hillert, J.A.; Moreau, Y.; et al. Updated Results of the COVID-19 in MS Global Data Sharing Initiative: Anti-CD20 and Other Risk Factors Associated With COVID-19 Severity. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e200021. [Google Scholar] [CrossRef] [PubMed]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Lone, N.I.; Baillie, J.K. Comorbidities, multimorbidity and COVID-19. Nat. Med. 2023, 29, 334–343. [Google Scholar] [CrossRef]
- Barzegar, M.; Manteghinejad, A.; Afshari-Safavi, A.; Mirmosayyeb, O.; Nasirian, M.; Bagherieh, S.; Mazaheri, S.; Rahimi, M.; Zabeti, A.; Javanmard, S.H.; et al. Effectiveness of BBIBP-CorV vaccine in preventing SARS-CoV2 infection and severe outcomes in people living with multiple sclerosis: A population-based study. Mult. Scler. Relat. Disord. 2023, 71, 104548. [Google Scholar] [CrossRef]
- Peeters, G.; Van Remoortel, A.; Nagels, G.; Van Schependom, J.; D’Haeseleer, M. Occurrence and Severity of Coronavirus Disease 2019 Are Associated with Clinical Disability Worsening in Patients with Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200089. [Google Scholar] [CrossRef]
- Bertozzi, A.; Mariottini, A.; Marchi, L.; Cristinzi, M.D.; Nistri, R.; Damato, V.; Mechi, C.; Barilaro, A.; Massacesi, L.; Repice, A.M. Safety and effectiveness of the booster dose of mRNA COVID-19 vaccines in people with multiple sclerosis: A monocentric experience. Mult. Scler. Relat. Disord. 2023, 72, 104582. [Google Scholar] [CrossRef]
- Stefanou, M.I.; Palaiodimou, L.; Theodorou, A.; Christodoulou, M.V.; Tzartos, J.S.; Tzanetakos, D.; Kitsos, D.; Chondrogianni, M.; Zouvelou, V.; Dardiotis, E.; et al. Safety of COVID-19 vaccines in multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. 2023, 29, 585–594. [Google Scholar] [CrossRef]
- Rahmani, M.; Moghadasi, A.N.; Shahi, S.; Eskandarieh, S.; Azizi, H.; Hasanzadeh, A.; Ahmadzade, A.; Dehnavi, A.Z.; Farahani, R.H.; Aminianfar, M.; et al. COVID-19 and its implications on the clinico-radiological course of multiple sclerosis: A case-control study. Med. Clin. 2023, 160, 187–192. [Google Scholar] [CrossRef]
- Gad, A.H.E.; Ahmed, S.M.; Garadah, M.Y.A.; Dahshan, A. Multiple sclerosis patients’ response to COVID-19 pandemic and vaccination in Egypt. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 131. [Google Scholar] [CrossRef]
- Rahman, M.S.; Harun, M.G.D.; Sumon, S.A.; Mohona, T.M.; Abdullah, S.A.H.M.; Khan, M.N.H.; Gazi, M.I.; Islam, M.S.; Anwar, M.M.U. Hospitalization and Mortality by Vaccination Status among COVID-19 Patients Aged ≥ 25 Years in Bangladesh: Results from a Multicenter Cross-Sectional Study. Vaccines 2022, 10, 1987. [Google Scholar] [CrossRef]
- Bakirtzis, C.; Boziki, M.K.; Karakasi, M.V.; Moysiadis, T.; Grigoriadis, N. The impact of SARS-CoV-2 immunization on COVID-19 disease course in people with myasthenia gravis. Muscle Nerve 2023, 67, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. COVID-19 Vaccines for People Who Are Moderately or Severely Immunocompromised, Updated 31 May 2023. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html (accessed on 7 August 2023).
