Is Whole-Body Cryostimulation Useful in Modulating Spasticity in Adults with Cerebral Palsy? A Case Study
Abstract
1. Introduction
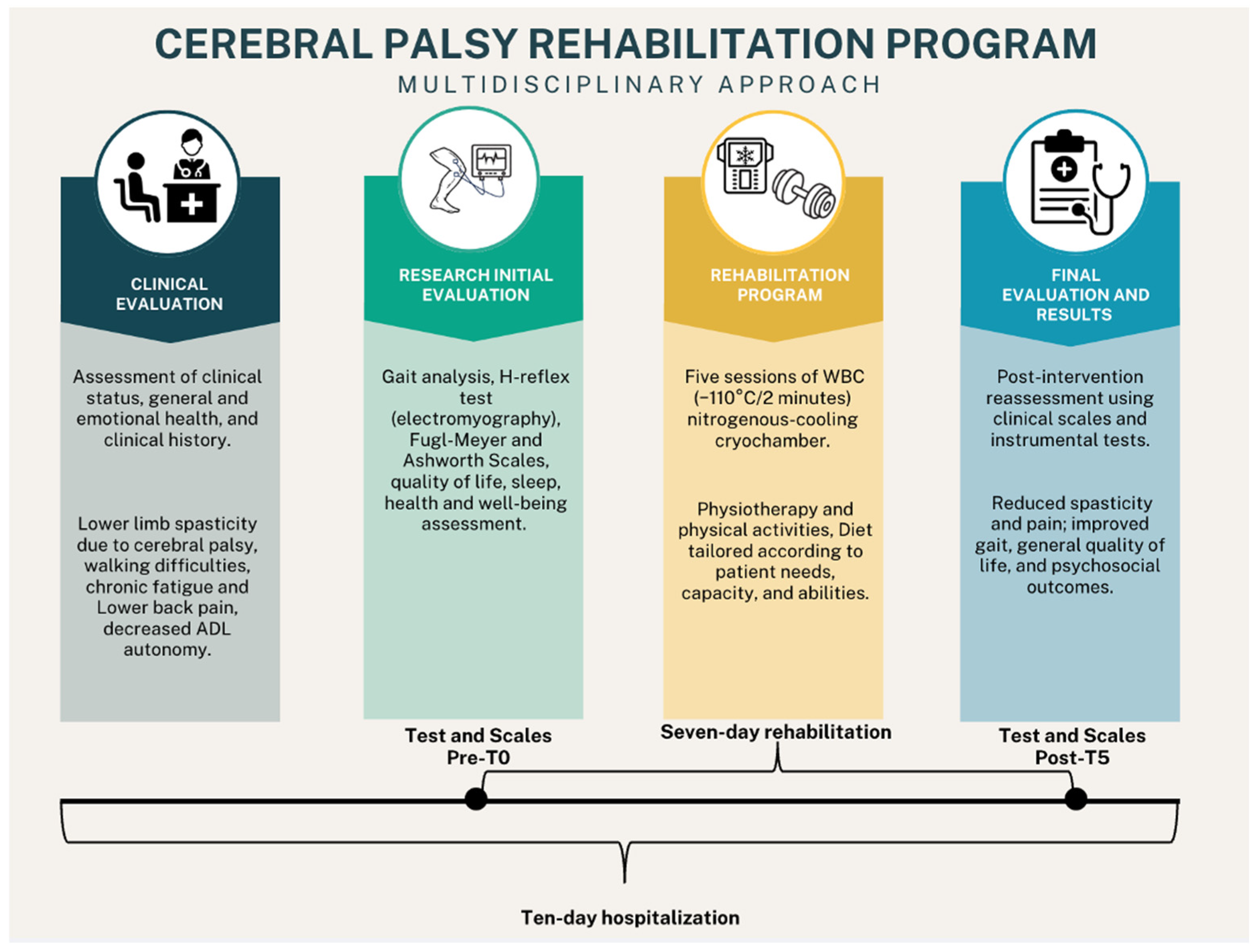
2. Materials and Methods
