Olfactory Receptors and Aortic Aneurysm: Review of Disease Pathways
Abstract
1. Introduction
2. Olfactory Receptors
2.1. Olfactory Receptors: Structure and Function
2.2. Olfactory Receptors: Ectopic Receptors and the Cardiovascular System
3. Aortic Aneurysm
3.1. Aortic Aneurysm: Definitions
3.2. Aortic Aneurysm: Etiology
3.2.1. Genetic Factors in Thoracic and Abdominal Aortic Aneurysm
3.2.2. Non-Genetic Factors in Thoracic and Abdominal Aortic Aneurysm
4. Aortic Aneurysm Pathophysiology
4.1. Extracellular Matrix (ECM)
4.2. Lipid Trafficking and Atherosclerosis
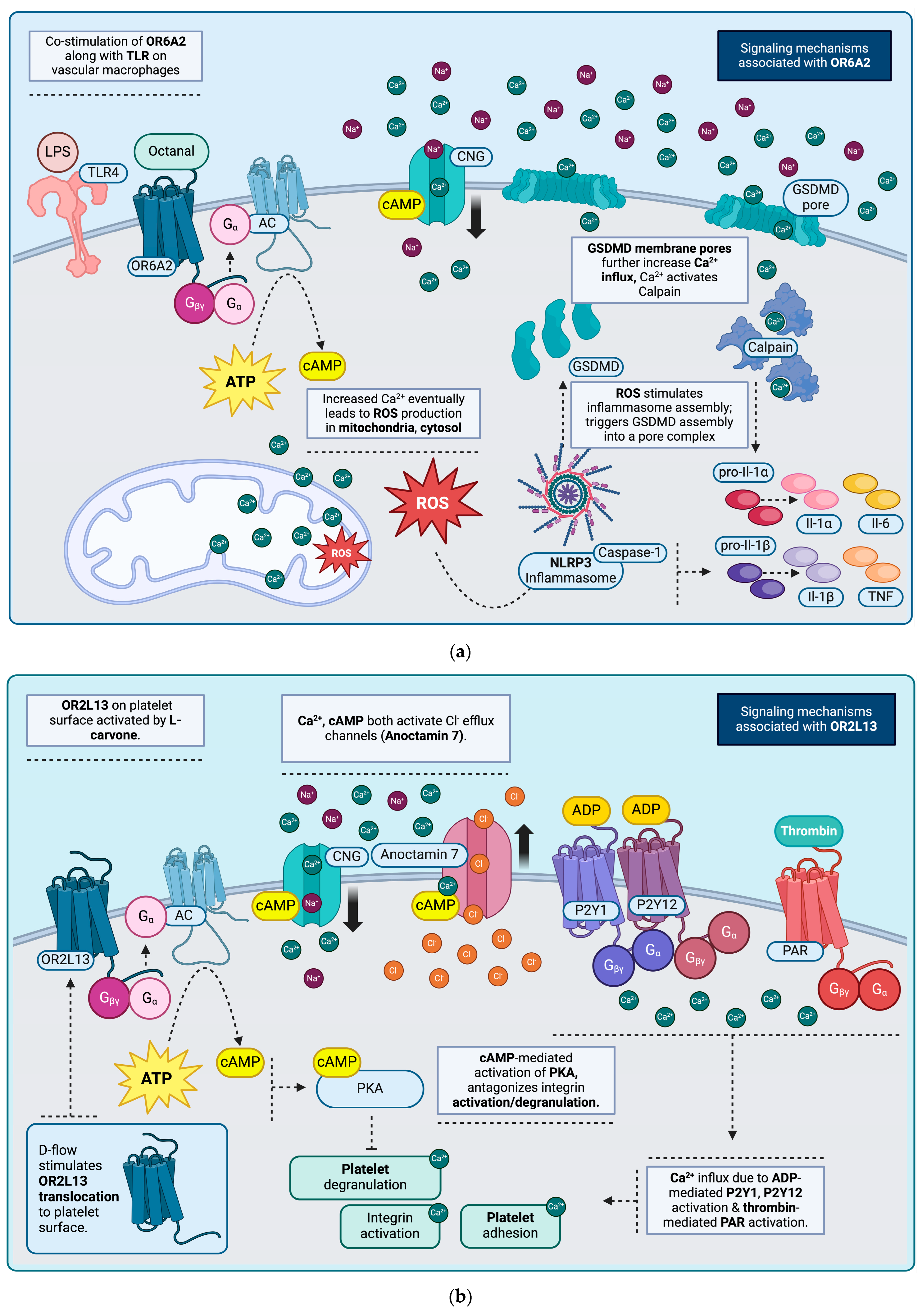
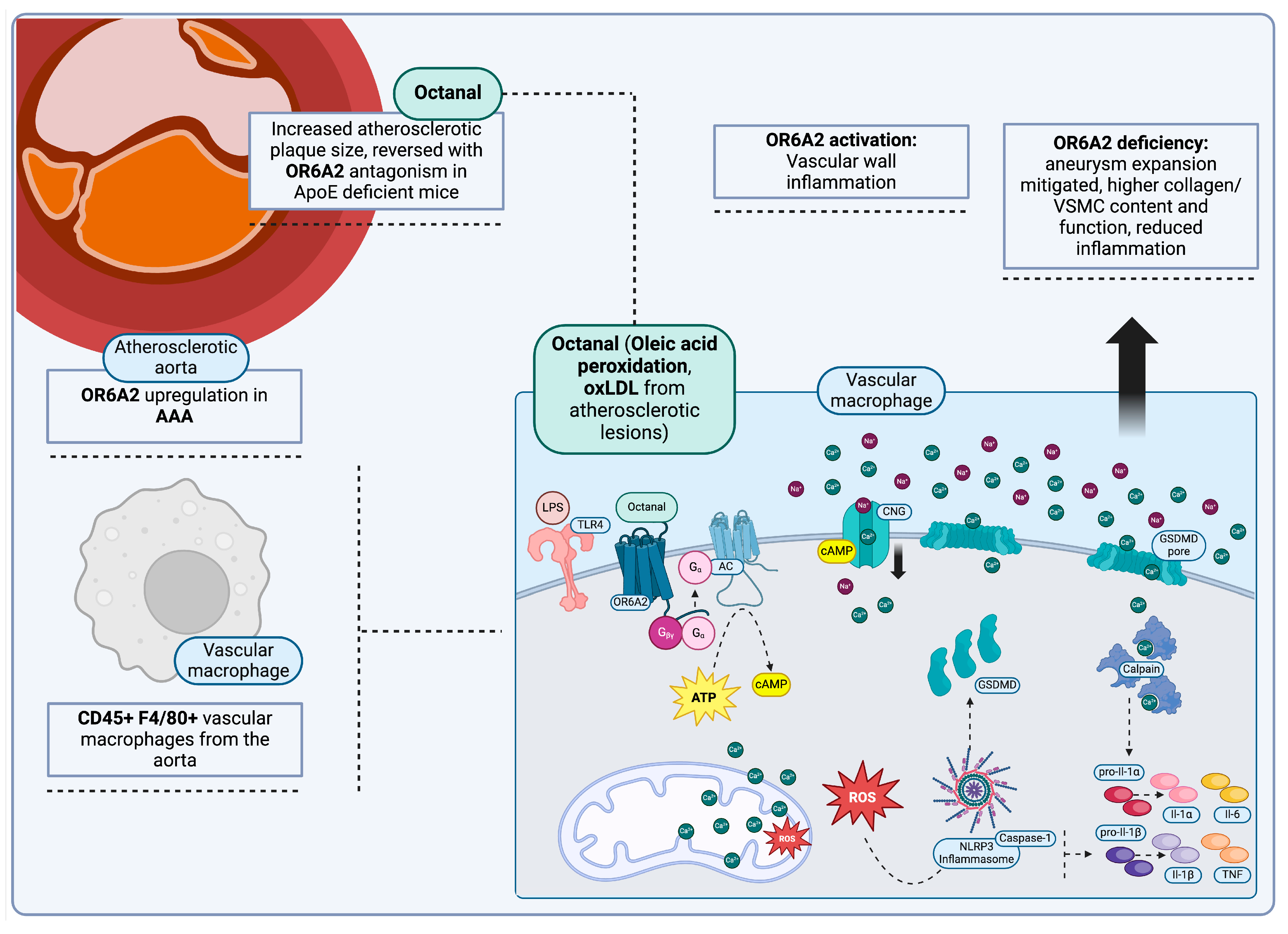
Olfactory Receptors, Lipid Trafficking and Atherosclerosis
4.3. Vascular Smooth Muscle Cells (VSMCs)
4.4. Inflammation
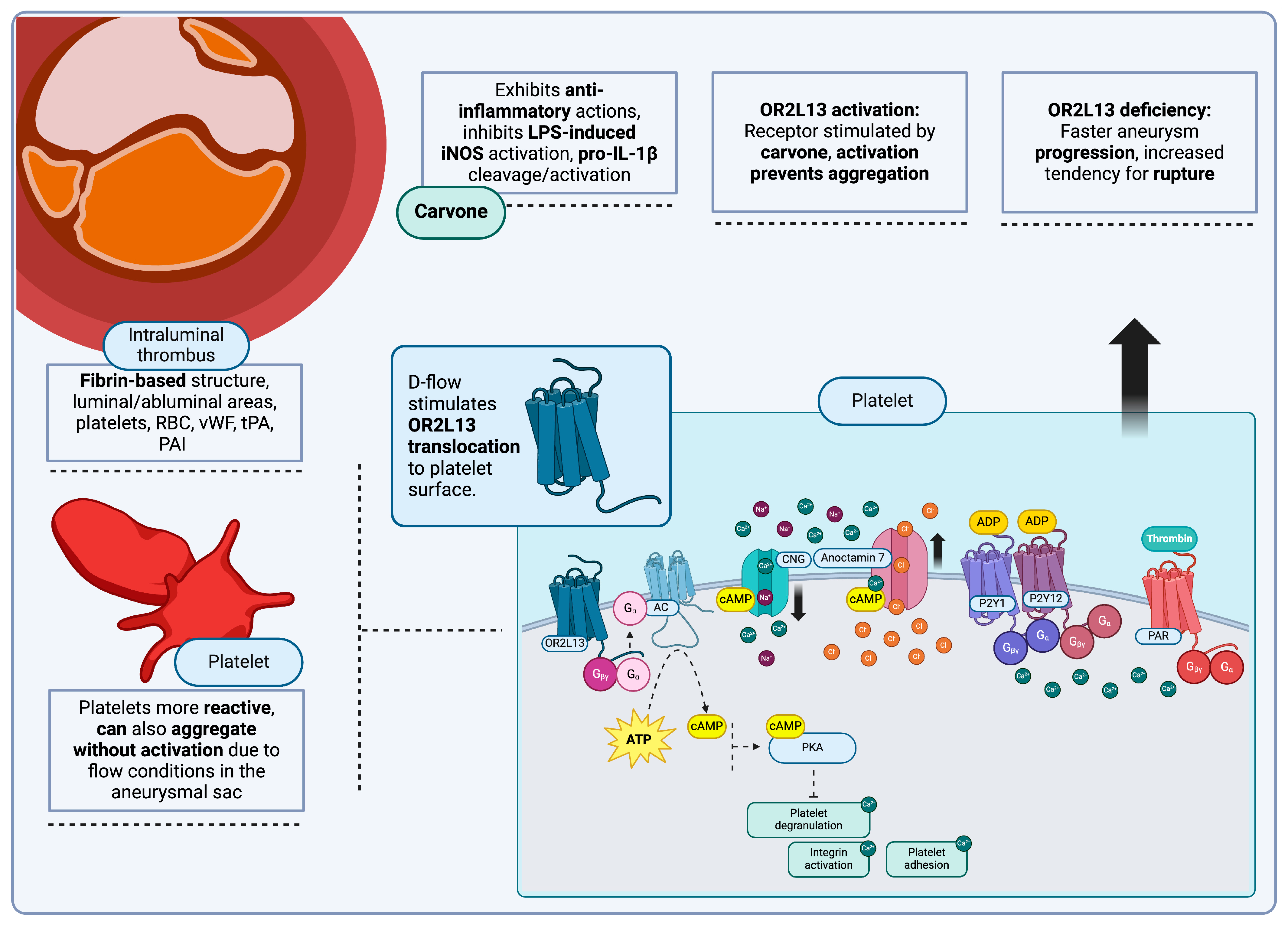
4.5. Platelets and Intraluminal Thrombus
Olfactory Receptors, Platelets and Intraluminal Thrombi
4.6. Endothelium
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bossone, E.; Eagle, K.A. Epidemiology and Management of Aortic Disease: Aortic Aneurysms and Acute Aortic Syndromes. Nat. Rev. Cardiol. 2021, 18, 331–348. [Google Scholar] [CrossRef]
- Writing Committee Members; Isselbacher, E.M.; Preventza, O.; Hamilton, B.I.J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease. J. Am. Coll. Cardiol. 2022, 80, e223–e393. [Google Scholar] [CrossRef]
- Sterpetti, A.V.; Gabriele, R.; Sapienza, P.; Marzo, L.D.; Borrelli, V. Mortality and Burden Related with Aortic Aneurysms and Dissections. The Importance of Information and Education. Curr. Probl. Cardiol. 2024, 49, 102384. [Google Scholar] [CrossRef]
- Chou, E.L.; Chaffin, M.; Simonson, B.; Pirruccello, J.P.; Akkad, A.-D.; Nekoui, M.; Lino Cardenas, C.L.; Bedi, K.C.; Nash, C.; Juric, D.; et al. Aortic Cellular Diversity and Quantitative Genome-Wide Association Study Trait Prioritization Through Single-Nuclear RNA Sequencing of the Aneurysmal Human Aorta. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1355–1374. [Google Scholar] [CrossRef] [PubMed]
- Pinard, A.; Jones, G.T.; Milewicz, D.M. Genetics of Thoracic and Abdominal Aortic Diseases. Circ. Res. 2019, 124, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Lee, M.-R.; Park, J.-G. Aortic Aneurysms: Current Pathogenesis and Therapeutic Targets. Exp. Mol. Med. 2023, 55, 2519–2530. [Google Scholar] [CrossRef]
- Fleischer, J.; Breer, H.; Strotmann, J. Mammalian Olfactory Receptors. Front. Cell. Neurosci. 2009, 3, 9. [Google Scholar] [CrossRef]
- Tirindelli, R. Coding of Pheromones by Vomeronasal Receptors. Cell Tissue Res. 2021, 383, 367–386. [Google Scholar] [CrossRef]
- Rivière, S.; Challet, L.; Fluegge, D.; Spehr, M.; Rodriguez, I. Formyl Peptide Receptor-like Proteins Are a Novel Family of Vomeronasal Chemosensors. Nature 2009, 459, 574–577. [Google Scholar] [CrossRef]
- Precone, V.; Paolacci, S.; Beccari, T.; Ragione, L.D.; Stuppia, L.; Baglivo, M.; Guerri, G.; Manara, E.; Tonini, G.; Herbst, K.L.; et al. Pheromone Receptors and Their Putative Ligands: Possible Role in Humans. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2140–2150. [Google Scholar] [PubMed]
- Lee, C.; Han, J.; Jung, Y. Formyl Peptide Receptor 2 Is an Emerging Modulator of Inflammation in the Liver. Exp. Mol. Med. 2023, 55, 325–332. [Google Scholar] [CrossRef]
- Chen, T.; Xiong, M.; Zong, X.; Ge, Y.; Zhang, H.; Wang, M.; Won Han, G.; Yi, C.; Ma, L.; Ye, R.D.; et al. Structural Basis of Ligand Binding Modes at the Human Formyl Peptide Receptor 2. Nat. Commun. 2020, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Jiao, Y.; Yang, L.; Yuan, G.; Jia, J. New Insights into the Roles of Olfactory Receptors in Cardiovascular Disease. Mol. Cell. Biochem. 2024, 479, 1615–1626. [Google Scholar] [CrossRef]
- Maßberg, D.; Hatt, H. Human Olfactory Receptors: Novel Cellular Functions Outside of the Nose. Physiol. Rev. 2018, 98, 1739–1763. [Google Scholar] [CrossRef]
- Rehman, S.; Rahimi, N.; Dimri, M. Biochemistry, G Protein Coupled Receptors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A Comprehensive Map of Molecular Drug Targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, M.; Dong, J.; Cui, W.; Yuan, S. The Structure and Function of Olfactory Receptors. Trends Pharmacol. Sci. 2024, 45, 268–280. [Google Scholar] [CrossRef]
- Schiöth, H.B.; Fredriksson, R. The GRAFS Classification System of G-Protein Coupled Receptors in Comparative Perspective. Gen. Comp. Endocrinol. 2005, 142, 94–101. [Google Scholar] [CrossRef]
- Nguyen, Q.A.T.; Rocha, A.; Chhor, R.; Yamashita, Y.; Stadler, C.; Pontrello, C.; Yang, H.; Haga-Yamanaka, S. Hypothalamic Representation of the Imminence of Predator Threat Detected by the Vomeronasal Organ in Mice. eLife 2024, 12, RP92982. [Google Scholar] [CrossRef] [PubMed]
- Brechbühl, J.; de Vallière, A.; Wood, D.; Nenniger Tosato, M.; Broillet, M.-C. The Grueneberg Ganglion Controls Odor-Driven Food Choices in Mice under Threat. Commun. Biol. 2020, 3, 533. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Marcos, A.; Halpern, M. Evolution of Olfactory and Vomeronasal Systems. In Encyclopedia of Neuroscience; Binder, M.D., Hirokawa, N., Windhorst, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1264–1269. ISBN 978-3-540-29678-2. [Google Scholar]
- Liberles, S.D. Trace Amine-Associated Receptors: Ligands, Neural Circuits, and Behaviors. Curr. Opin. Neurobiol. 2015, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.; Greer, C.A.; Mok, M.Y.; Mombaerts, P. A Putative Pheromone Receptor Gene Expressed in Human Olfactory Mucosa. Nat. Genet. 2000, 26, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Olender, T.; Jones, T.E.M.; Bruford, E.; Lancet, D. A Unified Nomenclature for Vertebrate Olfactory Receptors. BMC Evol. Biol. 2020, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ponissery-Saidu, S.; Yee, K.K.; Wang, H.; Chen, M.-L.; Iguchi, N.; Zhang, G.; Jiang, P.; Reisert, J.; Huang, L. Heterotrimeric G Protein Subunit Gγ13 Is Critical to Olfaction. J. Neurosci. 2013, 33, 7975–7984. [Google Scholar] [CrossRef] [PubMed]
- Billesbølle, C.B.; de March, C.A.; van der Velden, W.J.C.; Ma, N.; Tewari, J.; del Torrent, C.L.; Li, L.; Faust, B.; Vaidehi, N.; Matsunami, H.; et al. Structural Basis of Odorant Recognition by a Human Odorant Receptor. Nature 2023, 615, 742–749. [Google Scholar] [CrossRef]
- Floriano, W.B.; Vaidehi, N.; Goddard, W.A.; Singer, M.S.; Shepherd, G.M. Molecular Mechanisms Underlying Differential Odor Responses of a Mouse Olfactory Receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 10712–10716. [Google Scholar] [CrossRef]
- Maziarz, M.; Park, J.-C.; Leyme, A.; Marivin, A.; Garcia-Lopez, A.; Patel, P.P.; Garcia-Marcos, M. Revealing the Activity of Trimeric G-Proteins in Live Cells with a Versatile Biosensor Design. Cell 2020, 182, 770–785.e16. [Google Scholar] [CrossRef] [PubMed]
- Kida, H.; Fukutani, Y.; Mainland, J.D.; De March, C.A.; Vihani, A.; Li, Y.R.; Chi, Q.; Toyama, A.; Liu, L.; Kameda, M.; et al. Vapor Detection and Discrimination with a Panel of Odorant Receptors. Nat. Commun. 2018, 9, 4556. [Google Scholar] [CrossRef] [PubMed]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; LaMantia, A.-S.; McNamara, J.O.; Williams, S.M. The Transduction of Olfactory Signals. In Neuroscience, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Jimenez, R.C.; Casajuana-Martin, N.; García-Recio, A.; Alcántara, L.; Pardo, L.; Campillo, M.; Gonzalez, A. The Mutational Landscape of Human Olfactory G Protein-Coupled Receptors. BMC Biol. 2021, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Olender, T.; Waszak, S.M.; Viavant, M.; Khen, M.; Ben-Asher, E.; Reyes, A.; Nativ, N.; Wysocki, C.J.; Ge, D.; Lancet, D. Personal Receptor Repertoires: Olfaction as a Model. BMC Genom. 2012, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Gilad, Y.; Lancet, D. Population Differences in the Human Functional Olfactory Repertoire. Mol. Biol. Evol. 2003, 20, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, M.; Libert, F.; Schurmans, S.; Schiffmann, S.; Lefort, A.; Eggerickx, D.; Ledent, C.; Mollereau, C.; Gérard, C.; Perret, J.; et al. Expression of Members of the Putative Olfactory Receptor Gene Family in Mammalian Germ Cells. Nature 1992, 355, 453–455. [Google Scholar] [CrossRef]
- Kim, S.-H.; Yoon, Y.C.; Lee, A.S.; Kang, N.; Koo, J.; Rhyu, M.-R.; Park, J.-H. Expression of Human Olfactory Receptor 10J5 in Heart Aorta, Coronary Artery, and Endothelial Cells and Its Functional Role in Angiogenesis. Biochem. Biophys. Res. Commun. 2015, 460, 404–408. [Google Scholar] [CrossRef]
- Flegel, C.; Manteniotis, S.; Osthold, S.; Hatt, H.; Gisselmann, G. Expression Profile of Ectopic Olfactory Receptors Determined by Deep Sequencing. PLoS ONE 2013, 8, e55368. [Google Scholar] [CrossRef] [PubMed]
- Jovancevic, N.; Dendorfer, A.; Matzkies, M.; Kovarova, M.; Heckmann, J.C.; Osterloh, M.; Boehm, M.; Weber, L.; Nguemo, F.; Semmler, J.; et al. Medium-Chain Fatty Acids Modulate Myocardial Function via a Cardiac Odorant Receptor. Basic Res. Cardiol. 2017, 112, 13. [Google Scholar] [CrossRef]
- Saito, H.; Chi, Q.; Zhuang, H.; Matsunami, H.; Mainland, J.D. Odor Coding by a Mammalian Receptor Repertoire. Sci. Signal. 2009, 2, ra9. [Google Scholar] [CrossRef]
- Cherian, S.; Lopaschuk, G.D.; Carvalho, E. Cellular Cross-Talk between Epicardial Adipose Tissue and Myocardium in Relation to the Pathogenesis of Cardiovascular Disease. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E937–E949. [Google Scholar] [CrossRef] [PubMed]
- Labarthe, F.; Gélinas, R.; Des Rosiers, C. Medium-Chain Fatty Acids as Metabolic Therapy in Cardiac Disease. Cardiovasc. Drugs Ther. 2008, 22, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Airhart, S.; Cade, W.T.; Jiang, H.; Coggan, A.R.; Racette, S.B.; Korenblat, K.; Spearie, C.A.; Waller, S.; O’Connor, R.; Bashir, A.; et al. A Diet Rich in Medium-Chain Fatty Acids Improves Systolic Function and Alters the Lipidomic Profile in Patients With Type 2 Diabetes: A Pilot Study. J. Clin. Endocrinol. Metab. 2016, 101, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Kang, D.-K.; Kim, H.Y.; Kang, S.S.; Chang, S.-I. Angiogenin-Induced Protein Kinase B/Akt Activation Is Necessary for Angiogenesis but Is Independent of Nuclear Translocation of Angiogenin in HUVE Cells. Biochem. Biophys. Res. Commun. 2007, 352, 509–513. [Google Scholar] [CrossRef]
- Song, M.; Finley, S.D. ERK and Akt Exhibit Distinct Signaling Responses Following Stimulation by Pro-Angiogenic Factors. Cell Commun. Signal. 2020, 18, 114. [Google Scholar] [CrossRef]
- Yadunandanan Nair, N.; Samuel, V.; Ramesh, L.; Marib, A.; David, D.T.; Sundararaman, A. Actin Cytoskeleton in Angiogenesis. Biol. Open 2022, 11, bio058899. [Google Scholar] [CrossRef]
- Prasain, N.; Stevens, T. The Actin Cytoskeleton in Endothelial Cell Phenotypes. Microvasc. Res 2009, 77, 53–63. [Google Scholar] [CrossRef]
- Curtis, T.M.; Nilon, A.M.; Greenberg, A.J.; Besner, M.; Scibek, J.J.; Nichols, J.A.; Huie, J.L. Odorant Binding Causes Cytoskeletal Rearrangement, Leading to Detectable Changes in Endothelial and Epithelial Barrier Function and Micromotion. Biosensors 2023, 13, 329. [Google Scholar] [CrossRef] [PubMed]
- Beito, M.R.; Ashraf, S.; Odogwu, D.; Harmancey, R. Role of Ectopic Olfactory Receptors in the Regulation of the Cardiovascular–Kidney–Metabolic Axis. Life 2024, 14, 548. [Google Scholar] [CrossRef]