- Zaheer, R.; Amin, R.; Riddick, L.; Roy, S.; Wolff, S.; Nathanson, A.; Newsome, S. Impact of COVID-19 on prescribing patterns and treatment selection of disease modifying therapies in multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 71, 104575. [Google Scholar] [CrossRef]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; Geurts van Kessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. 2020, 26, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Bsteh, G.; Dürauer, S.; Assar, H.; Hegen, H.; Heschl, B.; Leutmezer, F.; Pauli, F.D.; Gradl, C.; Traxler, G.; Zulehner, G.; et al. Humoral immune response after COVID-19 in multiple sclerosis: A nation-wide Austrian study. Mult. Scler. 2021, 27, 2209–2218. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Wattengel, B.A.; Carter, M.T.; El-Solh, A.A.; Lesse, A.J.; Mergenhagen, K.A. Outcomes of multiple sclerosis patients admitted with COVID-19 in a large veteran cohort. Mult. Scler. Relat. Disord. 2022, 64, 103964. [Google Scholar] [CrossRef] [PubMed]
- Boross, P.; Leusen, J.H. Mechanisms of action of CD20 antibodies. Am. J. Cancer Res. 2012, 2, 676–690. [Google Scholar] [PubMed]
- Cotchett, K.R.; Dittel, B.N.; Obeidat, A.Z. Comparison of the Efficacy and Safety of Anti-CD20 B Cells Depleting Drugs in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2021, 49, 102787. [Google Scholar] [CrossRef]
- Gingele, S.; Jacobus, T.L.; Konen, F.F.; Hümmert, M.W.; Sühs, K.W.; Schwenkenbecher, P.; Ahlbrecht, J.; Möhn, N.; Müschen, L.H.; Bönig, L.; et al. Ocrelizumab Depletes CD20⁺ T Cells in Multiple Sclerosis Patients. Cells 2018, 8, 12. [Google Scholar] [CrossRef]
- Moser, T.; O’Sullivan, C.; Otto, F.; Hitzl, W.; Pilz, G.; Schwenker, K.; Mrazek, C.; Haschke-Becher, E.; Trinka, E.; Wipfler, P.; et al. Long-term immunological consequences of anti-CD20 therapies on humoral responses to COVID-19 vaccines in multiple sclerosis: An observational study. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221092092. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, L.; Chang, D.; Wang, J.; Hu, Y.; Chen, H.; Guo, L.; Wu, C.; Wang, C.; Wang, Y.; et al. The kinetics of humoral response and its relationship with the disease severity in COVID-19. Commun. Biol. 2020, 3, 780. [Google Scholar] [CrossRef]
- Benucci, M.; Quartuccio, L.; Li Gobbi, F.; Damiani, A.; Grossi, V.; Infantino, M.; Manfredi, M. Persistence of rT-PCR-SARS-CoV-2 infection and delayed serological response, as a possible effect of rituximab according to the hypothesis of Schulze-Koops et al. Ann. Rheum. Dis. 2020, 81, e184. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Choudhary, M.C.; Regan, J.; Sparks, J.A.; Padera, R.F.; Qiu, X.; Solomon, I.H.; Kuo, H.H.; Boucau, J.; Bowman, K.; et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N. Engl. J. Med. 2020, 383, 2291–2293. [Google Scholar] [CrossRef] [PubMed]
- Kos, I.; Balensiefer, B.; Roth, S.; Ahlgrimm, M.; Sester, M.; Schmidt, T.; Thurner, L.; Bewarder, M.; Bals, R.; Lammert, F.; et al. Prolonged Course of COVID-19-Associated Pneumonia in a B-Cell Depleted Patient After Rituximab. Front. Oncol. 2020, 10, 1578. [Google Scholar] [CrossRef]