2.1. Case Description and Clinical History
2.2. Symptoms Assessment
2.3. Intervention
2.3.1. Physiotherapy and Adapted Physical Activity
2.3.2. Whole-Body Cryostimulation (WBC)
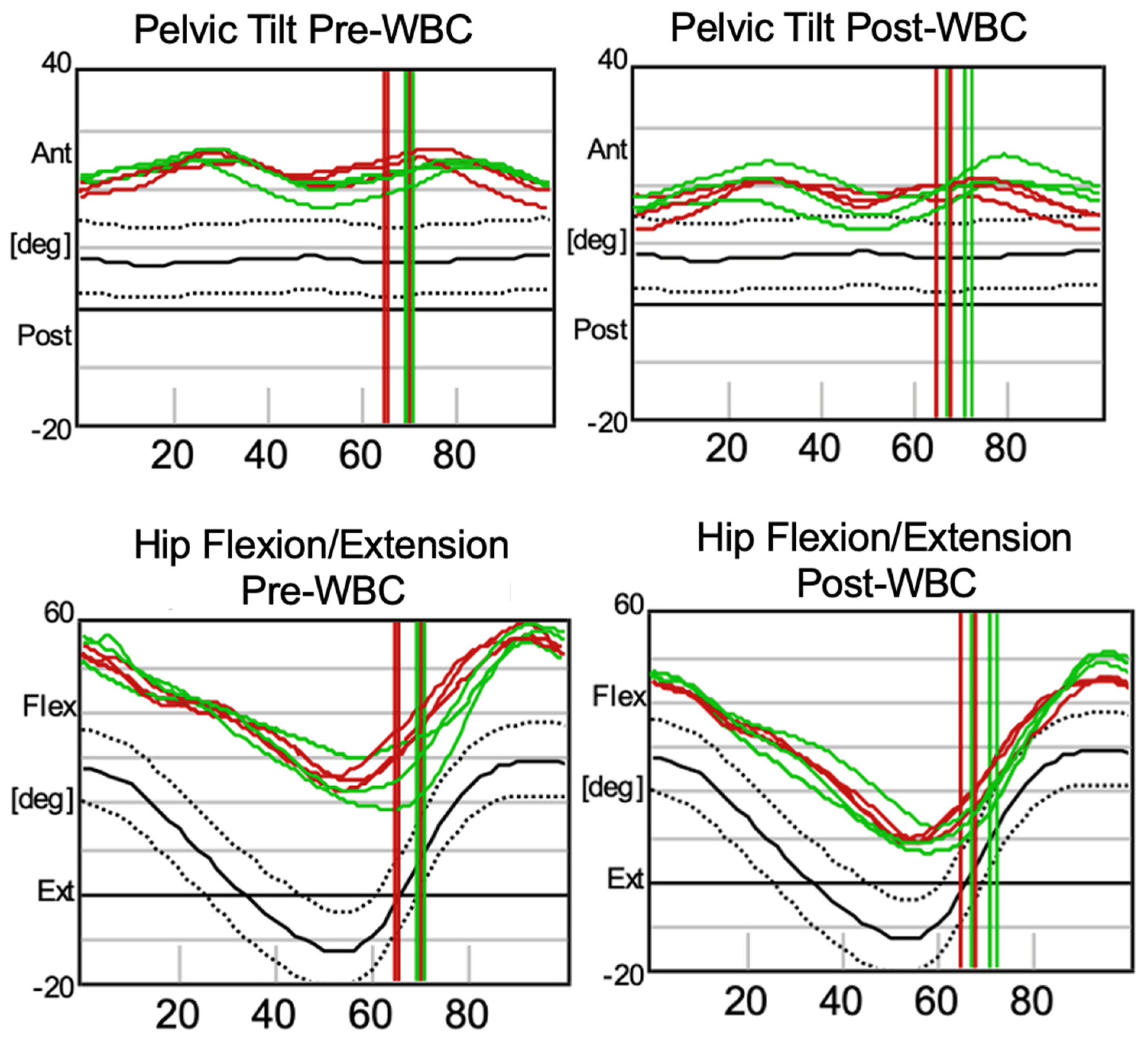