- Poll, B.G.; Xu, J.; Gupta, K.; Shubitowski, T.B.; Pluznick, J.L. Olfactory Receptor 78 Modulates Renin but Not Baseline Blood Pressure. Physiol. Rep. 2021, 9, e15017. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.-X.; Rey, F.; Wang, T.; et al. Olfactory Receptor Responding to Gut Microbiota-Derived Signals Plays a Role in Renin Secretion and Blood Pressure Regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Di, Y.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of Inflammatory Responses by Gut Microbiota and Chemoattractant Receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the Gut Microbiota on Host Adiposity Are Modulated by the Short-Chain Fatty-Acid Binding G Protein-Coupled Receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Kobiyama, K.; Winkels, H.; Ghosheh, Y.; McArdle, S.; Mikulski, Z.; Kiosses, W.B.; Fan, Z.; Wen, L.; Jung, Y.; et al. Olfactory Receptor 2 in Vascular Macrophages Drives Atherosclerosis by NLRP3-Dependent IL-1 Production. Science 2022, 375, 214–221. [Google Scholar] [CrossRef]
- He, Z.; Wang, D.W. Olfactory Receptor 2 Activation in Macrophages: Novel Mediator of Atherosclerosis Progression. Signal Transduct. Target. Ther. 2022, 7, 247. [Google Scholar] [CrossRef]
- McArdle, S.; Buscher, K.; Ghosheh, Y.; Pramod, A.B.; Miller, J.; Winkels, H.; Wolf, D.; Ley, K. Migratory and Dancing Macrophage Subsets in Atherosclerotic Lesions. Circ. Res. 2019, 125, 1038–1051. [Google Scholar] [CrossRef]
- Wang, C.; Andreasson, K.I. Odorant Receptors in Macrophages: Potential Targets for Atherosclerosis. Trends Immunol. 2022, 43, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Kodi, T.; Sankhe, R.; Gopinathan, A.; Nandakumar, K.; Kishore, A. New Insights on NLRP3 Inflammasome: Mechanisms of Activation, Inhibition, and Epigenetic Regulation. J. Neuroimmune Pharmacol. 2024, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Hosojima, S.; Hara, H.; Kushiyama, H.; Mahib, M.R.; Kinoshita, T.; Suda, T. Gasdermin D Mediates the Maturation and Release of IL-1α Downstream of Inflammasomes. Cell Rep. 2021, 34, 108887. [Google Scholar] [CrossRef]
- Morrell, C.N.; Mix, D.; Aggarwal, A.; Bhandari, R.; Godwin, M.; Owens, P.; Lyden, S.P.; Doyle, A.; Krauel, K.; Rondina, M.T.; et al. Platelet Olfactory Receptor Activation Limits Platelet Reactivity and Growth of Aortic Aneurysms. J. Clin. Investig. 2022, 132, e152373. [Google Scholar] [CrossRef]
- Bozzi, S.; Dominissini, D.; Redaelli, A.; Passoni, G. The Effect of Turbulence Modelling on the Assessment of Platelet Activation. J. Biomech. 2021, 128, 110704. [Google Scholar] [CrossRef]
- Geithe, C.; Protze, J.; Kreuchwig, F.; Krause, G.; Krautwurst, D. Structural Determinants of a Conserved Enantiomer-Selective Carvone Binding Pocket in the Human Odorant Receptor OR1A1. Cell. Mol. Life Sci. 2017, 74, 4209–4229. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Heo, Y.; Kwon, I.; Jo, S.; Jeon, H.; Lee, Y.; Kim, J.; Heo, J.H.; Namkung, W. Gestodene, a Novel Positive Allosteric Modulator of PAR1, Enhances PAR1-Mediated Human Platelet Aggregation. Front. Pharmacol. 2024, 15, 1430548. [Google Scholar] [CrossRef]
- Neuhaus, E.M.; Zhang, W.; Gelis, L.; Deng, Y.; Noldus, J.; Hatt, H. Activation of an Olfactory Receptor Inhibits Proliferation of Prostate Cancer Cells. J. Biol. Chem. 2009, 284, 16218–16225. [Google Scholar] [CrossRef]
- Schelemei, P.; Picard, F.; Nemade, H.; Mehrkens, D.; Grimm, S.; Tinaz, K.; Wagner, E.; Kreuzberg, W.; Orecchioni, M.; Wagenhäuser, M.; et al. Olfactory Receptor 2 Deficiency Protects from Experimental Abdominal Aortic Aneurysm Formation. Atherosclerosis 2023, 379, S62. [Google Scholar] [CrossRef]
- Schelemei, P.; Picard, F.; Nemade, H.; Mehrkens, D.; Grimm, S.; Tinaz, K.; Wagner, E.; Orrechioni, M.; Wagenhaeuser, M.; Schelzig, H.; et al. Olfactory Receptor 2 Signaling Drives Experimental Abdominal Aortic Aneurysm Formation. Eur. Heart J. 2023, 44, ehad655.3267. [Google Scholar] [CrossRef]
- Anderson, R.H.; Bamforth, S.D. Morphogenesis of the Mammalian Aortic Arch Arteries. Front. Cell Dev. Biol. 2022, 10, 892900. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.N.; Miyoshi, T.; Addetia, K.; Citro, R.; Daimon, M.; Fajardo, P.G.; Kasliwal, R.R.; Kirkpatrick, J.N.; Monaghan, M.J.; Muraru, D.; et al. Normal Values of Aortic Root Size According to Age, Sex and Race: Results of the World Alliance of Societies of Echocardiography Study. J. Am. Soc. Echocardiogr. 2022, 35, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.D.; Arora, Y.; Mahajan, K. Anatomy, Blood Vessels. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wang, Z.; You, Y.; Yin, Z.; Bao, Q.; Lei, S.; Yu, J.; Xie, C.; Ye, F.; Xie, X. Burden of Aortic Aneurysm and Its Attributable Risk Factors from 1990 to 2019: An Analysis of the Global Burden of Disease Study 2019. Front. Cardiovasc. Med. 2022, 9, 901225. [Google Scholar] [CrossRef]
- Jana, S.; Hu, M.; Shen, M.; Kassiri, Z. Extracellular Matrix, Regional Heterogeneity of the Aorta, and Aortic Aneurysm. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Saeyeldin, A.; Zafar, M.A.; Velasquez, C.A.; Ip, K.; Gryaznov, A.; Brownstein, A.J.; Li, Y.; Rizzo, J.A.; Erben, Y.; Ziganshin, B.A.; et al. Natural History of Aortic Root Aneurysms in Marfan Syndrome. Ann. Cardiothorac. Surg. 2017, 6, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Shalhub, S.; Black, J.H.; Cecchi, A.C.; Xu, Z.; Griswold, B.F.; Safi, H.J.; Milewicz, D.M.; McDonnell, N.B. Molecular Diagnosis in Vascular Ehlers-Danlos Syndrome Predicts Pattern of Arterial Involvement and Outcomes. J. Vasc. Surg. 2014, 60, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Used-Gavín, A.; Larrañaga-Moreira, J.M.; Lago-Cascudo, R.; Mosquera-Rodríguez, V.X.; Barriales-Villa, R. Giant Ascending Aortic Aneurysm with Impending Rupture as Presentation of Cutis Laxa 1B: A Case Report. Eur. Heart J. Case Rep. 2023, 7, ytad530. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Morita, H.; Fujita, D.; Inuzuka, R.; Taniguchi, Y.; Imai, Y.; Hirata, Y.; Komuro, I. Congenital Contractural Arachnodactyly Complicated with Aortic Dilatation and Dissection: Case Report and Review of Literature. Am. J. Med. Genet. A 2015, 167A, 2382–2387. [Google Scholar] [CrossRef] [PubMed]
- Gouda, P.; Kay, R.; Habib, M.; Aziz, A.; Aziza, E.; Welsh, R. Clinical Features and Complications of Loeys-Dietz Syndrome: A Systematic Review. Int. J. Cardiol. 2022, 362, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Heald, B.; Rigelsky, C.; Moran, R.; LaGuardia, L.; O’Malley, M.; Burke, C.A.; Zahka, K. Prevalence of Thoracic Aortopathy in Patients with Juvenile Polyposis Syndrome-Hereditary Hemorrhagic Telangiectasia Due to SMAD4. Am. J. Med. Genet. A 2015, 167A, 1758–1762. [Google Scholar] [CrossRef] [PubMed]
- Regalado, E.S.; Mellor-Crummey, L.; De Backer, J.; Braverman, A.C.; Ades, L.; Benedict, S.; Bradley, T.J.; Brickner, M.E.; Chatfield, K.C.; Child, A.; et al. Clinical History and Management Recommendations of the Smooth Muscle Dysfunction Syndrome Due to ACTA2 Arginine 179 Alterations. Genet. Med. 2018, 20, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Padang, R.; Bagnall, R.D.; Semsarian, C. Genetic Basis of Familial Valvular Heart Disease. Circ. Cardiovasc. Genet. 2012, 5, 569–580. [Google Scholar] [CrossRef]
- Rooprai, J.; Boodhwani, M.; Beauchesne, L.; Chan, K.; Dennie, C.; Nagpal, S.; Messika-Zeitoun, D.; Coutinho, T. Thoracic Aortic Aneurysm Growth in Bicuspid Aortic Valve Patients: Role of Aortic Stiffness and Pulsatile Hemodynamics. J. Am. Heart Assoc. 2019, 8, e010885. [Google Scholar] [CrossRef] [PubMed]
- Diletta, L.; Enrico, R.; Germano, M. Thoracoabdominal Aortic Aneurysm in Connective Tissue Disorder Patients. Indian J. Thorac. Cardiovasc. Surg. 2022, 38, 146–156. [Google Scholar] [CrossRef] [PubMed]
- di Gioia, C.R.T.; Ascione, A.; Carletti, R.; Giordano, C. Thoracic Aorta: Anatomy and Pathology. Diagnostics 2023, 13, 2166. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, L.I.; Russu, G.; Luca, A.-C.; Sandu, C.; Trandafir, L.M.; Vasiliu, I.; Popa, S.; Ghiga, G.; Bălănescu, L.; Țarcă, E. Identification of Genetic Variants Associated with Hereditary Thoracic Aortic Diseases (HTADs) Using Next Generation Sequencing (NGS) Technology and Genotype–Phenotype Correlations. Int. J. Mol. Sci. 2024, 25, 11173. [Google Scholar] [CrossRef]
- Summers, K.M. Genetic Models of Fibrillinopathies. Genetics 2024, 226, iyad189. [Google Scholar] [CrossRef] [PubMed]
- Krarup, N.T.; Hvidbjerg, M.; Zaremba, T.; Sommerlund, M.; Christensen, M.K. Autosomal Dominant Cutis Laxa and Critical Stenosis of the Left Main Coronary Artery in a 21-Year-Old Female with an Intronic Mutation in the Elastin Gene. Am. J. Med. Genet. A 2023, 191, 1059–1064. [Google Scholar] [CrossRef]
- Hoareau, M.; El Kholti, N.; Debret, R.; Lambert, E. Characterization of the Zebrafish Elastin a (Elnasa12235) Mutant: A New Model of Elastinopathy Leading to Heart Valve Defects. Cells 2023, 12, 1436. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Xie, X.; Wu, Q.; Shi, F.; Chen, Y.; Yuan, S.; Xing, K.; Li, X.; Zhu, Q.; Li, B.; et al. S-Adenosylmethionine Attenuates Angiotensin II-Induced Aortic Dissection Formation by Inhibiting Vascular Smooth Muscle Cell Phenotypic Switch and Autophagy. Biochem. Pharmacol. 2024, 219, 115967. [Google Scholar] [CrossRef]
- Wang, Q.; An, J.; Zhou, W.; Zhang, Y.; Huang, J.; Liao, G.; Wang, M.; Xia, L.; Le, A.; Zhu, J. S-Adenosyl-L-Methionine Supplementation Alleviates Aortic Dissection by Decreasing Inflammatory Infiltration. Nutr. Metab. 2024, 21, 67. [Google Scholar] [CrossRef]
- Hamandi, M.; Bolin, M.L.; Fan, J.; Lanfear, A.T.; Woolbert, S.K.; Baxter, R.D.; DiMaio, J.M.; Brinkman, W.T. A Newly Discovered Genetic Disorder Associated With Life-Threatening Aortic Disease in a 6-Year-Old Boy. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620909234. [Google Scholar] [CrossRef] [PubMed]
- Boerio, M.L.; Engelhardt, N.M.; Cuddapah, S.; Gold, J.I.; Marin, I.C.; Pinard, A.; Guo, D.; Prakash, S.K.; Milewicz, D.M. Further Evidence That ARIH1 Rare Variants Predispose to Thoracic Aortic Disease. Circ. Genom. Precis. Med. 2022, 15, e003707. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.L.; Haelterman, N.A.; Kwartler, C.S.; Regalado, E.S.; Lee, P.-T.; Nagarkar-Jaiswal, S.; Guo, D.; Duraine, L.; Wangler, M.F.; University of Washington Center for Mendelian Genomics; et al. Ari-1 Regulates Myonuclear Organization Together with Parkin and Is Associated with Aortic Aneurysms. Dev. Cell 2018, 45, 226–244.e8. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, T.; Kim, E.; Riehl, B.D.; Esfahani, A.M.; Rosenbohm, J.; Yang, R.; Duan, B.; Lim, J.Y. The LINC Complex, Mechanotransduction, and Mesenchymal Stem Cell Function and Fate. J. Biol. Eng. 2019, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Raunsø, J.; Song, R.J.; Vasan, R.S.; Bourdillon, M.T.; Nørager, B.; Torp-Pedersen, C.; Gislason, G.H.; Xanthakis, V.; Andersson, C. Familial Clustering of Aortic Size, Aneurysms, and Dissections in the Community. Circulation 2020, 142, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Gurung, R.; Choong, A.M.; Woo, C.C.; Foo, R.; Sorokin, V. Genetic and Epigenetic Mechanisms Underlying Vascular Smooth Muscle Cell Phenotypic Modulation in Abdominal Aortic Aneurysm. Int. J. Mol. Sci. 2020, 21, 6334. [Google Scholar] [CrossRef]
