Effect of the COVID-19 Pandemic on the Management of Breast Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Type, Duration, and Location of the Study
2.2. Determination of the Research Universe and Study Group
2.3. Inclusion and Exclusion Criteria
2.4. Definitions, Parameters, and Variables Used in the Study
2.5. Study Protocol and Ethics Committee Approval
2.6. Statistical Analysis
3. Results
3.1. Analysis of Breast Cancer Screening Data Based on Pre-COVID-19 and COVID-19 Era
3.2. Analysis of Data from the Entire Study Group
3.3. Comparison of Pre-COVID-19 and COVID-19 Era Groups
3.3.1. Univariate Analysis
3.3.2. Multivariate Analysis
3.4. Comparison of Surviving and Non-Surviving Patients with Brest Cancer
3.4.1. Univariate Analysis
3.4.2. Multivariate Analysis
3.5. Comparison of the Survival of Pre-COVID-19 and COVID-19 Era Groups
4. Discussion
5. Lessons Learned and Future Perspective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Sahin, T.; Akbulut, S.; Yilmaz, S. COVID-19 pandemic: Its impact on liver disease and liver transplantation. World J. Gastroenterol. 2020, 26, 2987–2999. [Google Scholar] [CrossRef] [PubMed]
- Abera, A.; Fenta, E.; Woldehanna, B.; Wolde, F.; Legesse, M.; Regassa, L.; Mor, S.; Kaba, M. Impact of COVID-19 on essential healthcare services in Addis Ababa, Ethiopia: Implications for future pandemics. PLoS ONE 2024, 19, e0308861. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, S.; Tuncer, A.; Ogut, Z.; Sahin, T.T.; Koc, C.; Guldogan, E.; Karabulut, E.; Tanriverdi, E.S.; Ozer, A. Effect of the COVID-19 pandemic on patients with presumed diagnosis of acute appendicitis. World J. Clin. Cases 2022, 10, 10487–10500. [Google Scholar] [CrossRef]
- Dursun, A.; Tuncer, K.; Kılınç, G.m.; Demirli Atıcı, S.; Eyduran, B.; Esin, H.; Sert, I.; Emiroğlu, M. Management of Emergency and Elective Oncological Surgeries at a Tertiary General Surgery Clinic in COVID-19 Pandemic. Anatol. J. Gen. Med. Res. 2022, 32, 384–392. [Google Scholar] [CrossRef]
- Tosun, Y.; Çetin, K. General surgery practice under the COVID-19 pandemic: The experience of a pandemic hospital in Istanbul. Ulus. Travma Acil Cerrahi Derg. 2022, 28, 175–179. [Google Scholar] [CrossRef]
- Al-Shamsi, H.; Alhazzani, W.; Alhuraiji, A.; Coomes, E.; Chemaly, R.; Almuhanna, M.; Wolff, R.; Ibrahim, N.; Chua, M.; Hotte, S.; et al. A Practical Approach to the Management of Cancer Patients During the Novel Coronavirus Disease 2019 (COVID-19) Pandemic: An International Collaborative Group. Oncologist 2020, 25, e936–e945. [Google Scholar] [CrossRef]
- de Azambuja, E.; Trapani, D.; Loibl, S.; Delaloge, S.; Senkus, E.; Criscitiello, C.; Poortman, P.; Gnant, M.; Di Cosimo, S.; Cortes, J.; et al. ESMO Management and treatment adapted recommendations in the COVID-19 era: Breast Cancer. ESMO Open 2020, 5, e000793. [Google Scholar] [CrossRef]
- Dietz, J.; Moran, M.; Isakoff, S.; Kurtzman, S.; Willey, S.; Burstein, H.; Bleicher, R.; Lyons, J.; Sarantou, T.; Baron, P.; et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. the COVID-19 pandemic breast cancer consortium. Breast Cancer Res. Treat. 2020, 181, 487–497. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Vegunta, S.; Bhatt, A.A.; Choudhery, S.A.; Pruthi, S.; Kaur, A.S. Identifying women with increased risk of breast cancer and implementing risk-reducing strategies and supplemental imaging. Breast Cancer 2022, 29, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Miller, K.; Fuchs, H.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Feron Agbo, C.; Assogba, E.; Bertaut, A.; Mamguem Kamga, A.; Coutant, C.; Desmoulins, I.; Dancourt, V.; Sandrine Dabakuyo Yonli, T. Impact of Covid-19 on breast cancer stage at discovery, and time to treatment in Cote d’Or, France. Prev. Med. Rep. 2023, 34, 102248. [Google Scholar] [CrossRef] [PubMed]