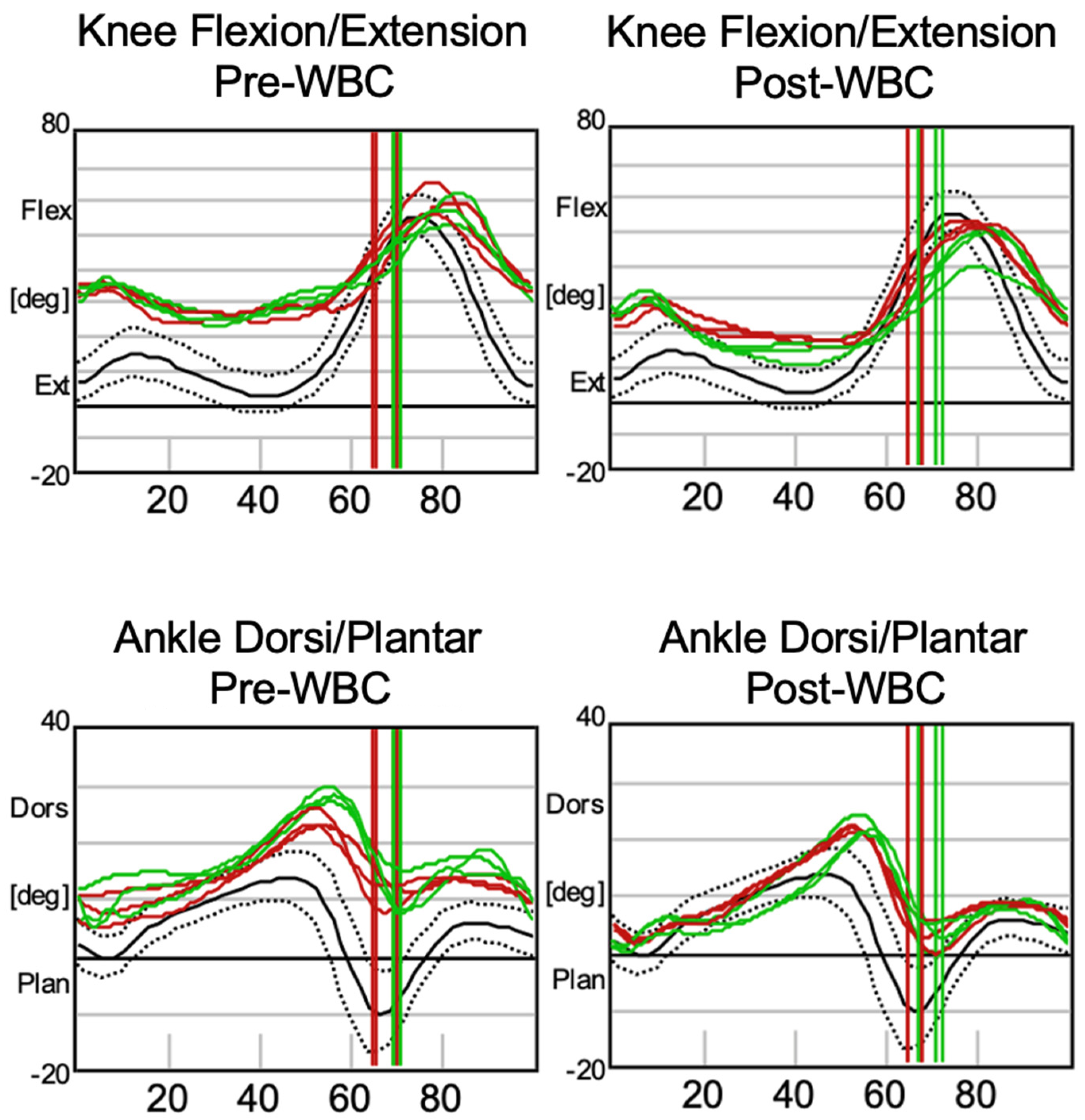
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC Cerebral Palsy (CP). Available online: https://www.cdc.gov/cerebral-palsy/index.html (accessed on 29 October 2024).