- Takayama, T.; Miyata, T.; Nagawa, H. True Abdominal Aortic Aneurysm in Marfan Syndrome. J. Vasc. Surg. 2009, 49, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Filippetti, L.; Dufrost, V.; Busby-Venner, H.; Hanna, N.; Arnaud, P.; Settembre, N.; Mandry, D.; Malikov, S.; Wahl, D.; Zuily, S. A Giant Abdominal Aortic Aneurysm Revealing a Marfan Syndrome With a New FBN1 Mutation. Can. J. Cardiol. 2021, 37, 1870–1872. [Google Scholar] [CrossRef] [PubMed]
- Hinterseher, I.; Tromp, G.; Kuivaniemi, H. Genes and Abdominal Aortic Aneurysm. Ann. Vasc. Surg. 2010, 25, 388–412. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Hsieh, C.-H.; Alonso, L.C. ANRIL: A lncRNA at the CDKN2A/B Locus with Roles in Cancer and Metabolic Disease. Front. Endocrinol. 2018, 9, 405. [Google Scholar] [CrossRef]
- Zhou, X.; Han, X.; Wittfeldt, A.; Sun, J.; Liu, C.; Wang, X.; Gan, L.-M.; Cao, H.; Liang, Z. Long Non-Coding RNA ANRIL Regulates Inflammatory Responses as a Novel Component of NF-κB Pathway. RNA Biol. 2015, 13, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Gretarsdottir, S.; Baas, A.F.; Thorleifsson, G.; Holm, H.; den Heijer, M.; de Vries, J.-P.P.M.; Kranendonk, S.E.; Zeebregts, C.J.A.M.; van Sterkenburg, S.M.; Geelkerken, R.H.; et al. Genome-Wide Association Study Identifies a Sequence Variant within the DAB2IP Gene Conferring Susceptibility to Abdominal Aortic Aneurysm. Nat. Genet. 2010, 42, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.C.; Chan, J.; Tarkas, T.N.; Meharban, N.; Munir, W.; Bashir, M. The Association of ANRIL With Coronary Artery Disease And Aortic Aneurysms, How Far Does The Gene Desert Go? Ann. Vasc. Surg. 2022, 80, 345–357. [Google Scholar] [CrossRef]
- Paige, E.; Clément, M.; Lareyre, F.; Sweeting, M.; Raffort, J.; Grenier, C.; Finigan, A.; Harrison, J.; Peters, J.E.; Sun, B.B.; et al. Interleukin-6 Receptor Signaling and Abdominal Aortic Aneurysm Growth Rates. Circ. Genom. Precis. Med. 2019, 12, e002413. [Google Scholar] [CrossRef]
- Bown, M.J.; Jones, G.T.; Harrison, S.C.; Wright, B.J.; Bumpstead, S.; Baas, A.F.; Gretarsdottir, S.; Badger, S.A.; Bradley, D.T.; Burnand, K.; et al. Abdominal Aortic Aneurysm Is Associated with a Variant in Low-Density Lipoprotein Receptor-Related Protein 1. Am. J. Hum. Genet. 2011, 89, 619–627. [Google Scholar] [CrossRef]
- Au, D.T.; Ying, Z.; Hernández-Ochoa, E.O.; Fondrie, W.E.; Hampton, B.; Migliorini, M.; Galisteo, R.; Schneider, M.F.; Daugherty, A.; Rateri, D.L.; et al. LRP1 (Low-Density Lipoprotein Receptor–Related Protein 1) Regulates Smooth Muscle Contractility by Modulating Ca2+ Signaling and Expression of Cytoskeleton-Related Proteins. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2651–2664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Migliorini, M.; Hampton, B.; Ni, F.; Ucuzian, A.; Strickland, D. Abstract 292: LRP1 Prevents Aortic and SMA Aneurysms by Modulating Excessive Ang-II Signaling. Arterioscler. Thromb. Vasc. Biol. 2022, 42, A292. [Google Scholar] [CrossRef]
- Karasaki, K.; Kokubo, H.; Bumdelger, B.; Kaji, N.; Sakai, C.; Ishida, M.; Yoshizumi, M. Angiotensin II Type 1 Receptor Blocker Prevents Abdominal Aortic Aneurysm Progression in Osteoprotegerin-Deficient Mice via Upregulation of Angiotensin (1–7). J. Am. Heart Assoc. 2023, 12, e027589. [Google Scholar] [CrossRef] [PubMed]
- Toghill, B.J.; Saratzis, A.; Freeman, P.J.; Sylvius, N.; UKAGS collaborators; Bown, M.J. SMYD2 Promoter DNA Methylation Is Associated with Abdominal Aortic Aneurysm (AAA) and SMYD2 Expression in Vascular Smooth Muscle Cells. Clin. Epigenet. 2018, 10, 29. [Google Scholar] [CrossRef]
- Xu, G.; Liu, G.; Xiong, S.; Liu, H.; Chen, X.; Zheng, B. The Histone Methyltransferase Smyd2 Is a Negative Regulator of Macrophage Activation by Suppressing Interleukin 6 (IL-6) and Tumor Necrosis Factor α (TNF-α) Production. J. Biol. Chem. 2015, 290, 5414–5423. [Google Scholar] [CrossRef]
- Botts, S.R.; Khyzha, N.; Kumaragurubaran, R.; Wu, R.; Raju, S.; Prajapati, K.; Howe, K.L.; Fish, J.E. Abstract P104: The Transcription Factor Erg Governs Chromatin Accessibility and Gene Expression Programs Underlying Vascular Dysfunction In Aortic Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2021, 41, AP104. [Google Scholar] [CrossRef]
- Pisano, C.; Terriaca, S.; Scioli, M.G.; Nardi, P.; Altieri, C.; Orlandi, A.; Ruvolo, G.; Balistreri, C.R. The Endothelial Transcription Factor ERG Mediates a Differential Role in the Aneurysmatic Ascending Aorta with Bicuspid or Tricuspid Aorta Valve: A Preliminary Study. Int. J. Mol. Sci. 2022, 23, 10848. [Google Scholar] [CrossRef]
- Tang, W.; Saratzis, A.; Pattee, J.; Smith, J.; Pankratz, N.; Leavy, O.C.; Guan, W.; Dudbridge, F.; Pankow, J.S.; Kitas, G.D.; et al. Replication of Newly Identified Genetic Associations Between Abdominal Aortic Aneurysm and SMYD2, LINC00540, PCIF1/MMP9/ZNF335, and ERG. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.T.; Tromp, G.; Kuivaniemi, H.; Gretarsdottir, S.; Baas, A.F.; Giusti, B.; Strauss, E.; Van’t Hof, F.N.G.; Webb, T.R.; Erdman, R.; et al. Meta-Analysis of Genome-Wide Association Studies for Abdominal Aortic Aneurysm Identifies Four New Disease-Specific Risk Loci. Circ. Res. 2017, 120, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.T.; Bown, M.J.; Gretarsdottir, S.; Romaine, S.P.; Helgadottir, A.; Yu, G.; Tromp, G.; Norman, P.E.; Jin, C.; Baas, A.F.; et al. A Sequence Variant Associated with Sortilin-1 (SORT1) on 1p13.3 Is Independently Associated with Abdominal Aortic Aneurysm. Hum. Mol. Genet. 2013, 22, 2941–2947. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.T.; Hughes, A.E.; Badger, S.A.; Jones, G.T.; Harrison, S.C.; Wright, B.J.; Bumpstead, S.; Baas, A.F.; Grétarsdóttir, S.; Burnand, K.; et al. A Variant in LDLR Is Associated with Abdominal Aortic Aneurysm. Circ. Cardiovasc. Genet. 2013, 6, 498–504. [Google Scholar] [CrossRef]
- DePaolo, J.; Levin, M.G.; Tcheandjieu, C.; Priest, J.R.; Gill, D.; Burgess, S.; Damrauer, S.M.; Chirinos, J.A. Relationship Between Ascending Thoracic Aortic Diameter and Blood Pressure: A Mendelian Randomization Study. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 359–366. [Google Scholar] [CrossRef]
- Chun, C.; Qi, X.; Wang, F.; Madrid, K.B.; Saldarriaga, L.A.; Fisch, M.R.; Brantly, M.L.; Gilbert R Upchurch, J.; Jiang, Z. Nicotine Exacerbates TAAD Formation Induced by Smooth Muscle-Specific Deletion of the TGF-β Receptor 2. J. Immunol. Res. 2021, 2021, 6880036. [Google Scholar] [CrossRef]
- Kunwor, R.; Canelas, A. Rare Case of Cocaine-Induced Aortic Aneurysm: A Near Dissection Event. Case Rep. Cardiol. 2017, 2017, 1785410. [Google Scholar] [CrossRef] [PubMed]
- Jata, B.; Jahollari, A.; Kojqiqi, A.; Huti, G. Coarctation of Aorta and Post-Stenotic Dissecting Aortic Aneurysm Successfully Treated with Endovascular Stent. Int. Med. Case Rep. J. 2020, 13, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Antequera-González, B.; Martínez-Micaelo, N.; Alegret, J.M. Bicuspid Aortic Valve and Endothelial Dysfunction: Current Evidence and Potential Therapeutic Targets. Front. Physiol. 2020, 11, 1015. [Google Scholar] [CrossRef]
- Huckaby, L.V.; Leshnower, B.G. Sex and Gender Differences in Aortic Disease. US Cardiol. Rev. 2023, 17, e14. [Google Scholar] [CrossRef] [PubMed]
- Fritze, O.; Romero, B.; Schleicher, M.; Jacob, M.P.; Oh, D.-Y.; Starcher, B.; Schenke-Layland, K.; Bujan, J.; Stock, U.A. Age-Related Changes in the Elastic Tissue of the Human Aorta. J. Vasc. Res. 2011, 49, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Corsini, A.; Pacini, D.; Corti, B.; Lorenzini, M.; Laus, V.; Foà, A.; Reggiani, M.L.B.; Marco, L.D.; Rapezzi, C. The Complex Interplay among Atherosclerosis, Inflammation, and Degeneration in Ascending Thoracic Aortic Aneurysms. J. Thorac. Cardiovasc. Surg. 2020, 160, 1434–1443.e6. [Google Scholar] [CrossRef] [PubMed]
- Jessula, S.; Hull, T.D.; Isselbacher, E.M.; Bellomo, T.; Ghoshhajra, B.; Dua, A.; Eagleton, M.J.; Mohebali, J.; Jassar, A.S.; Zacharias, N. Infectious Aortitis of Thoracic Aortic Aneurysm from Clostridium Septicum. JACC Case Rep. 2023, 10, 101783. [Google Scholar] [CrossRef]
- Espitia, O.; Blonz, G.; Urbanski, G.; Landron, C.; Connault, J.; Lavigne, C.; Roblot, P.; Maillot, F.; Audemard-Verger, A.; Artifoni, M.; et al. Symptomatic Aortitis at Giant Cell Arteritis Diagnosis: A Prognostic Factor of Aortic Event. Arthritis Res. Ther. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Echeverria-Ortegon, E.; Millet-Herrera, J.L.; Méndez-Molina, R.; Casillas, J. Exceptional Multisite Aneurysms in Takayasu Arteritis: A Unique and Challenging Case. Radiol. Case Rep. 2024, 19, 3345–3348. [Google Scholar] [CrossRef]
- Wang, L.W.; Omari, A.; Emmett, L.; Jansz, P.C.; Huilgol, R.; Rainer, S.; Subbiah, R.N. Granulomatous Sarcoid Aortitis: A Serious Complication of a Well-Known Multisystem Disease. Lancet 2015, 385, 2014. [Google Scholar] [CrossRef]
- Gravos, A.; Katsifa, K.; Tselioti, P.; Grammatikopoulou, V.; Sakellaridis, K.; Kanakaki, S.; Tsapas, C.; Destounis, A.; Moschouris, H.; Athanasiadou, I.; et al. Ruptured Arterial Aneurysm in Wegener’s Granulomatosis: A Case Report. J. Med. Case Rep. 2021, 15, 343. [Google Scholar] [CrossRef] [PubMed]
- Shovman, O.; Tiosano, S.; Comaneshter, D.; Cohen, A.D.; Amital, H.; Sherf, M. Aortic Aneurysm Associated with Rheumatoid Arthritis: A Population-Based Cross-Sectional Study. Clin. Rheumatol. 2016, 35, 2657–2661. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.H.; Moore, R.; Kim, B.; Mosher, D.; Alvarez, N. Inflammatory Aortic Aneurysm in a Young Patient with Ankylosing Spondylitis. J. Vasc. Surg. 2017, 66, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Corominas, H.; Tsokos, M.; Quezado, M.; Tsokos, G.C. Aneurysm of the Ascending Aorta in Systemic Lupus Erythematosus: Case Report and Review of the Literature. Eur. J. Rheumatol. 2017, 4, 133–135. [Google Scholar] [CrossRef]
- Kwon, H.; Han, Y.; Son, D.H.; Cho, Y.-P.; Kwon, T.-W. Abdominal Aortic Aneurysm in Giant Cell Arteritis. Ann. Surg. Treat. Res. 2015, 89, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Kadian-Dodov, D.; Seo, P.; Robson, P.M.; Fayad, Z.A.; Olin, J.W. Inflammatory Diseases of the Aorta: JACC Focus Seminar, Part 2. J. Am. Coll. Cardiol. 2022, 80, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Safal, S.; Tyagi, D. Aortitis and Aortic Aneurysm in Systemic Vasculitis. Indian J. Thorac. Cardiovasc. Surg. 2019, 35, 47–56. [Google Scholar] [CrossRef]
- Abdelazeem, B.; Kambalapalli, S.; Lahmar, A.; Yousaf, A.; Kusz, H. Infectious Aortitis: Case Report and Literature Review. Cureus 2022, 14, e23198. [Google Scholar] [CrossRef]
- Jaffer, U.; Gibbs, R. Mycotic Thoracoabdominal Aneurysms. Ann. Cardiothorac. Surg. 2012, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Han, Y.; Lu, Q.; An, J.; Zhu, H.; Xie, Z.; Song, P.; Zou, M.-H. Peroxynitrite-Mediated SIRT (Sirtuin)-1 Inactivation Contributes to Nicotine-Induced Arterial Stiffness in Mice. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Tilson, M.D. Decline of the Atherogenic Theory of the Etiology of the Abdominal Aortic Aneurysm and Rise of the Autoimmune Hypothesis. J. Vasc. Surg. 2016, 64, 1523–1525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tilson, M.D. Autoimmunity in the Abdominal Aortic Aneurysm and Its Association with Smoking. AORTA J. 2017, 5, 159–167. [Google Scholar] [CrossRef]