- Leipe, J.; Wilke, E.L.; Ebert, M.P.; Teufel, A.; Reindl, W. Long, relapsing, and atypical symptomatic course of COVID-19 in a B-cell-depleted patient after rituximab. Semin. Arthritis Rheum. 2020, 50, 1087–1088. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Tsukune, Y.; Watanabe, N.; Sugimoto, K.; Uchimura, A.; Tateyama, M.; Miyashita, Y.; Ochi, Y.; Komatsu, N. Persistent COVID-19 Pneumonia and Failure to Develop Anti-SARS-CoV-2 Antibodies During Rituximab Maintenance Therapy for Follicular Lymphoma. Clin. Lymphoma Myeloma Leuk. 2020, 20, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liang, B.; Chen, C.; Wang, H.; Fang, Y.; Shen, S.; Yang, X.; Wang, B.; Chen, L.; Chen, Q.; et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat. Commun. 2021, 12, 1813. [Google Scholar] [CrossRef]
- Iannetta, M.; Landi, D.; Cola, G.; Malagnino, V.; Teti, E.; Fraboni, D.; Buccisano, F.; Grelli, S.; Coppola, L.; Campogiani, L.; et al. T-cell responses to SARS-CoV-2 in multiple sclerosis patients treated with ocrelizumab healed from COVID-19 with absent or low anti-spike antibody titers. Mult. Scler. Relat. Disord. 2021, 55, 103157. [Google Scholar] [CrossRef]
- Sormani, M.P.; De Rossi, N.; Schiavetti, I.; Carmisciano, L.; Cordioli, C.; Moiola, L.; Radaelli, M.; Immovilli, P.; Capobianco, M.; Trojano, M.; et al. Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann. Neurol. 2021, 89, 780–789. [Google Scholar] [CrossRef]
- Parrotta, E.; Kister, I.; Charvet, L.; Sammarco, C.; Saha, V.; Charlson, R.E.; Howard, J.; Gutman, J.M.; Gottesman, M.; Abou-Fayssal, N.; et al. COVID-19 outcomes in MS: Observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e835. [Google Scholar] [CrossRef]
- Montero-Escribano, P.; Matías-Guiu, J.; Gómez-Iglesias, P.; Porta-Etessam, J.; Pytel, V.; Matias-Guiu, J.A. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: A case series of 60 patients from Madrid, Spain. Mult. Scler. Relat. Disord. 2020, 42, 102185. [Google Scholar] [CrossRef]
- Soresina, A.; Moratto, D.; Chiarini, M.; Paolillo, C.; Baresi, G.; Focà, E.; Bezzi, M.; Baronio, B.; Giacomelli, M.; Badolato, R. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr. Allergy Immunol. 2020, 31, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Avouac, J.; Drumez, E.; Hachulla, E.; Seror, R.; Georgin-Lavialle, S.; El Mahou, S.; Pertuiset, E.; Pham, T.; Marotte, H.; Servettaz, A.; et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: A cohort study. Lancet Rheumatol. 2021, 3, e419–e426. [Google Scholar] [CrossRef] [PubMed]
- Brill, L.; Rechtman, A.; Zveik, O.; Haham, N.; Oiknine-Djian, E.; Wolf, D.G.; Levin, N.; Raposo, C.; Vaknin-Dembinsky, A. Humoral and T-Cell Response to SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis Treated with Ocrelizumab. JAMA Neurol. 2021, 78, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Disanto, G.; Sacco, R.; Bernasconi, E.; Martinetti, G.; Keller, F.; Gobbi, C.; Zecca, C. Association of Disease-Modifying Treatment and Anti-CD20 Infusion Timing with Humoral Response to 2 SARS-CoV-2 Vaccines in Patients with Multiple Sclerosis. JAMA Neurol. 2021, 78, 1529–1531. [Google Scholar] [CrossRef]
- Richter, D.; Faissner, S.; Bartig, D.; Tönges, L.; Hellwig, K.; Ayzenberg, I.; Krogias, C.; Gold, R. Multiple sclerosis is not associated with an increased risk for severe COVID-19: A nationwide retrospective cross-sectional study from Germany. Neurol. Res. Pract. 2021, 3, 42. [Google Scholar] [CrossRef]