- Vitrikas, K.; Dalton, H.; Breish, D. Cerebral Palsy: An Overview. Am. Fam. Phys. 2020, 101, 213–220. [Google Scholar]
- Stoknes, M.; Andersen, G.L.; Elkamil, A.I.; Irgens, L.M.; Skranes, J.; Salvesen, K.Å.; Vik, T. The Effects of Multiple Pre- and Perinatal Risk Factors on the Occurrence of Cerebral Palsy. A Norwegian Register Based Study. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2012, 16, 56–63. [Google Scholar] [CrossRef]
- Jacobsson, B.; Hagberg, G. Antenatal Risk Factors for Cerebral Palsy. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 425–436. [Google Scholar] [CrossRef]
- Blair, E.M.; Nelson, K.B. Fetal Growth Restriction and Risk of Cerebral Palsy in Singletons Born after at Least 35 Weeks’ Gestation. Am. J. Obstet. Gynecol. 2015, 212, 520. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.M. Diagnosis, Treatment, and Prevention of Cerebral Palsy. Clin. Obstet. Gynecol. 2008, 51, 816–828. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Hines, M.; Goldsmith, S.; Barclay, R. Clinical Prognostic Messages from a Systematic Review on Cerebral Palsy. Pediatrics 2012, 130, e1285–e1312. [Google Scholar] [CrossRef] [PubMed]
- Stadskleiv, K. Cognitive Functioning in Children with Cerebral Palsy. Dev. Med. Child Neurol. 2020, 62, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Jenks, K.M.; van Lieshout, E.C.D.M.; de Moor, J.M.H. Cognitive Correlates of Mathematical Achievement in Children with Cerebral Palsy and Typically Developing Children. Br. J. Educ. Psychol. 2012, 82, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Fluss, J.; Lidzba, K. Cognitive and Academic Profiles in Children with Cerebral Palsy: A Narrative Review. Ann. Phys. Rehabil. Med. 2020, 63, 447–456. [Google Scholar] [CrossRef]
- Nordmark, E.; Hägglund, G.; Lagergren, J. Cerebral Palsy in Southern Sweden I. Prevalence and Clinical Features. Acta Paediatr. Oslo Nor. 2001, 90, 1271–1276. [Google Scholar] [CrossRef]
- Ostensjø, S.; Carlberg, E.B.; Vøllestad, N.K. Motor Impairments in Young Children with Cerebral Palsy: Relationship to Gross Motor Function and Everyday Activities. Dev. Med. Child Neurol. 2004, 46, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Trisnowiyanto, B.; Andriani, I. Cerebral Palsy Types Based on Kind of Disability Correlated with The Functional Independence. J. Kebidanan 2020, 10, 74–79. [Google Scholar] [CrossRef]
- Javed, M.; Ali, M.H. Epidemiological Burden of Lower Limb Spasticity in Adults: A Systematic Review. J. Med. Res. Innov. 2020, 4, e000195. [Google Scholar] [CrossRef]
- Soyuer, F.; Oztürk, A. The Effect of Spasticity, Sense and Walking Aids in Falls of People after Chronic Stroke. Disabil. Rehabil. 2007, 29, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Lomax, M.R.; Shrader, M.W. Orthopedic Conditions in Adults with Cerebral Palsy. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.K.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.-P.; Damiano, D.L.; Becher, J.G.; Gaebler-Spira, D.; Colver, A.; Reddihough, D.S.; et al. Cerebral Palsy. Nat. Rev. Dis. Primer 2016, 2, 15082. [Google Scholar] [CrossRef]
- Reebye, R.; Jacinto, L.J.; Balbert, A.; Biering-Sørensen, B.; Carda, S.; Draulans, N.; Molteni, F.; O’Dell, M.W.; Picelli, A.; Santamato, A.; et al. Multimodal Therapy and Use of Adjunctive Therapies to BoNT-A in Spasticity Management: Defining Terminology to Help Enhance Spasticity Treatment. Front. Neurol. 2024, 15, 1432330. [Google Scholar] [CrossRef] [PubMed]