- Ohmori, H.; Kanayama, N. Immunogenicity of an Inflammation-Associated Product, Tyrosine Nitrated Self-Proteins. Autoimmun. Rev. 2005, 4, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Borné, Y.; Bao, X.; Persson, M.; Gottsäter, A.; Acosta, S.; Engström, G. Comparisons of Risk Factors for Abdominal Aortic Aneurysm and Coronary Heart Disease: A Prospective Cohort Study. Angiology 2021, 72, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Rapacchietta, L.; Profeta, V.F.; Fagnano, R. Risk Factors for Abdominal Aortic Aneurysm in Population-Based Studies: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 2805. [Google Scholar] [CrossRef]
- Newton, E.R.; Akerman, A.W.; Strassle, P.D.; Kibbe, M.R. Association of Fluoroquinolone Use With Short-Term Risk of Development of Aortic Aneurysm. JAMA Surg. 2021, 156, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; El Helou, M.L.; Vellipuram, A.R. Fluoroquinolones and the Risk of Aortic Aneurysm or Aortic Dissection: A Systematic Review and Meta-Analysis. Cardiovasc. Hematol. Agents Med. Chem. 2019, 17, 3–10. [Google Scholar] [CrossRef]
- Zhou, Z.; Yue, Y.; Ma, K.; Hua, Z.; Li, Z. Congenital Abdominal Aortic Aneurysm: A Case Report and Literature Review. Front. Pediatr. 2022, 10, 853517. [Google Scholar] [CrossRef] [PubMed]
- Golombek, S.; Doll, I.; Kaufmann, L.; Lescan, M.; Schlensak, C.; Avci-Adali, M. A Novel Strategy for the Treatment of Aneurysms: Inhibition of MMP-9 Activity through the Delivery of TIMP-1 Encoding Synthetic mRNA into Arteries. Int. J. Mol. Sci. 2024, 25, 6599. [Google Scholar] [CrossRef]
- Stepien, K.L.; Bajdak-Rusinek, K.; Fus-Kujawa, A.; Kuczmik, W.; Gawron, K. Role of Extracellular Matrix and Inflammation in Abdominal Aortic Aneurysm. Int. J. Mol. Sci. 2022, 23, 11078. [Google Scholar] [CrossRef]
- Hu, M.; Meganathan, I.; Zhu, J.; MacArthur, R.; Kassiri, Z. Loss of TIMP3, but Not TIMP4, Exacerbates Thoracic and Abdominal Aortic Aneurysm. J. Mol. Cell. Cardiol. 2023, 184, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Deleeuw, V.; Van Impe, M.; Logghe, G.; Vanhomwegen, M.; Olbinado, M.; Stampanoni, M.; Segers, P.; Muino-Mosquera, L.; Sakai, L.; Sips, P.; et al. Local TGF-Beta Sequestration by Fibrillin-1 Regulates Vascular Wall Homeostasis in the Thoracic Aorta. Eur. Heart J. 2022, 43, ehac544.1934. [Google Scholar] [CrossRef]
- Carlson, E.J.; Rushkin, M.; Darby, D.; Chau, T.; Shirley, R.L.; King, J.S.; Nguyen, K.; Landry, G.J.; Moneta, G.L.; Abraham, C.; et al. Circulating Fibrillin Fragment Concentrations in Patients with and without Aortic Pathology. JVS Vasc. Sci. 2022, 3, 389–402. [Google Scholar] [CrossRef]
- Wang, X.; LeMaire, S.A.; Chen, L.; Shen, Y.H.; Gan, Y.; Bartsch, H.; Carter, S.A.; Utama, B.; Ou, H.; Coselli, J.S.; et al. Increased Collagen Deposition and Elevated Expression of Connective Tissue Growth Factor in Human Thoracic Aortic Dissection. Circulation 2006, 114, I200-5. [Google Scholar] [CrossRef]
- Abdul-Hussien, H.; Soekhoe, R.G.V.; Weber, E.; von der Thüsen, J.H.; Kleemann, R.; Mulder, A.; van Bockel, J.H.; Hanemaaijer, R.; Lindeman, J.H.N. Collagen Degradation in the Abdominal Aneurysm: A Conspiracy of Matrix Metalloproteinase and Cysteine Collagenases. Am. J. Pathol. 2007, 170, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.G.; Romary, D.J.; Kerr, K.E.; Gorazd, N.E.; Wigand, M.M.; Patnaik, S.S.; Finol, E.A.; Cox, A.D.; Goergen, C.J. Experimental Aortic Aneurysm Severity and Growth Depend on Topical Elastase Concentration and Lysyl Oxidase Inhibition. Sci. Rep. 2022, 12, 99. [Google Scholar] [CrossRef]
- Cikach, F.S.; Koch, C.D.; Mead, T.J.; Galatioto, J.; Willard, B.B.; Emerton, K.B.; Eagleton, M.J.; Blackstone, E.H.; Ramirez, F.; Roselli, E.E.; et al. Massive Aggrecan and Versican Accumulation in Thoracic Aortic Aneurysm and Dissection. JCI Insight 2018, 3, e97167. [Google Scholar] [CrossRef] [PubMed]
- Didangelos, A.; Yin, X.; Mandal, K.; Saje, A.; Smith, A.; Xu, Q.; Jahangiri, M.; Mayr, M. Extracellular Matrix Composition and Remodeling in Human Abdominal Aortic Aneurysms: A Proteomics Approach*. Mol. Cell. Proteom. 2011, 10, M111.008128. [Google Scholar] [CrossRef]
- Chen, M.; Cavinato, C.; Hansen, J.; Tanaka, K.; Ren, P.; Hassab, A.; Li, D.S.; Youshao, E.; Tellides, G.; Iyengar, R.; et al. Fibronectin-Integrin A5 Signaling Promotes Thoracic Aortic Aneurysm in a Mouse Model of Marfan Syndrome. Arterioscler. Thromb. Vasc. Biol. 2023, 43, e132–e150. [Google Scholar] [CrossRef] [PubMed]
- Plana, E.; Oto, J.; Medina, P.; Herranz, R.; Fernández-Pardo, Á.; Requejo, L.; Miralles, M. Thrombospondins in Human Aortic Aneurysms. IUBMB Life 2022, 74, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, T.; Sorenson, C.M.; Sheibani, N.; Liu, B. Myeloid-Derived Thrombospondin-1 Contributes to Abdominal Aortic Aneurysm through Suppressing Tissue Inhibitor of Metalloproteinases-1. Arter. Thromb. Vasc. Biol. 2020, 40, e350–e366. [Google Scholar] [CrossRef]
- Seim, B.E.; Holt, M.F.; Ratajska, A.; Michelsen, A.; Ringseth, M.M.; Halvorsen, B.E.; Skjelland, M.; Kvitting, J.-P.E.; Lundblad, R.; Krohg-Sørensen, K.; et al. Markers of Extracellular Matrix Remodeling and Systemic Inflammation in Patients with Heritable Thoracic Aortic Diseases. Front. Cardiovasc. Med. 2022, 9, 1073069. [Google Scholar] [CrossRef] [PubMed]
- Bazzazi, H.; Zhang, Y.; Jafarnejad, M.; Isenberg, J.S.; Annex, B.H.; Popel, A.S. Computer Simulation of TSP1 Inhibition of VEGF–Akt–eNOS: An Angiogenesis Triple Threat. Front. Physiol. 2018, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tsao, P.S.; Pan, C. Abdominal Aortic Aneurysm and Cardiometabolic Traits Share Strong Genetic Susceptibility to Lipid Metabolism and Inflammation. Nat. Commun. 2024, 15, 5652. [Google Scholar] [CrossRef] [PubMed]
- De Castro-Orós, I.; Pocoví, M.; Civeira, F. The Genetic Basis of Familial Hypercholesterolemia: Inheritance, Linkage, and Mutations. Appl. Clin. Genet. 2010, 3, 53–64. [Google Scholar] [PubMed]
- Smit, J.W.A.; Havekes, L.M. Mixed Lipemias. In Encyclopedia of Endocrine Diseases; Martini, L., Ed.; Elsevier: New York, NY, USA, 2004; pp. 258–262. ISBN 978-0-12-475570-3. [Google Scholar]
- Pirahanchi, Y.; Sinawe, H.; Dimri, M. Biochemistry, LDL Cholesterol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Khalil, Y.A.; Rabès, J.-P.; Boileau, C.; Varret, M. APOE Gene Variants in Primary Dyslipidemia. Atherosclerosis 2021, 328, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Bouchareychas, L.; Raffai, R.L. Apolipoprotein E and Atherosclerosis: From Lipoprotein Metabolism to MicroRNA Control of Inflammation. J. Cardiovasc. Dev. Dis. 2018, 5, 30. [Google Scholar] [CrossRef]
- Lumsden, A.L.; Mulugeta, A.; Zhou, A.; Hyppönen, E. Apolipoprotein E (APOE) Genotype-Associated Disease Risks: A Phenome-Wide, Registry-Based, Case-Control Study Utilising the UK Biobank. eBioMedicine 2020, 59, 102954. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.C.; Holmes, M.V.; Burgess, S.; Asselbergs, F.W.; Jones, G.T.; Baas, A.F.; van ’t Hof, F.N.; de Bakker, P.I.W.; Blankensteijn, J.D.; Powell, J.T.; et al. Genetic Association of Lipids and Lipid Drug Targets with Abdominal Aortic Aneurysm: A Meta-Analysis. JAMA Cardiol. 2018, 3, 26–33. [Google Scholar] [CrossRef]
- Weng, L.-C.; Roetker, N.S.; Lutsey, P.L.; Alonso, A.; Guan, W.; Pankow, J.S.; Folsom, A.R.; Steffen, L.M.; Pankratz, N.; Tang, W. Evaluation of the Relationship between Plasma Lipids and Abdominal Aortic Aneurysm: A Mendelian Randomization Study. PLoS ONE 2018, 13, e0195719. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.T.; Chan, Y.C.; Cheuk, B.L.Y.; Cheng, S.W.K. Clearance of Matrix Metalloproteinase-9 Is Dependent on Low-Density Lipoprotein Receptor-Related Protein-1 Expression Downregulated by microRNA-205 in Human Abdominal Aortic Aneurysm. J. Vasc. Surg. 2017, 65, 509–520. [Google Scholar] [CrossRef]
- Munshaw, S.; Bruche, S.; Redpath, A.N.; Jones, A.; Patel, J.; Dubé, K.N.; Lee, R.; Hester, S.S.; Davies, R.; Neal, G.; et al. Thymosin β4 Protects against Aortic Aneurysm via Endocytic Regulation of Growth Factor Signaling. J. Clin. Investig. 2021, 131, e127884. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Takayama, Y.; Boucher, P.; Tallquist, M.D.; Herz, J. LRP1 Regulates Architecture of the Vascular Wall by Controlling PDGFRβ-Dependent Phosphatidylinositol 3-Kinase Activation. PLoS ONE 2009, 4, e6922. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, M.; Zhang, C. Vascular Macrophages Sense Octanal and Drive Athero-Inflammation. Cell. Mol. Immunol. 2022, 19, 1077–1078. [Google Scholar] [CrossRef]
- Thomas, C.E.; Jackson, R.L.; Ohlweiler, D.F.; Ku, G. Multiple Lipid Oxidation Products in Low Density Lipoproteins Induce Interleukin-1 Beta Release from Human Blood Mononuclear Cells. J. Lipid Res. 1994, 35, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Schelemei, P.; Picard, F.S.R.; Nemade, H.; Mehrkens, D.; Tinaz, K.; Grimm, S.; Orecchioni, M.; Wagenhäuser, M.; Schelzig, H.; Roy, J.; et al. Olfactory Receptor 2 Signaling Drives Abdominal Aortic Aneurysm Formation. Atherosclerosis 2024, 395, 118390. [Google Scholar] [CrossRef]
- Chakraborty, R.; Chatterjee, P.; Dave, J.M.; Ostriker, A.C.; Greif, D.M.; Rzucidlo, E.M.; Martin, K.A. Targeting Smooth Muscle Cell Phenotypic Switching in Vascular Disease. JVS Vasc. Sci. 2021, 2, 79–94. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, X.; Friesel, R.E.; Vary, C.P.H.; Liaw, L. Mechanisms of TGF-β-Induced Differentiation in Human Vascular Smooth Muscle Cells. J. Vasc. Res. 2011, 48, 485–494. [Google Scholar] [CrossRef]
- Gao, J.; Cao, H.; Hu, G.; Wu, Y.; Xu, Y.; Cui, H.; Lu, H.S.; Zheng, L. The Mechanism and Therapy of Aortic Aneurysms. Signal Transduct. Target. Ther. 2023, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.J.; Redmond, E.M.; Gillespie, D.L.; Knight, P.A.; Cullen, J.P.; Cahill, P.A.; Morrow, D.J. Differential Expression of Hedgehog/Notch and Transforming Growth Factor-β in Human Abdominal Aortic Aneurysms. J. Vasc. Surg. 2015, 62, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-B.; Zhu, J.; Zhou, Z.-Z.; Xi, E.-P.; Wang, R.-P.; Zhang, Y. TGF-Β1 Induces Human Aortic Vascular Smooth Muscle Cell Phenotype Switch through PI3K/AKT/ID2 Signaling. Am. J. Transl. Res. 2015, 7, 2764–2774. [Google Scholar]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How Vascular Smooth Muscle Cell Phenotype Switching Contributes to Vascular Disease. Cell Commun. Signal. 2022, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Petsophonsakul, P.; Furmanik, M.; Forsythe, R.; Dweck, M.; Schurink, G.W.; Natour, E.; Reutelingsperger, C.; Jacobs, M.; Mees, B.; Schurgers, L. Role of Vascular Smooth Muscle Cell Phenotypic Switching and Calcification in Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1351–1368. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Li, Y.; Ren, P.; Dawson, A.; Vasquez, H.G.; Ageedi, W.; Zhang, C.; Luo, W.; Chen, R.; Li, Y.; Kim, S.; et al. Single-Cell Transcriptome Analysis Reveals Dynamic Cell Populations and Differential Gene Expression Patterns in Control and Aneurysmal Human Aortic Tissue. Circulation 2020, 142, 1374–1388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Lu, H.; Chang, Z.; Zhao, Y.; Zhu, T.; Chang, L.; Guo, Y.; Garcia-Barrio, M.T.; Chen, Y.E.; Zhang, J. Single-Cell RNA Sequencing Reveals the Cellular Heterogeneity of Aneurysmal Infrarenal Abdominal Aorta. Cardiovasc. Res. 2021, 117, 1402–1416. [Google Scholar] [CrossRef]