- Bakirtzis, C.; Nikolaidis, I.; Boziki, M.-K.; Grigoriadou, E.; Karakasi, M.-V.; Moysiadis, T.; Kesidou, E.; Papazisis, G.; Grigoriadis, N. Epidemiological Insights on Medication Concurrency and Polypharmacy in People with Multiple Sclerosis in Greece. Int. J. MS Care 2023, 25, 140–144. [Google Scholar] [CrossRef]
- Montini, F.; Nozzolillo, A.; Rancoita, P.M.V.; Zanetta, C.; Moiola, L.; Cugnata, F.; Esposito, F.; Rocca, M.A.; Martinelli, V.; Filippi, M. Modifiable risk factors of COVID-19 in patients with multiple sclerosis: A single-centre case-control study. J. Neurol. 2023, 270, 1835–1842. [Google Scholar] [CrossRef]
| Unvaccinated (n = 1204) | Vaccinated (n = 1147) | |
|---|---|---|
| Age (mean, SD) | 44.0 (12.5) | 42.9 (12.6) |
| Male sex | 430 (35.7%) | 390 (34%) |
| Disease-modifying treatment | ||
| None | 168 (14.0%) | 120 (10.5%) |
| Glatiramer acetate | 151 (12.5%) | 124 (10.8%) |
| Interferons | 237 (19.7%) | 205 (17.9%) |
| Teriflunomide | 86 (7.1%) | 78 (6.8%) |
| Dimethyl fumarate | 248 (20.6%) | 238 (20.7%) |
| Fingolimod | 202 (16.8%) | 259 (22.6%) |
| Cladribine | 34 (2.8%) | 43 (3.7%) |
| Azathioprine | 14 (1.2%) | 11 (1.0%) |
| Alemtuzumab | 2 (0.2%) | 0 (0.0%) |
| Natalizumab | 36 (3.0%) | 26 (2.3%) |
| Ocrelizumab | 21 (1.7%) | 35 (3.1%) |
| Rituximab | 5 (0.4%) | 8 (0.7%) |
| Number of comorbidities | ||
| 0 | 913 (75.8%) | 872 (76.0%) |
| 1 | 174 (14.5%) | 173 (15.1%) |
| 2 | 65 (5.4%) | 57 (5.0%) |
| 3 | 36 (3.0%) | 38 (3.3%) |
| 4+ | 16 (1.3%) | 7 (0.6%) |
| Outcome | Independent Factors | Odds Ratio | 95% C.I. for Odds Ratio | p-Value |
|---|---|---|---|---|
| Hospitalization | ||||
| Age | 1.076 | 1.064–1.089 | <0.001 | |
| Male sex | 1.587 | 1.223–2.060 | 0.001 | |
| DMT | <0.001 | |||
| ALEMTUZUMAB * | - | - | - | |
| AZATHIOPRINE * | 0.455 | 0.152–1.365 | 0.160 | |
| CLADRIBINE * | 0.166 | 0.065–0.425 | <0.001 | |
| DMF * | 0.197 | 0.129–0.300 | <0.001 | |
| FINGOLIMOD * | 0.291 | 0.197–0.428 | <0.001 | |
| GLATIRAMER ACETATE * | 0.208 | 0.125–0.344 | <0.001 | |
| INTERFERON * | 0.143 | 0.089–0.231 | <0.001 | |
| NATALIZUMAB * | 0.039 | 0.005–0.287 | 0.001 | |
| OCRELIZUMAB * | 0.584 | 0.288–1.183 | 0.135 | |
| RITUXIMAB * | 0.716 | 0.192–2.668 | 0.619 | |
| TERIFLUNOMIDE * | 0.276 | 0.157–0.455 | <0.001 | |
| Number of comorbidities | <0.001 | |||
| 1 ** | 2.449 | 1.770–3.390 | <0.001 | |
| 2 ** | 2.387 | 1.448–3.937 | 0.001 | |
| 3 ** | 6.989 | 4.240–11.522 | <0.001 | |
| 4+ ** | 5.024 | 2.033–12.411 | <0.001 | |
| Partially or fully vaccinated *** | 0.418 | 0.317–0.552 | <0.001 | |
| Death due to COVID-19 | ||||
| Age | 1.122 | 1.084–1.161 | <0.001 | |
| Male sex | 2.039 | 0.926–4.490 | 0.077 | |
| DMT | 0.091 | |||
| ALEMTUZUMAB * | - | - | - | |
| AZATHIOPRINE * | - | - | - | |
| CLADRIBINE * | 0.331 | 0.042–2.607 | 0.294 | |
| DMF * | 0.156 | 0.043–0.565 | 0.005 | |
| FINGOLIMOD * | 0.276 | 0.095–0.803 | 0.018 | |
| GLATIRAMER ACETATE * | 0.092 | 0.012–0.717 | 0.023 | |
| INTERFERON * | 0.114 | 0.025–0.520 | 0.005 | |
| NATALIZUMAB * | - | - | - | |
| OCRELIZUMAB * | - | - | - | |
| RITUXIMAB * | - | - | - | |
| TERIFLUNOMIDE * | 0.311 | 0.068–1.420 | 0.132 | |
| Number of comorbidities | <0.001 | |||
| 1 ** | 5.915 | 2.266–15.439 | <0.001 | |
| 2 ** | 3.702 | 0.778–17.626 | 0.100 | |
| 3 ** | 12.693 | 3.733–43.155 | <0.001 | |
| 4+ ** | 21.155 | 4.237–105.633 | <0.001 | |
| Partially or fully vaccinated *** | 0.490 | 0.211–1.141 | 0.098 |