- Niemi, S. Muscle Relaxants and Antispasticity Drugs. In Pain: A Review Guide; Abd-Elsayed, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 279–283. [Google Scholar]
- Pavone, V.; Testa, G.; Restivo, D.A.; Cannavò, L.; Condorelli, G.; Portinaro, N.M.; Sessa, G. Botulinum Toxin Treatment for Limb Spasticity in Childhood Cerebral Palsy. Front. Pharmacol. 2016, 7, 29. [Google Scholar] [CrossRef]
- Peck, J.; Urits, I.; Kassem, H.; Lee, C.; Robinson, W.; Cornett, E.M.; Berger, A.A.; Herman, J.; Jung, J.W.; Kaye, A.D.; et al. Interventional Approaches to Pain and Spasticity Related to Cerebral Palsy. Psychopharmacol. Bull. 2020, 50, 108–120. [Google Scholar]
- Williams, G.; Singer, B.J.; Ashford, S.; Hoare, B.; Hastings-Ison, T.; Fheodoroff, K.; Berwick, S.; Sutherland, E.; Hill, B. A Synthesis and Appraisal of Clinical Practice Guidelines, Consensus Statements and Cochrane Systematic Reviews for the Management of Focal Spasticity in Adults and Children. Disabil. Rehabil. 2022, 44, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, S.; Yılmaz, E. The Effect of Soft Tissue Injury Cold Application Duration on Symptoms, Edema, Joint Mobility, and Patient Satisfaction: A Randomized Controlled Trial. J. Emerg. Nurs. 2020, 46, 449–459. [Google Scholar] [CrossRef]
- Brosseau, L.; Yonge, K.A.; Robinson, V.; Marchand, S.; Judd, M.; Wells, G.; Tugwell, P. Thermotherapy for Treatment of Osteoarthritis. Cochrane Database Syst. Rev. 2003, 2003, CD004522. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Maksoud, G.M.; Sharaf, M.A.; Rezk-Allah, S.S. Efficacy of Cold Therapy on Spasticity and Hand Function in Children with Cerebral Palsy. J. Adv. Res. 2011, 2, 319–325. [Google Scholar] [CrossRef]
- Varallo, G.; Piterà, P.; Fontana, J.M.; Gobbi, M.; Arreghini, M.; Giusti, E.M.; Franceschini, C.; Plazzi, G.; Castelnuovo, G.; Capodaglio, P. Is Whole-Body Cryostimulation an Effective Add-On Treatment in Individuals with Fibromyalgia and Obesity? A Randomized Controlled Clinical Trial. J. Clin. Med. 2022, 11, 4324. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.M.; Bozgeyik, S.; Gobbi, M.; Piterà, P.; Giusti, E.M.; Dugué, B.; Lombardi, G.; Capodaglio, P. Whole-Body Cryostimulation in Obesity. A Scoping Review. J. Therm. Biol. 2022, 106, 103250. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, P.; Cremascoli, R.; Piterà, P.; Fontana, J.M. Whole-Body Cryostimulation: A Rehabilitation Booster. J. Rehabil. Med. Clin. Commun. 2022, 5, 2810. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Kostka, J.; Włodarczyk, T.; Dugué, B. Whole-Body Cryostimulation (Cryotherapy) Provides Benefits for Fatigue and Functional Status in Multiple Sclerosis Patients. A Case-Control Study. Acta Neurol. Scand. 2016, 134, 420–426. [Google Scholar] [CrossRef]
- Miller, E. Cryostimulation factor supporting rehabilitation patients with multiple sclerosis and fatigue syndrome. Wiadomosci Lek. Wars. Pol. 2010, 63, 41–45. [Google Scholar]
- Radecka, A.; Knyszyńska, A.; Łuczak, J.; Lubkowska, A. Adaptive Changes in Muscle Activity after Cryotherapy Treatment: Potential Mechanism for Improvement the Functional State in Patients with Multiple Sclerosis. NeuroRehabilitation 2021, 48, 119–131. [Google Scholar] [CrossRef]