- Li, G.; Wang, M.; Caulk, A.W.; Cilfone, N.A.; Gujja, S.; Qin, L.; Chen, P.-Y.; Chen, Z.; Yousef, S.; Jiao, Y.; et al. Chronic mTOR Activation Induces a Degradative Smooth Muscle Cell Phenotype. J. Clin. Investig. 2020, 130, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Qin, L.; Li, G.; Malagon-Lopez, J.; Wang, Z.; Bergaya, S.; Gujja, S.; Caulk, A.W.; Murtada, S.-I.; Zhang, X.; et al. Smooth Muscle Cell Reprogramming in Aortic Aneurysms. Cell Stem Cell 2020, 26, 542–557.e11. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.; Li, Y.; Li, Y.; Ren, P.; Vasquez, H.G.; Zhang, C.; Rebello, K.R.; Ageedi, W.; Azares, A.R.; Mattar, A.B.; et al. Single-Cell Analysis of Aneurysmal Aortic Tissue in Patients with Marfan Syndrome Reveals Dysfunctional TGF-β Signaling. Genes 2021, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Ganizada, B.H.; Veltrop, R.J.A.; Akbulut, A.C.; Koenen, R.R.; Accord, R.; Lorusso, R.; Maessen, J.G.; Reesink, K.; Bidar, E.; Schurgers, L.J. Unveiling Cellular and Molecular Aspects of Ascending Thoracic Aortic Aneurysms and Dissections. Basic Res. Cardiol. 2024, 119, 371–395. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Nash, J.; Syed, M.B.J.; Macaskill, M.G.; Tavares, A.A.S.; Walker, N.; Salcudean, H.; Leipsic, J.A.; Lim, K.H.H.; Madine, J.; et al. Microcalcification and Thoracic Aortopathy: A Window into Disease Severity. Arter. Thromb. Vasc. Biol. 2022, 42, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Cui, S.; Wang, J.; Xu, T.; Du, H.; Yue, H.; Ye, H.; Guo, J.; Zhang, J.; Li, P.; et al. Early Progression of Abdominal Aortic Aneurysm Is Decelerated by Improved Endothelial Barrier Function via ALDH2-LIN28B-ELK3 Signaling. Adv. Sci. 2023, 10, 2302231. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Ren, J.; Li, X.; Wang, Z.; Xue, L.; Cui, S.; Sang, W.; Xu, T.; Zhang, J.; Yu, J.; et al. Prevention of Aortic Dissection and Aneurysm via an ALDH2-Mediated Switch in Vascular Smooth Muscle Cell Phenotype. Eur. Heart J. 2020, 41, 2442–2453. [Google Scholar] [CrossRef]
- Airhart, N.; Brownstein, B.H.; Cobb, J.P.; Schierding, W.; Arif, B.; Ennis, T.L.; Thompson, R.W.; Curci, J.A. Smooth Muscle Cells from Abdominal Aortic Aneurysms Are Unique and Can Independently and Synergistically Degrade Insoluble Elastin. J. Vasc. Surg. 2014, 60, 1033–1041, discussion 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Saaoud, F.; Lu, Y.; Pu, Y.; Xu, K.; Shao, Y.; Jiang, X.; Wu, S.; Yang, L.; Tian, Y.; et al. Innate Immunity of Vascular Smooth Muscle Cells Contributes to Two-Wave Inflammation in Atherosclerosis, Twin-Peak Inflammation in Aortic Aneurysms and Trans-Differentiation Potential into 25 Cell Types. Front. Immunol. 2024, 14, 1348238. [Google Scholar] [CrossRef]
- Lu, H.; Du, W.; Ren, L.; Hamblin, M.H.; Becker, R.C.; Chen, Y.E.; Fan, Y. Vascular Smooth Muscle Cells in Aortic Aneurysm: From Genetics to Mechanisms. J. Am. Heart Assoc. 2021, 10, e023601. [Google Scholar] [CrossRef]
- Cao, G.; Xuan, X.; Li, Y.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. Single-Cell RNA Sequencing Reveals the Vascular Smooth Muscle Cell Phenotypic Landscape in Aortic Aneurysm. Cell Commun. Signal. 2023, 21, 113. [Google Scholar] [CrossRef] [PubMed]
- Pedroza, A.J.; Tashima, Y.; Shad, R.; Cheng, P.; Wirka, R.; Churovich, S.; Nakamura, K.; Yokoyama, N.; Cui, J.Z.; Iosef, C.; et al. Single-Cell Transcriptomic Profiling of Vascular Smooth Muscle Cell Phenotype Modulation in Marfan Syndrome Aortic Aneurysm. Arter. Thromb. Vasc. Biol. 2020, 40, 2195–2211. [Google Scholar] [CrossRef]
- Badran, A.; Nasser, S.A.; Mesmar, J.; El-Yazbi, A.F.; Bitto, A.; Fardoun, M.M.; Baydoun, E.; Eid, A.H. Reactive Oxygen Species: Modulators of Phenotypic Switch of Vascular Smooth Muscle Cells. Int. J. Mol. Sci. 2020, 21, 8764. [Google Scholar] [CrossRef]
- Vendrov, A.E.; Silveira Fernandes Chamon, J.; Levin, J.; Hayami, T.; Runge, M.S.; Madamanchi, N.R. Abstract 16902: Molecular Basis of the Vascular Cell Phenotypic Switch and Wall Remodeling in Abdominal Aortic Aneurysm: NOX4 NADPH Oxidase-Derived Mitochondrial Oxidative Stress and DNA Damage. Circulation 2023, 148, A16902. [Google Scholar] [CrossRef]
- Kazaleh, M.; Gioscia-Ryan, R.; Ailawadi, G.; Salmon, M. Oxidative Stress and the Pathogenesis of Aortic Aneurysms. Biomedicines 2024, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Stefens, S.J.M.; van Vliet, N.; IJpma, A.; Burger, J.; Li, Y.; van Heijningen, P.M.; Lindeman, J.H.N.; Majoor-Krakauer, D.; Verhagen, H.J.M.; Kanaar, R.; et al. Increased Vascular Smooth Muscle Cell Senescence in Aneurysmal Fibulin-4 Mutant Mice. Npj Aging 2024, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Balint, B.; Yin, H.; Nong, Z.; Arpino, J.-M.; O’Neil, C.; Rogers, S.R.; Randhawa, V.K.; Fox, S.A.; Chevalier, J.; Lee, J.J.; et al. Seno-Destructive Smooth Muscle Cells in the Ascending Aorta of Patients with Bicuspid Aortic Valve Disease. EBioMedicine 2019, 43, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Budbazar, E.; Sulser Ponce De Leon, S.; Tsukahara, Y.; Liu, H.; Huangfu, Y.; Wang, Y.; Seabra, P.M.; Yang, X.; Goodman, J.B.; Wan, X.; et al. Redox Dysregulation of Vascular Smooth Muscle Sirtuin-1 in Thoracic Aortic Aneurysm in Marfan Syndrome. Arterioscler. Thromb. Vasc. Biol. 2023, 43, e339–e357. [Google Scholar] [CrossRef] [PubMed]
- Clément, M.; Chappell, J.; Raffort, J.; Lareyre, F.; Vandestienne, M.; Taylor, A.L.; Finigan, A.; Harrison, J.; Bennett, M.R.; Bruneval, P.; et al. Vascular Smooth Muscle Cell Plasticity and Autophagy in Dissecting Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Raffort, J.; Lareyre, F.; Clément, M.; Hassen-Khodja, R.; Chinetti, G.; Mallat, Z. Monocytes and Macrophages in Abdominal Aortic Aneurysm. Nat. Rev. Cardiol. 2017, 14, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, A.; Neumayer, C.; Bolliger, M.; Burghuber, C.; Klinger, M.; Demyanets, S.; Nanobachvili, J.; Huk, I. Insight into the Expression of Toll-like Receptors 2 and 4 in Patients with Abdominal Aortic Aneurysm. Mol. Biol. Rep. 2020, 47, 2685–2692. [Google Scholar] [CrossRef]
- Yuan, Z.; Lu, Y.; Wei, J.; Wu, J.; Yang, J.; Cai, Z. Abdominal Aortic Aneurysm: Roles of Inflammatory Cells. Front. Immunol. 2021, 11, 609161. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Eilenberg, W.; Neumayer, C.; Brostjan, C. Neutrophil Extracellular Traps in Cardiovascular and Aortic Disease: A Narrative Review on Molecular Mechanisms and Therapeutic Targeting. Int. J. Mol. Sci. 2024, 25, 3983. [Google Scholar] [CrossRef]
- Hinterseher, I.; Schworer, C.M.; Lillvis, J.H.; Stahl, E.; Erdman, R.; Gatalica, Z.; Tromp, G.; Kuivaniemi, H. Immunohistochemical Analysis of the Natural Killer Cell Cytotoxicity Pathway in Human Abdominal Aortic Aneurysms. Int. J. Mol. Sci. 2015, 16, 11196–11212. [Google Scholar] [CrossRef]
- Good, E.; Åkerman, L.; Nyström, S.; Jonasson, L.; Ernerudh, J.; de Muinck, E. Changes in Natural Killer and T Lymphocyte Phenotypes in Response to Cardiovascular Risk Management. Sci. Rep. 2023, 13, 20810. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Ishimori, N.; Tokuhara, S.; Homma, T.; Nishikawa, M.; Iwabuchi, K.; Tsutsui, H. Activation of Invariant Natural Killer T Cells by α-Galactosylceramide Attenuates the Development of Angiotensin II-Mediated Abdominal Aortic Aneurysm in Obese Ob/Ob Mice. Front. Cardiovasc. Med. 2021, 8, 659418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, M.-X.; Shen, Y.H.; Burks, J.K.; Zhang, Y.; Wang, J.; LeMaire, S.A.; Yoshimura, K.; Aoki, H.; Coselli, J.S.; et al. TNF-α Suppresses Prolyl-4-Hydroxylase A1 Expression via the ASK1–JNK–NonO Pathway. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1760–1767. [Google Scholar] [CrossRef]
- Chen, Y.; He, Y.; Wei, X.; Jiang, D.-S. Targeting Regulated Cell Death in Aortic Aneurysm and Dissection Therapy. Pharmacol. Res. 2022, 176, 106048. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xin, S.; Yan, Y.; Lun, Y.; Yang, X.; Zhang, J. Abnormal Acetylation of FOXP3 Regulated by SIRT-1 Induces Treg Functional Deficiency in Patients with Abdominal Aortic Aneurysms. Atherosclerosis 2018, 271, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Hamaidi, I.; Kim, S. Sirtuins Are Crucial Regulators of T Cell Metabolism and Functions. Exp. Mol. Med. 2022, 54, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ageedi, W.; Zhang, C.; Frankel, W.C.; Dawson, A.; Li, Y.; Coselli, J.S.; Shen, H.Y.; LeMaire, S.A. AIM2 Inflammasome Activation Contributes to Aortic Dissection in a Sporadic Aortic Disease Mouse Model. J. Surg. Res. 2022, 272, 105–116. [Google Scholar] [CrossRef]
- Pi, S.; Xiong, S.; Yuan, Y.; Deng, H. The Role of Inflammasome in Abdominal Aortic Aneurysm and Its Potential Drugs. Int. J. Mol. Sci. 2024, 25, 5001. [Google Scholar] [CrossRef]
- Dihlmann, S.; Erhart, P.; Mehrabi, A.; Nickkholgh, A.; Lasitschka, F.; Böckler, D.; Hakimi, M. Increased Expression and Activation of Absent in Melanoma 2 Inflammasome Components in Lymphocytic Infiltrates of Abdominal Aortic Aneurysms. Mol. Med. 2014, 20, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, P.J.; Jessen, M.L.; Eiberg, J.; Ghulam, Q. Hypoxia Inducible Factor 1-Alpha in the Pathogenesis of Abdominal Aortic Aneurysms in Vivo: A Narrative Review. JVS-Vasc. Sci. 2024, 5, 100189. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Shibasaki, F. Hypoxia-Inducible Factor as an Angiogenic Master Switch. Front. Pediatr. 2015, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Kessler, K.; Borges, L.F.; Ho-Tin-Noé, B.; Jondeau, G.; Michel, J.-B.; Vranckx, R. Angiogenesis and Remodelling in Human Thoracic Aortic Aneurysms. Cardiovasc. Res. 2014, 104, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, D.; Yu, J.; Jiang, W.; Liu, Y.; Li, F.; Zeng, R.; Wan, Z.; Liao, X. Angiogenesis in Aortic Aneurysm and Dissection: A Literature Review. RCM 2023, 24, 223. [Google Scholar] [CrossRef]
- Kaneko, H.; Anzai, T.; Takahashi, T.; Kohno, T.; Shimoda, M.; Sasaki, A.; Shimizu, H.; Nagai, T.; Maekawa, Y.; Yoshimura, K.; et al. Role of Vascular Endothelial Growth Factor-A in Development of Abdominal Aortic Aneurysm. Cardiovasc. Res. 2011, 91, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Bleichert, S.; Klopf, J.; Kurzreiter, G.; Hayden, H.; Knöbl, V.; Artner, T.; Krall, M.; Stiglbauer-Tscholakoff, A.; Oehler, R.; et al. Reducing Abdominal Aortic Aneurysm Progression by Blocking Neutrophil Extracellular Traps Depends on Thrombus Formation. JACC Basic Transl. Sci. 2024, 9, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xia, S.; Liu, G.; Song, C. The Detrimental Role of Intraluminal Thrombus Outweighs Protective Advantage in Abdominal Aortic Aneurysm Pathogenesis: The Implications for the Anti-Platelet Therapy. Biomolecules 2022, 12, 942. [Google Scholar] [CrossRef] [PubMed]
- Lenting, P.J.; Christophe, O.D.; Denis, C.V. Von Willebrand Factor Biosynthesis, Secretion, and Clearance: Connecting the Far Ends. Blood 2015, 125, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Alkarithi, G.; Duval, C.; Shi, Y.; Macrae, F.L.; Ariëns, R.A.S. Thrombus Structural Composition in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2370–2383. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet Integrin αIIbβ3: Signal Transduction, Regulation, and Its Therapeutic Targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar] [CrossRef]
- Dam, E.; Dams, S.; Peters, G.; Rutten, M.; Schurink, G.; Buth, J.; van de Vosse, F. Non-Linear Viscoelastic Behavior of Abdominal Aortic Aneurysm Thrombus. Biomech. Model. Mechanobiol. 2008, 7, 127–137. [Google Scholar] [CrossRef]