| Outcome | Independent Factors | Odds Ratio | 95% C.I. for Odds Ratio | p-Value |
|---|---|---|---|---|
| Hospitalization | ||||
| Age | 1.065 | 1.050–1.081 | <0.001 | |
| Male sex | 1.639 | 1.231–2.184 | 0.001 | |
| DMT | <0.001 | |||
| ALEMTUZUMAB * | - | - | - | |
| AZATHIOPRINE * | 0.248 | 0.075–0.819 | 0.022 | |
| CLADRIBINE * | 0.442 | 0.165–1.186 | 0.105 | |
| DMF * | 0.423 | 0.266–0.673 | <0.001 | |
| FINGOLIMOD * | 0.589 | 0.384–0.904 | 0.016 | |
| GLATIRAMER ACETATE * | 0.388 | 0.225–0.667 | 0.001 | |
| INTERFERON * | 0.231 | 0.139–0.383 | <0.001 | |
| NATALIZUMAB * | 0.078 | 0.010–0.588 | 0.013 | |
| OCRELIZUMAB * | 1.471 | 0.675–3.207 | 0.332 | |
| RITUXIMAB * | 1.507 | 0.366–6.200 | 0.570 | |
| TERIFLUNOMIDE * | 0.391 | 0.215–0.711 | 0.002 | |
| Number of comorbidities | 0.007 | |||
| 1 ** | 1.437 | 0.996–2.072 | 0.052 | |
| 2 ** | 0.790 | 0.444–1.404 | 0.422 | |
| 3 ** | 2.569 | 1.402–4.707 | 0.002 | |
| 4+ ** | 0.980 | 0.360–2.673 | 0.969 | |
| Partially or fully vaccinated *** | 0.380 | 0.281–0.513 | <0.001 | |
| Death due to COVID-19 | ||||
| Age | 1.110 | 1.061–1.162 | <0.001 | |
| Male sex | 2.103 | 0.909–4.866 | 0.082 | |
| DMT | 0.944 | |||
| ALEMTUZUMAB * | - | - | - | |
| AZATHIOPRINE * | - | - | - | |
| CLADRIBINE * | 2.026 | 0.226–18.155 | 0.528 | |
| DMF * | 0.648 | 0.158–2.657 | 0.547 | |
| FINGOLIMOD * | 0.964 | 0.294–3.165 | 0.952 | |
| GLATIRAMER ACETATE * | 0.235 | 0.028–1.984 | 0.183 | |
| INTERFERON * | 0.302 | 0.062–1.469 | 0.138 | |
| NATALIZUMAB * | - | - | - | |
| OCRELIZUMAB * | - | - | - | |
| RITUXIMAB * | - | - | - | |
| TERIFLUNOMIDE * | 0.684 | 0.138–3.391 | 0.642 | |
| Number of comorbidities | 0.255 | |||
| 1 ** | 2.684 | 0.965–7.469 | 0.059 | |
| 2 ** | 0.705 | 0.124–4.024 | 0.695 | |
| 3 ** | 1.996 | 0.479–8.309 | 0.342 | |
| 4+ ** | 2.304 | 0.370–14.358 | 0.371 | |
| Partially or fully vaccinated *** | 0.488 | 0.201–1.185 | 0.113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakirtzis, C.; Konstantinidou, N.; Stavropoulou De Lorenzo, S.; Moysiadis, T.; Boziki, M.-K.; Grigoriadou, E.; Kesidou, E.; Theotokis, P.; Thireos, E.; Mitrou, P.; et al. COVID-19 Vaccination and Disease Course in People with Multiple Sclerosis in Greece. J. Clin. Med. 2023, 12, 5460. https://doi.org/10.3390/jcm12175460
Bakirtzis C, Konstantinidou N, Stavropoulou De Lorenzo S, Moysiadis T, Boziki M-K, Grigoriadou E, Kesidou E, Theotokis P, Thireos E, Mitrou P, et al. COVID-19 Vaccination and Disease Course in People with Multiple Sclerosis in Greece. Journal of Clinical Medicine. 2023; 12(17):5460. https://doi.org/10.3390/jcm12175460
Chicago/Turabian StyleBakirtzis, Christos, Natalia Konstantinidou, Sotiria Stavropoulou De Lorenzo, Theodoros Moysiadis, Marina-Kleopatra Boziki, Eleni Grigoriadou, Evangelia Kesidou, Paschalis Theotokis, Eleftherios Thireos, Panagiota Mitrou, and et al. 2023. "COVID-19 Vaccination and Disease Course in People with Multiple Sclerosis in Greece" Journal of Clinical Medicine 12, no. 17: 5460. https://doi.org/10.3390/jcm12175460
APA StyleBakirtzis, C., Konstantinidou, N., Stavropoulou De Lorenzo, S., Moysiadis, T., Boziki, M.-K., Grigoriadou, E., Kesidou, E., Theotokis, P., Thireos, E., Mitrou, P., & Grigoriadis, N. (2023). COVID-19 Vaccination and Disease Course in People with Multiple Sclerosis in Greece. Journal of Clinical Medicine, 12(17), 5460. https://doi.org/10.3390/jcm12175460