- Ptaszek, B.; Teległów, A.; Adamiak, J.; Głodzik, J.; Podsiadło, S.; Mucha, D.; Marchewka, J.; Halski, T.; Mucha, D. Effect of Whole-Body Cryotherapy on Morphological, Rheological and Biochemical Indices of Blood in People with Multiple Sclerosis. J. Clin. Med. 2021, 10, 2833. [Google Scholar] [CrossRef]
- Pawik, M.; Kowalska, J.; Rymaszewska, J. The Effectiveness of Whole-Body Cryotherapy and Physical Exercises on the Psychological Well-Being of Patients with Multiple Sclerosis: A Comparative Analysis. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2019, 28, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Alito, A.; Fontana, J.M.; Franzini Tibaldeo, E.; Verme, F.; Piterà, P.; Miller, E.; Cremascoli, R.; Brioschi, A.; Capodaglio, P. Whole-Body Cryostimulation in Multiple Sclerosis: A Scoping Review. J. Clin. Med. 2024, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Krukowska, J.; Dalewski, M.; Czernicki, J. Evaluation of effectiveness of local cryotherapy in patients with post-stroke spasticity. Wiad. Lek. Wars. Pol. 2014, 67, 71–75. [Google Scholar]
- Alcantara, C.C.; Blanco, J.; De Oliveira, L.M.; Ribeiro, P.F.S.; Herrera, E.; Nakagawa, T.H.; Reisman, D.S.; Michaelsen, S.M.; Garcia, L.C.; Russo, T.L. Cryotherapy Reduces Muscle Hypertonia, but Does Not Affect Lower Limb Strength or Gait Kinematics Post-Stroke: A Randomized Controlled Crossover Study. Top. Stroke Rehabil. 2019, 26, 267–280. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.T.B.R.; de Oliveira, L.M. Use of Cryotherapy to Enhance Mouth Opening in Patients with Cerebral Palsy. Spec. Care Dent. Off. Publ. Am. Assoc. Hosp. Dent. Acad. Dent. Handicap. Am. Soc. Geriatr. Dent. 2004, 24, 232–234. [Google Scholar] [CrossRef]
- Legrand, F.D.; Dugué, B.; Costello, J.; Bleakley, C.; Miller, E.; Broatch, J.R.; Polidori, G.; Lubkowska, A.; Louis, J.; Lombardi, G.; et al. Evaluating Safety Risks of Whole-Body Cryotherapy/Cryostimulation (WBC): A Scoping Review from an International Consortium. Eur. J. Med. Res. 2023, 28, 387. [Google Scholar] [CrossRef]
- Joch, W.; Jonas, L.; Nöcker, K.; Papenfuss, W.; Samborski, W.; Savalli, L.; Schwenke, G.; Smuk, L.; Sobieska, M.; Teuber, J.; et al. Consensus Declaration on Whole-Body Cryotherapy (WBCT). In Proceedings of the Second Austrian Symposium on WBC, Bad Vöslau Health Resort, Bad Vöslau, Austria, 17–18 February 2006; Available online: https://www.polarior.com/images/manager/Hauptordner/Concensus%20Declaration%20on%20WBCT.pdf (accessed on 29 October 2024).
- Point, M.; Guilhem, G.; Hug, F.; Nordez, A.; Frey, A.; Lacourpaille, L. Cryotherapy Induces an Increase in Muscle Stiffness. Scand. J. Med. Sci. Sports 2018, 28, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Minton, J. 1992 Student Writing Contest-1st Runner-up: A Comparison of Thermotherapy and Cryotherapy in Enhancing Supine, Extended-Leg, Hip Flexion. J. Athl. Train. 1993, 28, 172–176. [Google Scholar]
- Price, R.; Lehmann, J.F. Influence of Muscle Cooling on the Viscoelastic Response of the Human Ankle to Sinusoidal Displacements. Arch. Phys. Med. Rehabil. 1990, 71, 745–748. [Google Scholar]