- Swedenborg, J.; Eriksson, P. The Intraluminal Thrombus as a Source of Proteolytic Activity. Ann. N. Y. Acad. Sci. 2006, 1085, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Piechota-Polanczyk, A.; Jozkowicz, A.; Nowak, W.; Eilenberg, W.; Neumayer, C.; Malinski, T.; Huk, I.; Brostjan, C. The Abdominal Aortic Aneurysm and Intraluminal Thrombus: Current Concepts of Development and Treatment. Front. Cardiovasc. Med. 2015, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Debski, A.; Hans, C.P.; Go, M.R.; Agarwal, G. Structurally Abnormal Collagen Fibrils in Abdominal Aortic Aneurysm Resist Platelet Adhesion. J. Thromb. Haemost. 2022, 20, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zheng, J.; Gao, Y. Targeting Platelet Activation in Abdominal Aortic Aneurysm: Current Knowledge and Perspectives. Biomolecules 2022, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Bontekoe, J.; Matsumura, J.; Liu, B. Thrombosis in the Pathogenesis of Abdominal Aortic Aneurysm. JVS Vasc. Sci. 2023, 4, 100106. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Bone Marrow. Circulation 2003, 108, 378–379. [Google Scholar] [CrossRef][Green Version]
- Fontaine, V.; Touat, Z.; Mtairag, E.M.; Vranckx, R.; Louedec, L.; Houard, X.; Andreassian, B.; Sebbag, U.; Palombi, T.; Jacob, M.-P.; et al. Role of Leukocyte Elastase in Preventing Cellular Re-Colonization of the Mural Thrombus. Am. J. Pathol. 2004, 164, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.; Escaig, R.; Erber, J.; Nicolai, L. Neutrophil-Platelet Interactions as Novel Treatment Targets in Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 8, 824112. [Google Scholar] [CrossRef]
- Lindquist Liljeqvist, M.; Silveira, A.; Hultgren, R.; Frebelius, S.; Lengquist, M.; Engström, J.; Gasser, T.C.; Eriksson, P.; Roy, J. Neutrophil Elastase-Derived Fibrin Degradation Products Indicate Presence of Abdominal Aortic Aneurysms and Correlate with Intraluminal Thrombus Volume. Thromb. Haemost. 2018, 118, 329–339. [Google Scholar] [CrossRef]
- Rao, J.; Brown, B.N.; Weinbaum, J.S.; Ofstun, E.L.; Makaroun, M.S.; Humphrey, J.D.; Vorp, D.A. Distinct Macrophage Phenotype and Collagen Organization within the Intraluminal Thrombus of Abdominal Aortic Aneurysm. J. Vasc. Surg. 2015, 62, 585–593. [Google Scholar] [CrossRef]
- Ashida, S.; Yamawaki-Ogata, A.; Tokoro, M.; Mutsuga, M.; Usui, A.; Narita, Y. Administration of Anti-Inflammatory M2 Macrophages Suppresses Progression of Angiotensin II-Induced Aortic Aneurysm in Mice. Sci. Rep. 2023, 13, 1380. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Tu, G.; Qin, L.; Wei, L.; Chen, J. Macrophage in Sporadic Thoracic Aortic Aneurysm and Dissection: Potential Therapeutic and Preventing Target. RCM 2023, 24, 340. [Google Scholar] [CrossRef]
- Liu, O.; Jia, L.; Liu, X.; Wang, Y.; Wang, X.; Qin, Y.; Du, J.; Zhang, H. Clopidogrel, a Platelet P2Y12 Receptor Inhibitor, Reduces Vascular Inflammation and Angiotensin II Induced-Abdominal Aortic Aneurysm Progression. PLoS ONE 2012, 7, e51707. [Google Scholar] [CrossRef]
- Rolling, C.C.; Barrett, T.J.; Berger, J.S. Platelet-Monocyte Aggregates: Molecular Mediators of Thromboinflammation. Front. Cardiovasc. Med. 2023, 10, 960398. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Jennings, C.L.; Manning, E.; Cameron, S.J. Platelets at the Vessel Wall in Non-Thrombotic Disease. Circ. Res. 2023, 132, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Wagenhäuser, M.U.; Mulorz, J.; Krott, K.J.; Bosbach, A.; Feige, T.; Rhee, Y.H.; Chatterjee, M.; Petzold, N.; Böddeker, C.; Ibing, W.; et al. Crosstalk of Platelets with Macrophages and Fibroblasts Aggravates Inflammation, Aortic Wall Stiffening, and Osteopontin Release in Abdominal Aortic Aneurysm. Cardiovasc. Res. 2024, 120, 417–432. [Google Scholar] [CrossRef]
- Wang, J.; He, C.; Chen, Y.; Hu, X.; Xu, H.; Liu, J.; Yang, Y.; Chen, L.; Li, T.; Fang, L.; et al. Platelet Factors Ameliorate Thoracic Aortic Aneurysm and Dissection by Inhibiting the FGF-FGFR Cascade Activation in Aortic-Endothelial Cell. iScience 2024, 27, 110953. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Meganathan, I.; MacAruthur, R.; Kassiri, Z. Inflammation in Abdominal Aortic Aneurysm: Cause or Comorbidity? Can. J. Cardiol. 2024, 40, 2378–2391. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Hao, H.; Naito, Y.; Oboshi, M.; Hirotani, S.; Mitsuno, M.; Miyamoto, Y.; Hirota, S.; Masuyama, T. Aortic Iron Overload With Oxidative Stress and Inflammation in Human and Murine Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.J.; Russell, H.M.; Owens, A.P., III. Antithrombotic Therapy in Abdominal Aortic Aneurysm: Beneficial or Detrimental? Blood 2018, 132, 2619–2628. [Google Scholar] [CrossRef]
- Sano, M.; Sasaki, T.; Hirakawa, S.; Sakabe, J.; Ogawa, M.; Baba, S.; Zaima, N.; Tanaka, H.; Inuzuka, K.; Yamamoto, N.; et al. Lymphangiogenesis and Angiogenesis in Abdominal Aortic Aneurysm. PLoS ONE 2014, 9, e89830. [Google Scholar] [CrossRef]
- Gäbel, G.; Northoff, B.H.; Weinzierl, I.; Ludwig, S.; Hinterseher, I.; Wilfert, W.; Teupser, D.; Doderer, S.A.; Bergert, H.; Schönleben, F.; et al. Molecular Fingerprint for Terminal Abdominal Aortic Aneurysm Disease. J. Am. Heart Assoc. 2017, 6, e006798. [Google Scholar] [CrossRef]
- Mackay, C.D.A.; Jadli, A.S.; Fedak, P.W.M.; Patel, V.B. Adventitial Fibroblasts in Aortic Aneurysm: Unraveling Pathogenic Contributions to Vascular Disease. Diagnostics 2022, 12, 871. [Google Scholar] [CrossRef]
- Yamakawa, M.; Liu, L.X.; Date, T.; Belanger, A.J.; Vincent, K.A.; Akita, G.Y.; Kuriyama, T.; Cheng, S.H.; Gregory, R.J.; Jiang, C. Hypoxia-Inducible Factor-1 Mediates Activation of Cultured Vascular Endothelial Cells by Inducing Multiple Angiogenic Factors. Circ. Res. 2003, 93, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.; Waliszewski, K.; Oszkinis, G.; Staniszewski, R. Polymorphisms of Genes Involved in the Hypoxia Signaling Pathway and the Development of Abdominal Aortic Aneurysms or Large-Artery Atherosclerosis. J. Vasc. Surg. 2015, 61, 1105–1113.e3. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.M.; Seto, S.W.; Jose, R.; Li, J.; Moxon, J.; Clancy, P.; Crossman, D.J.; Norman, P.; Emeto, T.I.; Golledge, J. High Serum Thrombospondin-1 Concentration Is Associated with Slower Abdominal Aortic Aneurysm Growth and Deficiency of Thrombospondin-1 Promotes Angiotensin II Induced Aortic Aneurysm in Mice. Clin. Sci. 2017, 131, 1261–1281. [Google Scholar] [CrossRef]
- Golledge, J.; Clancy, P.; Yeap, B.B.; Hankey, G.J.; Norman, P.E. Increased Serum Angiopoietin-2 Is Associated with Abdominal Aortic Aneurysm Prevalence and Cardiovascular Mortality in Older Men. Int. J. Cardiol. 2013, 167, 1159–1163. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
- Chappell, J.C.; Mouillesseaux, K.P.; Bautch, V.L. Flt-1 (Vascular Endothelial Growth Factor Receptor-1) Is Essential for the Vascular Endothelial Growth Factor–Notch Feedback Loop During Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Kappas, N.C.; Zeng, G.; Chappell, J.C.; Kearney, J.B.; Hazarika, S.; Kallianos, K.G.; Patterson, C.; Annex, B.H.; Bautch, V.L. The VEGF Receptor Flt-1 Spatially Modulates Flk-1 Signaling and Blood Vessel Branching. J. Cell Biol. 2008, 181, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Nakamura, S.; Sugimoto, N.; Shigemori, T.; Kato, Y.; Ohno, M.; Sakuma, S.; Ito, K.; Kumon, H.; Hirose, H.; et al. Turbulence Activates Platelet Biogenesis to Enable Clinical Scale Ex Vivo Production. Cell 2018, 174, 636–648.e18. [Google Scholar] [CrossRef]
- Moço, G.; Sousa, C.; Capitão, A.; MacKinnon, S.S.; Leitão, A.J.; Mendes, A.F. Synthesis of Carvone Derivatives and In Silico and In Vitro Screening of Anti-Inflammatory Activity in Murine Macrophages. Int. J. Mol. Sci. 2023, 24, 2263. [Google Scholar] [CrossRef] [PubMed]
- Rucker, D.; Dhamoon, A.S. Physiology, Thromboxane A2. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Millar, J.K.; Salmon, M.; Nasser, E.; Malik, S.; Kolli, P.; Lu, G.; Pinteaux, E.; Hawkins, R.B.; Ailawadi, G. Endothelial to Mesenchymal Transition in the Interleukin-1 Pathway during Aortic Aneurysm Formation. J. Thorac. Cardiovasc. Surg. 2024, 167, e146–e158. [Google Scholar] [CrossRef] [PubMed]
- Jauhiainen, S.; Kiema, M.; Hedman, M.; Laakkonen, J.P. Large Vessel Cell Heterogeneity and Plasticity: Focus in Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef]
- Verstraeten, A.; Fedoryshchenko, I.; Loeys, B. The Emerging Role of Endothelial Cells in the Pathogenesis of Thoracic Aortic Aneurysm and Dissection. Eur. Heart J. 2023, 44, 1262–1264. [Google Scholar] [CrossRef]
- Shen, M.; Hu, M.; Fedak, P.W.M.; Oudit, G.Y.; Kassiri, Z. Cell-Specific Functions of ADAM17 Regulate the Progression of Thoracic Aortic Aneurysm. Circ. Res. 2018, 123, 372–388. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Takayanagi, T.; Forrester, S.J.; Preston, K.J.; Obama, T.; Tsuji, T.; Kobayashi, T.; Boyer, M.J.; Cooper, H.A.; Kwok, H.F.; et al. Vascular ADAM17 (a Disintegrin and Metalloproteinase Domain 17) Is Required for Angiotensin II/β-Aminopropionitrile–Induced Abdominal Aortic Aneurysm. Hypertension 2017, 70, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Shirakura, K.; Okada, Y. Vascular Leakage Prevention by Roundabout 4 under Pathological Conditions. Biol. Pharm. Bull. 2021, 44, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Shirakura, K.; Ishiba, R.; Kashio, T.; Funatsu, R.; Tanaka, T.; Fukada, S.; Ishimoto, K.; Hino, N.; Kondoh, M.; Ago, Y.; et al. The Robo4–TRAF7 Complex Suppresses Endothelial Hyperpermeability in Inflammation. J. Cell Sci. 2019, 132, jcs220228. [Google Scholar] [CrossRef] [PubMed]
- Schoonderwoerd, M.J.A.; Goumans, M.-J.T.H.; Hawinkels, L.J.A.C. Endoglin: Beyond the Endothelium. Biomolecules 2020, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Adzraku, S.Y.; Cao, C.; Zhou, Q.; Yuan, K.; Hao, X.; Li, Y.; Yuan, S.; Huang, Y.; Xu, K.; Qiao, J.; et al. Endothelial Robo4 Suppresses Endothelial-to-Mesenchymal Transition Induced by Irradiation and Improves Hematopoietic Reconstitution. Cell Death Dis. 2024, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Poujade, F.-A.; Bergman, O.; Gådin, J.R.; Simon, N.; Lång, K.; Franco-Cereceda, A.; Body, S.C.; Björck, H.M.; Eriksson, P. Endothelial/Epithelial Mesenchymal Transition in Ascending Aortas of Patients With Bicuspid Aortic Valve. Front. Cardiovasc. Med. 2019, 6, 182. [Google Scholar] [CrossRef]
- Gould, R.A.; Aziz, H.; Woods, C.E.; Seman-Senderos, M.A.; Sparks, E.; Preuss, C.; Wünnemann, F.; Bedja, D.; Moats, C.R.; McClymont, S.A.; et al. ROBO4 Variants Predispose Individuals to Bicuspid Aortic Valve and Thoracic Aortic Aneurysm. Nat. Genet. 2019, 51, 42–50. [Google Scholar] [CrossRef]
- Lim, X.R.; Harraz, O.F. Mechanosensing by Vascular Endothelium. Annu. Rev. Physiol. 2024, 86, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Coon, B.G.; Baeyens, N.; Han, J.; Budatha, M.; Ross, T.D.; Fang, J.S.; Yun, S.; Thomas, J.-L.; Schwartz, M.A. Intramembrane Binding of VE-Cadherin to VEGFR2 and VEGFR3 Assembles the Endothelial Mechanosensory Complex. J. Cell Biol. 2015, 208, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, N.; Nicoli, S.; Coon, B.G.; Ross, T.D.; Van den Dries, K.; Han, J.; Lauridsen, H.M.; Mejean, C.O.; Eichmann, A.; Thomas, J.-L.; et al. Vascular Remodeling Is Governed by a VEGFR3-Dependent Fluid Shear Stress Set Point. eLife 2015, 4, e04645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Hutterer, E.; Hultin, S.; Bergman, O.; Kolbeinsdottir, S.; Jin, H.; Forteza, M.J.; Ketelhuth, D.F.J.; Roy, J.; et al. The VE-Cadherin/AmotL2 Mechanosensory Pathway Suppresses Aortic Inflammation and the Formation of Abdominal Aortic Aneurysms. Nat. Cardiovasc. Res. 2023, 2, 629–644. [Google Scholar] [CrossRef]