- Muraoka, T.; Omuro, K.; Wakahara, T.; Muramatsu, T.; Kanehisa, H.; Fukunaga, T.; Kanosue, K. Effects of Muscle Cooling on the Stiffness of the Human Gastrocnemius Muscle in Vivo. Cells Tissues Organs 2008, 187, 152–160. [Google Scholar] [CrossRef]
- Eldred, E.; Lindsley, D.F.; Buchwald, J.S. The Effect of Cooling on Mammalian Muscle Spindles. Exp. Neurol. 1960, 2, 144–157. [Google Scholar] [CrossRef]
- Ottoson, D. The Effects of Temperature on the Isolated Muscle Spindle. J. Physiol. 1965, 180, 636–648. [Google Scholar] [CrossRef]
- Knutsson, E.; Mattsson, E. Effects of Local Cooling on Monosynaptic Reflexes in Man. Scand. J. Rehabil. Med. 1969, 1, 126–132. [Google Scholar] [CrossRef]
- Michlovitz, S.L.; Smith, W.; Watkins, M. Ice and High Voltage Pulsed Stimulation in Treatment of Acute Lateral Ankle Sprains. J. Orthop. Sports Phys. Ther. 1988, 9, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Lippold, O.C.; Nicholls, J.G.; Redfearn, J.W. A Study of the Afferent Discharge Produced by Cooling a Mammalian Muscle Spindle. J. Physiol. 1960, 153, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Miglietta, O. Action of Cold on Spasticity. Am. J. Phys. Med. 1973, 52, 198–205. [Google Scholar] [PubMed]
- Urbscheit, N.; Johnston, R.; Bishop, B. Effects of Cooling on the Ankle Jerk and H-Response in Hemiplegic Patients. Phys. Ther. 1971, 51, 983–990. [Google Scholar] [CrossRef]
- Rymaszewska, J.; Ramsey, D.; Chładzińska-Kiejna, S. Whole-Body Cryotherapy as Adjunct Treatment of Depressive and Anxiety Disorders. Arch. Immunol. Ther. Exp. 2008, 56, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Vitenet, M.; Tubez, F.; Marreiro, A.; Polidori, G.; Taiar, R.; Legrand, F.; Boyer, F.C. Effect of Whole Body Cryotherapy Interventions on Health-Related Quality of Life in Fibromyalgia Patients: A Randomized Controlled Trial. Complement. Ther. Med. 2018, 36, 6–8. [Google Scholar] [CrossRef]
- Piterà, P.; Cremascoli, R.; Alito, A.; Bianchi, L.; Galli, F.; Verme, F.; Fontana, J.M.; Bigoni, M.; Priano, L.; Mauro, A.; et al. Whole-Body Cryostimulation as an Adjunctive Treatment for Neurophysiologic Tinnitus and Associated Disorders: Preliminary Evidence from a Case Study. J. Clin. Med. 2024, 13, 993. [Google Scholar] [CrossRef]
- Fontana, J.M.; Alito, A.; Piterà, P.; Verme, F.; Cattaldo, S.; Cornacchia, M.; Mai, S.; Brunani, A.; Capodaglio, P. Whole-Body Cryostimulation in Post-COVID Rehabilitation for Patients with Obesity: A Multidisciplinary Feasibility Study. Biomedicines 2023, 11, 3092. [Google Scholar] [CrossRef] [PubMed]
| Outcome and Range | Pre | Post | Δ% |
|---|---|---|---|
| SF-36 | |||
| Physical functioning | 30% | 65% | +35% |
| Role limitations due to physical health | 100% | 100% | 0 |
| Role limitations due to emotional problems | 100% | 100% | 0 |
| Energy/fatigue | 45% | 55% | +10% |
| Emotional well-being | 64% | 80% | +16% |
| Social functioning | 25% | 50% | +25% |
| Pain | 45% | 87.5% | +42.5% |
| General health | 55% | 60% | +5% |
| WHO-5 (0–100) | 68 | 92 | +24% |
| PSQI (0–21) | 7 | 3 | −19.04% |
| ESS (0–24) | 6 | 2 | −16.7% |
| BDI (0–63) | 7 | 2 | −7.9% |
| STAI 1 (20–80) | 33 | 33 | 0 |
| STAI 2 (20–80) | 38 | 35 | −3.8% |
| Outcome and Range | Pre | Post | Δ% |
|---|---|---|---|
| NRS pain (0–10) | 8 | 4 | −40% |
| FUGL-MEYER | |||
| Motor Function (0–34) | 17 | 20 | +8.8 |
| Sensation (0–12) | 8 | 10 | +16.6% |
| Passive Joint Movement (0–20) | 16 | 20 | +20% |
| Joint pain (0–20) | 0 | 4 | +20% |
| ASHWORTH SCALE (0–4) | 2 | 0 | −50% |
| NERVE | Test | Resp. No M Max | Resp. No H Max | Lat. H (ms) | H Amp. (mV) | H/M Amp. (%) | M Amp. M Max (mV) | M Amp. H Max (mV) | Lat. M M Max (ms) | Lat. MH Max (ms) |
|---|---|---|---|---|---|---|---|---|---|---|
| R SPI-SOLEUS | T0 | 8 | 8 | 25.5 | 1.6 | 95.3 | 1.7 | 1.7 | 9.45 | 9.45 |
| R SPI-SOLEUS | T5 | 10 | 10 | 24.25 | 3.7 | 56.2 | 6.6 | 6.6 | 2.95 | 2.95 |
| L SPI-SOLEUS | T0 | 7 | 7 | 24.25 | 0.8 | 18.7 | 4.3 | 4.3 | 7.4 | 7.4 |
| L SPI-SOLEUS | T5 | 1 | 1 | 26.7 | 0.9 | 30.3 | 3.1 | 3.1 | 3.5 | 3.5 |
| Parameter | Pre-WBC (Left) | Pre-WBC (Right) | Post-WBC (Left) | Post-WBC (Right) | Normal Range |
|---|---|---|---|---|---|
| Cadence (steps/min) | 96.2 ± 9.72 | 36.2 ± 2.41 | 98.0 ± 1.99 | 37.3 ± 2.13 | 113 ± 12.4 |
| Double Support (%) | 35.1 ± 4.23 | 36.2 ± 2.41 | 37.7 ± 1.63 | 37.3 ± 2.13 | 23.6 ± 3.75 |
| Foot Off (%) | 66.7 ± 2.60 | 69.6 ± 0.62 | 66.6 ± 1.41 | 69.8 ± 2.31 | 61.6 ± 2.39 |
| Opposite Foot Contact (%) | 48.0 ± 2.12 | 51.5 ± 1.27 | 48.1 ± 0.46 | 50.7 ± 0.55 | 50.1 ± 2.24 |
| Opposite Foot Off (%) | 16.5 ± 4.17 | 18.1 ± 1.74 | 19.3 ± 1.44 | 18.2 ± 1.64 | 11.9 ± 2.16 |
| Single Support (%) | 31.5 ± 2.13 | 33.4 ± 2.98 | 28.8 ± 1.00 | 32.4 ± 1.15 | 38.2 ± 2.60 |
| Step Length (m) | 0.30 ± 0.058 | 0.30 ± 0.053 | 0.38 ± 0.024 | 0.35 ± 0.024 | 0.62 ± 0.081 |
| Step Time (s) | 0.66 ± 0.058 | 0.61 ± 0.060 | 0.64 ± 0.010 | 0.60 ± 0.010 | 0.49 ± 0.24 |
| Step Width (m) | 0.15 ± 0.012 | 0.14 ± 0.011 | 0.14 ± 0.0016 | 0.15 ± 0.015 | 0.11 ± 0.02 |
| Stride Length (m) | 0.60 ± 0.12 | 0.61 ± 0.11 | 0.75 ± 0.033 | 0.69 ± 0.033 | 1.22 ± 0.15 |
| Stride Time (s) | 1.27 ± 0.15 | 1.25 ± 0.098 | 1.23 ± 0.027 | 1.22 ± 0.034 | 1.06 ± 0.12 |
| Walking Speed (m/s) | 0.48 ± 0.11 | 0.49 ± 0.12 | 0.61 ± 0.023 | 0.57 ± 0.043 | 1.17 ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piterà, P.; Bigoni, M.; Prina, E.; Barrera, B.; Yavuz, D.C.; Verme, F.; Fontana, J.M.; Priano, L.; Mauro, A.; Capodaglio, P. Is Whole-Body Cryostimulation Useful in Modulating Spasticity in Adults with Cerebral Palsy? A Case Study. J. Clin. Med. 2024, 13, 7674. https://doi.org/10.3390/jcm13247674
Piterà P, Bigoni M, Prina E, Barrera B, Yavuz DC, Verme F, Fontana JM, Priano L, Mauro A, Capodaglio P. Is Whole-Body Cryostimulation Useful in Modulating Spasticity in Adults with Cerebral Palsy? A Case Study. Journal of Clinical Medicine. 2024; 13(24):7674. https://doi.org/10.3390/jcm13247674
Chicago/Turabian StylePiterà, Paolo, Matteo Bigoni, Elisa Prina, Boris Barrera, Duru Ceren Yavuz, Federica Verme, Jacopo Maria Fontana, Lorenzo Priano, Alessandro Mauro, and Paolo Capodaglio. 2024. "Is Whole-Body Cryostimulation Useful in Modulating Spasticity in Adults with Cerebral Palsy? A Case Study" Journal of Clinical Medicine 13, no. 24: 7674. https://doi.org/10.3390/jcm13247674
APA StylePiterà, P., Bigoni, M., Prina, E., Barrera, B., Yavuz, D. C., Verme, F., Fontana, J. M., Priano, L., Mauro, A., & Capodaglio, P. (2024). Is Whole-Body Cryostimulation Useful in Modulating Spasticity in Adults with Cerebral Palsy? A Case Study. Journal of Clinical Medicine, 13(24), 7674. https://doi.org/10.3390/jcm13247674