- Ritsvall, O.; Albinsson, S. Emerging Role of YAP/TAZ in Vascular Mechanotransduction and Disease. Microcirculation 2024, 31, e12838. [Google Scholar] [CrossRef]
- Chuntharpursat-Bon, E.; Povstyan, O.V.; Ludlow, M.J.; Carrier, D.J.; Debant, M.; Shi, J.; Gaunt, H.J.; Bauer, C.C.; Curd, A.; Simon Futers, T.; et al. PIEZO1 and PECAM1 Interact at Cell-Cell Junctions and Partner in Endothelial Force Sensing. Commun. Biol. 2023, 6, 358. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, A.-L.; Vignes, H.; Vermot, J. Mechanically Activated Piezo Channels Modulate Outflow Tract Valve Development through the Yap1 and Klf2-Notch Signaling Axis. eLife 2019, 8, e44706. [Google Scholar] [CrossRef] [PubMed]
- DeRoo, E.; Stranz, A.; Yang, H.; Hsieh, M.; Se, C.; Zhou, T. Endothelial Dysfunction in the Pathogenesis of Abdominal Aortic Aneurysm. Biomolecules 2022, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.C.; Anderson, C.W.; Agala, C.B.; Tasoudis, P.; Collins, E.N.; Ding, Y.; Blackwell, J.W.; Willcox, D.E.; Farivar, B.S.; Kibbe, M.R.; et al. Paradoxical Changes: EMMPRIN Tissue and Plasma Levels in Marfan Syndrome-Related Thoracic Aortic Aneurysms. J. Clin. Med. 2024, 13, 1548. [Google Scholar] [CrossRef] [PubMed]
- Lizarbe, T.R.; Tarín, C.; Gómez, M.; Lavin, B.; Aracil, E.; Orte, L.M.; Zaragoza, C. Nitric Oxide Induces the Progression of Abdominal Aortic Aneurysms through the Matrix Metalloproteinase Inducer EMMPRIN. Am. J. Pathol. 2009, 175, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Guzik, B.; Sagan, A.; Ludew, D.; Mrowiecki, W.; Chwała, M.; Bujak-Gizycka, B.; Filip, G.; Grudzien, G.; Kapelak, B.; Żmudka, K.; et al. Mechanisms of Oxidative Stress in Human Aortic Aneurysms—Association with Clinical Risk Factors for Atherosclerosis and Disease Severity. Int. J. Cardiol. 2013, 168, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Salmon, M. NADPH Oxidases in Aortic Aneurysms. Antioxidants 2022, 11, 1830. [Google Scholar] [CrossRef]
- Peshkova, I.O.; Schaefer, G.; Koltsova, E.K. Atherosclerosis and Aortic Aneurysm—Is Inflammation a Common Denominator? FEBS J. 2016, 283, 1636–1652. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Benaiges, D.; Chillarón, J.J.; Flores-Le Roux, J.A.; Pedro-Botet, J. Diabetes Mellitus as a Protective Factor of Abdominal Aortic Aneurysm: Possible Mechanisms. Clin. Investig. Arter. 2018, 30, 181–187. [Google Scholar] [CrossRef]
- van Loosdregt, J.; Brunen, D.; Fleskens, V.; Pals, C.E.G.M.; Lam, E.W.F.; Coffer, P.J. Rapid Temporal Control of Foxp3 Protein Degradation by Sirtuin-1. PLoS ONE 2011, 6, e19047. [Google Scholar] [CrossRef] [PubMed]
- Budbazar, E.; Rodriguez, F.; Sanchez, J.M.; Seta, F. The Role of Sirtuin-1 in the Vasculature: Focus on Aortic Aneurysm. Front. Physiol. 2020, 11, 1047. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.R.; Gallo, A.; Coady, M.A.; Tellides, G.; Botta, D.M.; Burke, B.; Coe, M.P.; Kopf, G.S.; Elefteriades, J.A. Novel Measurement of Relative Aortic Size Predicts Rupture of Thoracic Aortic Aneurysms. Ann. Thorac. Surg. 2006, 81, 169–177. [Google Scholar] [CrossRef]
- Rosenblum, J.M.; Chen, E.P. Thoracoabdominal Aortic Aneurysm Repair: Open, Endovascular, or Hybrid? Gen. Thorac. Cardiovasc. Surg. 2019, 67, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, R.; Rinaldi, E.; Kahlberg, A.; Tinaglia, S.; Santoro, A.; Colacchio, G.; Melissano, G. Outcomes Following Management of Complex Thoracoabdominal Aneurysm by an Open Approach. J. Clin. Med. 2023, 12, 3193. [Google Scholar] [CrossRef] [PubMed]
- Gloviczki, P. Surgical Repair of Thoracoabdominal Aneurysms: Patient Selection, Techniques and Results. Cardiovasc. Surg. 2002, 10, 434–441. [Google Scholar] [CrossRef]
- Tanaka, A.; Smith, H.N.; Safi, H.J.; Estrera, A.L. Open Treatments for Thoracoabdominal Aortic Aneurysm Repair. Methodist Debakey Cardiovasc. J. 2023, 19, 49–58. [Google Scholar] [CrossRef]
- Oderich, G.S.; Tenorio, E.R.; Mendes, B.C.; Lima, G.B.B.; Marcondes, G.B.; Saqib, N.; Hofer, J.; Wong, J.; Macedo, T.A. Midterm Outcomes of a Prospective, Nonrandomized Study to Evaluate Endovascular Repair of Complex Aortic Aneurysms Using Fenestrated-Branched Endografts. Ann. Surg. 2021, 274, 491–499. [Google Scholar] [CrossRef]
- Rizza, A.; Trimarchi, G.; Di Sibio, S.; Bastiani, L.; Murzi, M.; Palmieri, C.; Foffa, I.; Berti, S. Preliminary Outcomes of Zone 2 Thoracic Endovascular Aortic Repair Using Castor Single-Branched Stent Grafts: A Single-Center Experience. J. Clin. Med. 2023, 12, 7593. [Google Scholar] [CrossRef] [PubMed]
- Derycke, L.; Avril, S.; Vermunt, J.; Perrin, D.; El Batti, S.; Alsac, J.-M.; Albertini, J.-N.; Millon, A. Computational Prediction of Proximal Sealing in Endovascular Abdominal Aortic Aneurysm Repair with Unfavorable Necks. Comput. Methods Programs Biomed. 2024, 244, 107993. [Google Scholar] [CrossRef] [PubMed]
- Houser, A.; Martinez, C.; Tassiopoulos, A. The Challenge of Treating Abdominal Aortic Aneurysms with Hostile Neck Anatomy: An Overview. J. Clin. Med. 2024, 13, 1460. [Google Scholar] [CrossRef] [PubMed]
- Conroy, P.D.; Rastogi, V.; Yadavalli, S.D.; Solomon, Y.; Romijn, A.-S.; Dansey, K.; Verhagen, H.J.M.; Giles, K.A.; Lombardi, J.V.; Schermerhorn, M.L. The Rise of Endovascular Repair for Abdominal, Thoracoabdominal, and Thoracic Aortic Aneurysms. J. Vasc. Surg. 2024, 81, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Wooster, M. Hybrid and Endovascular Management of Aortic Arch Pathology. J. Clin. Med. 2024, 13, 6248. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.M.; Ferreiro-Marzal, A.; Esteban-Molina, M.; Rodríguez-Morata, A.; Rodríguez-Serrano, F. Endovascular Technique for Ascending Aorta Repair Based on TEVAR and TAVI Procedures. J. Endovasc. Ther. 2024, 31, 360–365. [Google Scholar] [CrossRef] [PubMed]
| Receptor | Characteristics/Function | References |
|---|---|---|
| OR51E1 (Olfr558) | Fetal, adult cardiomyocytes; negative inotropic, chronotropic effects | [37] |
| Agonists: MCFA (nonanoic, dodecanoic, tetradecanoic), some endogenously stored and secreted by epicardial fat tissue | ||
| OR51E2 (Olfr78) | VSMCs in resistance vessels of heart, skeletal muscle, skin; modulation of arterial pressure, tissue perfusion | [47,49,50,51,52,63] |
| VSMCs in the JGA afferent arteriole, counterregulation of hypotension mediated via propionate-induced activation of Gpr41, Gpr43 (in murine models used to evaluate function of the Olfr78 receptor) | ||
| Agonists: SCFA (acetate, propionate), androgens, β-ionone | ||
| OR10J5 (Olfr16) | Human aorta, coronary arteries, HUVEC; microtubule disassembly/cortical rim actin assembly, cell–cell junction disruption, endothelial cell migration (AKT signaling), angiogenesis | [35,46] |
| Agonists: Lyral, α-cedrene | ||
| OR6A2 (Olfr2) | CD45+ F4/80+ vascular macrophages (aorta), CD45- CD31+ endothelial cells (aorta), CD45- CD31- VSMCs (aorta) | [53,54,64,65] |
| ROS production in cytosol, mitochondria, NLRP3 inflammasome activation, release of IL-1β, IL-1α, IL-6 and TNF, deficiency mitigates aortic aneurysm formation, remodeling and inflammation (murine models) | ||
| Agonists: Octanal | ||
| OR2L13 (Olfr168) | Platelets, megakaryocytes; stored in α-granules, co-localizes with P-selectin, upregulated by D-flow | [59] |
| Counterregulates platelet aggregation induced by other mechanisms, deficiency leads to increased aneurysm growth, early rupture (murine models) | ||
| Agonists: vanillin, myrrh, frankincense, carvone ((−) enantiomer producing stronger effect), octanoate |
| Gene | Functional Group | TAA (AD)/AAA | References |
|---|---|---|---|
| Fbn1, Lox, Bgn, Eln, Col3a1, Lrp1 | ECM homeostasis | TAA | [5,83,84,85,105] |
| Col3a1, Fbn1, Lrp1, Erg, Mmp | AAA | [5,93,95,105,108] | |
| Acta1, Myh11, Mylk, Flna, Lrp1 | VSMC | TAA | [5,103] |
| Lrp1, Smyd2 | AAA | [5,103,106] | |
| Tgfbr1, Tgfbr2, Tgfb2, Smad3, Smad4 | TGFβ signaling | TAA | [5] |
| Tgfbr1, Tgfbr2 | AAA | [96] | |
| Mat2a | SAM metabolism | TAD (AD), TAA | [5,86,87] |
| Arih1 | Cytoskeleton | TAD (AD), TAA | [5] |
| Sort1, Ldlr | Lipid trafficking/metabolism | TAA, AAA | [5,112,113] |
| Cdkn2b-As1/Anril, Cdkn2b, Dab2ip, Il6r, Smyd2, Erg, Il6, Il1α, Il1β | Inflammation | AAA | [5,96,100,101,107,108] |
| Erg | Endothelial dysfunction | TAA, AAA | [5,108,109] |
| TAA | References | AAA | References |
|---|---|---|---|
| HTN | [114] | HTN | [139] |
| Atherosclerosis | [121] | Atherosclerosis | [121] |
| Age | [120,121] | Age | [119] |
| Smoking | [115] | Smoking | [135] |
| Congenital disease (CoA, BAV) | [117,118] | Congenital disease (cAAA) | [143] |
| Approx. equal sex distribution, unless other risk factors present | [119] | Male sex | [119] |
| Cocaine | [116] | Fluoroquinolones | [141,142] |
| Infectious aortitis | [122] | Infectious aortitis | [122,133,134] |
| Non-infectious aortitis (GCA, TA, IgG4-related aortitis, SLE, sarcoidosis, GPA, RA, AS) | [81,123,124,125,127,128,129,131] | Non-infectious aortitis (TA, GCA, AS, IgG4-related aortitis) | [124,128,130,131] |
| IAAA (chronic idiopathic periaortitis, idiopathic retroperitoneal fibrosis) | [132] |
| Component | Description | References |
|---|---|---|
| MMP | Various enzymes including ADAM-TS (TAA), ADAM (TAA, AAA), Cathepsin (AAA), upregulation due to inflammatory cytokines, macrophages, neutrophils | [70] |
| TIMP | Generally reduced expression or complete deficiency of TIMP enzymes, TIMP3 overexpression | [70,145,146] |
| Elastin | Elastin fragmentation, structural derangements due to genetic etiologies (Fbn1); TGFβ signaling disruption | [147,148] |
| Collagen | Disruption in normal collagen concentrations, structural derangements in triple helix structure and cross-linking (LOX) | [149,151] |
| Proteoglycans | Increased versican and aggrecan production (TAA), decreased versican, perlecan and aggrecan production (AAA), accumulation of fibronectin, thrombospondin, dysregulation in VSMC/ECM interactions | [152,153,154] |
| Process | Description | References |
|---|---|---|
| Phenotype Switch | Klf4 upregulation, Myocd, SRF downregulation, suppression of contractile phenotype genes, upregulation of phagocytic-like markers (PDGF-BB, oxidized phospholipids) | [179,180] |
| VSMC types include contractile, inflammatory, stressed, proliferative (synthetic/contractile phenotype), fibromyocytic group, degradative (proteolytic/phagocytic gene phenotype), MSC-like, osteoblastic | [182,183,184,185,187] | |
| ALDH2 deficiency prevents phenotype switch, maintains contractile characteristics | [190] | |
| Secretory characteristics | Increased secretion of MMP (MMP9, MMP2), ECM components (i.e., collagen), inflammatory cytokines (TNF, IL-1β, IL-6) | [191,192,193,194,195] |
| Oxidative stress | ROS production due to upregulation of ROS-producing enzymes (NOX4, iNOS) | [196,198] |
| Senescence | DNA damage, telomere shortening, epigenetic changes, dysregulated proteostasis, increased production of SA-β-gal, p21, p16, decreased expression of antioxidant enzymes such as sirtuins, impaired autophagy (SASP) | [193,199,201,202] |
| Component | Description | References |
|---|---|---|
| Macrophage | M1 macrophages (adventitia) propagate vascular wall inflammation, M2 macrophages (anti-inflammatory) contribute angiogenesis, ECM deposition | [205] |
| CD45+ F4/80+ vascular macrophages propagate wall inflammation via activation of surface ORs by atherosclerotic and pro-inflammatory ligands | [54] | |
| Neutrophil | Phagocytosis, degranulation, formation of NETs | [206] |
| NETs contribute to aortic wall damage via protease secretion and as part of the ILT | [206,222] | |
| NKT | Stimulation of cytotoxic pathways affecting VSMC survival and aggravation of atherosclerotic changes, iNKTs antagonize inflammation via reduction of inflammatory cell infiltrate and stimulation of the M2 macrophage phenotype. | [207,209] |
| T cell | CD4+ Th1 (TNF, IL-2), Th17 (IL-17) stimulate macrophage activity, modulate ECM collagen concentrations, Th2 contribute to VSMC apoptosis (Fas–FasL interactions), FOXP3+ Treg (IL-10, IL-35) modulate/reduce inflammation | [182,205,210] |
| FOXP3 deacetylation causes its proteolytic degradation and reduced Treg functionality, FOXP3 deacetylation carried out by SIRT1 | [212] | |
| Inflammasome | NLRP3, AIM2 inflammasomes associated with AA, formation induced by cellular debris, OR activation on surface of vascular macrophages, stimulate MMP9 activity | [56,205,215,216] |
| HIF-1α | Secreted in response to inflammation, implicated in signaling pathways within macrophages, adventitial fibroblasts, VSMCs; HIF-1α induces activation of downstream angiogenic effectors (VEGF, SDF1, Ang1, Ang2, PDGFB), angiogenesis also augmented by inflammatory cytokines (mMCP-4, IFNI), eventually inducing formation of microvessels in the aortic wall | [217,218,219,220,221] |
| Component | Description | References |
|---|---|---|
| Platelets | Activation due to flow conditions, adhesion/attachment (activation not always required for adhesion/aggregation), aggregation via αΙIbβ3 integrin–fibrinogen interactions | [226,229,230,231] |
| Secretion of factors facilitating recruitment of inflammatory cells (MCP-1, β2-microglobulin), VSMC apoptosis (PDGF), ECM degradation (MMP9) and aortic wall hypoxia, some platelet factors protective (PF4 stabilizing the endothelium in experimental TAA models) | [180,223,240,244] | |
| Neutrophils | Adhesion due to platelet aggregation and platelet (P-Selectin)–neutrophil (PSGL-1) interactions, neutrophil activation and generation of NETs, death upon fibrin contact, release of MMP, inflammatory cytokines, neutrophil elastase (fibronectin degradation) and enzymes inducing endothelial detachment from subendothelial layers with temporal ILT propagation | [235,236] |
| Macrophages | Canaliculi macrophages secrete inflammatory cytokines, MMPs, and luminal anti-inflammatory macrophages that may mitigate ILT-associated inflammation | [223] |
| RBCs | RBC–platelet, RBC–fibrinogen binding, RBC lysis leads to heme and Fe2+ release, aggravation of oxidative stress, thrombus propagation | [232,246] |
| Soluble factors | vWF (produced by platelets, endothelial cells), tPA, PAI, IFNγ, IL-1α, platelet products (PF4, PDGF), TGFβ, neutrophil products (uPA, proteinase 3, cathepsins, MPO, neutrophil elastase) | [245] |
| Fibrin | Fibrinogen converted to fibrin by thrombin (coagulation cascade), fibrin monomers assemble and cross-link with other coagulation factors | [232] |
| HIF-1α | Secreted in response to hypoxia brought on by ILT mass, implicated in signaling pathways within macrophages, adventitial fibroblasts, VSMCs; activation of downstream angiogenic effectors (VEGF, SDF1, Ang1, Ang2, PDGFB) and inducing formation of microvessels in the aortic wall | [220,249,250,251] |
| Event | Description | References |
|---|---|---|
| Phenotype switch | EndMT (TGFβ, oxidative stress, IL-1β) with acquisition of mesenchymal characteristics (contractile phenotype, VSMC markers, collagen, loss of endothelial cell–cell junctions), higher propensity for migration, disruption of endothelial barrier, entry of circulating immune cells/factors (vascular wall inflammation) | [261,262] |
| Endothelial phenotype switch normally suppressed by ROBO4 | [269] | |
| Endothelial barrier dysfunction | ADAM17-mediated proteolytic shedding of transmembrane proteins (VE-cadherin, junctional adhesion molecule A, claudin-5), ADAM17 secreted by endothelial cells (VE-cadherin cleavage, junction disruption) and VSMCs (phenotypic switch, apoptosis) | [264,265,266] |
| ROBO4 dysregulation (gene variants, mutations) with disruption in VEGF/VEGFR2 signaling downregulation, disruption in ROBO4–TRAF7 complex formation (VE-cadherin redistribution via TNF–TNFR interactions), disinhibition of endothelial phenotype switch | [264,267,268,270,271] | |
| Mechanosensation | VE-cadherin-PECAM1–VEGFR2/VEGFR3 stimulation by shear stress and vascular remodeling | [275] |
| AmotL2 absence causes tunica intima inflammation, aortic aneurysm (endothelial barrier dysregulation) due to absence of actin filament and VE-cadherin modulation (dysregulation of endothelial shape and alignment with flow) | [277,278] | |
| Piezo1 (ion channel) associates with VE-cadherin/PECAM1, modulates Ca2+ homeostasis, cytoskeleton configuration in response to flow; under disturbed flow, the activation of VE-cadherin-PECAM1–VEGFR2/VEGFR3 leads to NF-κB upregulation and inflammation | [279,280] | |
| Oxidative stress | eNOS uncoupling from BH4, leads to superoxide production (BH4 dependent on DHFR), NO also associated with AA progression via EMMPRIN-mediated MMP13 activation (higher in MFS aneurysmal wall) | [281,282,283,284] |
| Increase in oxidative stress markers (systemic MDA, superoxide locally) | [285] |
| Receptor | Description | References |
|---|---|---|
| OR6A2 (Olfr2) | Increased Olfr2 mRNA expression in ApoE−/− murine aortas (relative expression of ~4) (peaking after 2 weeks of WD) compared to WT (relative expression of ~0.01), Olfr2 mRNA expression is generally observed in ApoE−/− aortic vascular CD45+ F4/80 macrophages, CD45- CD31+ ECs, CD45- CD31- VSMCs with most Olfr2 cells identified as macrophages (~ 28%), Olfr2−/− mice exhibit smaller atherosclerotic regions by ~50%, compared to WT | [53] |
| OR6A2 (Olfr2 ortholog) expression correlates with macrophage content (high macrophage content associated with ~0.1 increase in mean OR6A2 expression), atherosclerotic plaque samples generally associated with higher macrophage and OR6A2 expression levels | [53] | |
| Octanal can be detected in plasma of WT mice (baseline at ~2 μM, doubles after WD) and Apoe−/− mice (baseline at ~7 μM, increases to ~9 μM after WD), absence of octanal in the diet indicates that it is not derived directly from diet but can be produced by lipid peroxidation of oleic acid in the atherosclerotic aorta (culturing of atherosclerotic aortic tissue with oleic acid increases octanal content to ~ 35%), endogenous human octanal concentrations exhibit positive correlation with total CH, HDL, LDL, TG levels | [53] | |
| In ApoE−/− mice, octanal treatment (4 weeks) doubles aortic atherosclerotic plaque size, induces an inflammatory response evident by a systemic increase in mean TNFα (by ~10 pg/mL), IL-1β (by ~5 pg/mL) concentrations in plasma but has no effect on total CH, HDL, LDL, TG levels; use of the odorant olfactory receptor antagonist citral induces a ~40% reduction in atherosclerotic plaque size but has no effects on systemic levels of lipids, leukocytes | [53] | |
| Separate treatment with LPS and octanal increases relative Olfr2 aortic expression by ~3 (LPS) and ~1 (octanal), CD45+ F4/80 Olfr2 vascular macrophage MFI by ~500 (LPS), Olfr2 BMDM MFI by ~4000 (LPS) and relative Olfr2 expression in BMDMs by ~0.5 (LPS) (octanal) compared to untreated samples; conversely, combined treatment with both LPS and octanal further increases the responses observed, with increases in relative Olfr2 aortic expression by ~6, CD45+ F4/80 Olfr2 vascular macrophage MFI by ~900, Olfr2 BMDM MFI by ~5000 and relative BMDM Olfr2 expression by ~1.5 | [53] | |
| OR6A2 receptor expression increased in human AAA tissue (human macrophage surface) compared to controls | [64,65,173] | |
| Murine AAA models exhibit a peak in upregulation of the OR6A2 ortholog, Olfr2, on day 7, which returns to baseline on day 28 (upregulated on the surface of pro-inflammatory monocytes, pro-inflammatory macrophages and macrophages with mixed resident/migratory behavior); no Olfr2 receptors detected on the surface of resident macrophages | [64,65,173] | |
| Olfr2 KO mice exhibit reduced aortic macrophage populations, increased VSMC populations and collagen content as well as reduced pro-inflammatory cytokine levels (TNFa, IFNγ) (day 28) compared to WT; transcriptome analysis also reveals upregulation of signaling pathways related to VSMC contractile function and downregulation of signaling pathways related to leukocyte activation | [64,65,173] | |
| OR2L13 (Olfr168) | Platelets in AAA patients are more reactive compared to those in healthy controls (increased reactivity via PAR1, TP with no significant difference in receptor density), upregulate OR2L13 (~0.7 increase in mean expression over controls) and increase its localization to the surface, especially under D-flow conditions (~20 increase in platelet surface OR2L13 MFI over controls, which increases to ~300 if platelets are subjected to D-flow) | [59] |
| Various ligands have been screened for their ability to activate OR2L13 receptors (vanillin, myrrh, octanoate, frankincense and both carvone enantiomers),with (−) carvone producing the highest luciferase activity ratio (highest downstream cAMP activity) at ~3.0 compared to (+) carvone and all other ligands; activation of the OR2L13 receptors by these ligands increases intracellular cAMP, leads to Cl− efflux via activation of Anoctamin channels and inhibits platelet activation and aggregation via upregulation of PKA | [59] | |
| (−) Carvone administration mitigates AAA growth and MMP2 activity, with both daily doses of 100 mg/kg (−) carvone and 30 mg/L aspirin producing a decrease in aortic diameter by ~ 1.6 mm (4 weeks), while the reduction in aortic MMP2 activity associated with (−) carvone administration is ~2000 compared to the ~3000 decrease observed with aspirin administration; conversely, Olfr168 deletion augments AAA growth, evident by an increase in aortic diameter of ~0.88 mm compared to an increase of ~0.56 mm in WT mice over 4 weeks, increases mean MMP2 activity by ~5000 AU and reduces mouse survival by ~70% (4 weeks) | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stougiannou, T.M.; Christodoulou, K.C.; Karangelis, D. Olfactory Receptors and Aortic Aneurysm: Review of Disease Pathways. J. Clin. Med. 2024, 13, 7778. https://doi.org/10.3390/jcm13247778
Stougiannou TM, Christodoulou KC, Karangelis D. Olfactory Receptors and Aortic Aneurysm: Review of Disease Pathways. Journal of Clinical Medicine. 2024; 13(24):7778. https://doi.org/10.3390/jcm13247778
Chicago/Turabian StyleStougiannou, Theodora M., Konstantinos C. Christodoulou, and Dimos Karangelis. 2024. "Olfactory Receptors and Aortic Aneurysm: Review of Disease Pathways" Journal of Clinical Medicine 13, no. 24: 7778. https://doi.org/10.3390/jcm13247778
APA StyleStougiannou, T. M., Christodoulou, K. C., & Karangelis, D. (2024). Olfactory Receptors and Aortic Aneurysm: Review of Disease Pathways. Journal of Clinical Medicine, 13(24), 7778. https://doi.org/10.3390/jcm13247778