- de Bock, E.; Herman, E.S.; Bastian, O.W.; Filipe, M.D.; Vriens, M.R.; Richir, M.C. Systematic review and meta-analysis determining the effect of implemented COVID-19 guidelines on surgical oncology volumes and clinical outcomes. Surg. Oncol. 2022, 45, 101859. [Google Scholar] [CrossRef]
- Gathani, T.; Clayton, G.; MacInnes, E.; Horgan, K. The COVID-19 pandemic and impact on breast cancer diagnoses: What happened in England in the first half of 2020. Br. J. Cancer 2021, 124, 710–712. [Google Scholar] [CrossRef]
- Ramanakumar, A.; Annie, B.; Frederic, L.; Christine, B.; Cathy, R.; Jean, L. Evaluating the impact of COVID-19 on cancer declarations in Quebec, Canada. Cancer Med. 2023, 12, 6260–6269. [Google Scholar] [CrossRef]
- Terashima, T.; Konishi, H.; Sato, Y.; Igarashi, M.; Yanagibashi, T.; Konno, R.; Saya, H.; Doki, Y.; Kakizoe, T. Impact of coronavirus disease 2019 on the number of newly diagnosed cancer patients and examinations and surgeries performed for cancer in Japan: A nationwide study. BMC Cancer 2022, 22, 1303. [Google Scholar] [CrossRef]
- Vrdoljak, E.; Balja, M.P.; Marušić, Z.; Avirović, M.; Blažičević, V.; Tomasović, Č.; Čerina, D.; Bajić, Ž.; Miše, B.P.; Lovasić, I.B.; et al. COVID-19 Pandemic Effects on Breast Cancer Diagnosis in Croatia: A Population- and Registry-Based Study. Oncologist 2021, 26, e1156–e1160. [Google Scholar] [CrossRef]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
- Alkatout, I.; Biebl, M.; Momenimovahed, Z.; Giovannucci, E.; Hadavandsiri, F.; Salehiniya, H.; Allahqoli, L. Has COVID-19 Affected Cancer Screening Programs? A Systematic Review. Front. Oncol. 2021, 11, 675038. [Google Scholar] [CrossRef]
- Grimm, L.; Lee, C.; Rosenberg, R.; Burleson, J.; Simanowith, M.; Fruscello, T., Jr.; Pelzl, C.; Friedewald, S.; Moy, L.; Zuley, M. Impact of the COVID-19 Pandemic on Breast Imaging: An Analysis of the National Mammography Database. J. Am. Coll. Radiol. 2022, 19, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Mayo, M.; Potugari, B.; Bzeih, R.; Scheidel, C.; Carrera, C.; Shellenberger, R.A. Cancer Screening During the COVID-19 Pandemic: A Systematic Review and Meta-analysis. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Riera, R.; Bagattini, Â.M.; Pacheco, R.; Pachito, D.; Roitberg, F.; Ilbawi, A. Delays and Disruptions in Cancer Health Care Due to COVID-19 Pandemic: Systematic Review. JCO Glob. Oncol. 2021, 7, 311–323. [Google Scholar] [CrossRef]
- Hyder, T.; Bhattacharya, S.; Gade, K.; Nasrazadani, A.; Brufsky, A.M. Approaching Neoadjuvant Therapy in the Management of Early-Stage Breast Cancer. Breast Cancer (Dove Med Press) 2021, 13, 199–211. [Google Scholar] [CrossRef]
- Masood, S. Neoadjuvant chemotherapy in breast cancers. Womens Health 2016, 12, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Denecli, A. Breast-Conserving Surgery for Breast Cancer. Haydarpasa Numune Med. J. 2024, 64, 55–60. [Google Scholar] [CrossRef]
- Beck, A.; Morrow, M. Axillary lymph node dissection: Dead or still alive? Breast 2023, 69, 469–475. [Google Scholar] [CrossRef]
- Amin, M.; Greene, F.; Edge, S.; Compton, C.; Gershenwald, J.; Brookland, R.; Meyer, L.; Gress, D.; Byrd, D.; Winchester, D. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Zhu, H.; Dogan, B. American Joint Committee on Cancer’s Staging System for Breast Cancer, Eighth Edition: Summary for Clinicians. Eur. J. Breast Health 2021, 17, 234–238. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Rosenbaum, L. The Untold Toll—The Pandemic’s Effects on Patients without COVID-19. N. Engl. J. Med. 2020, 382, 2368–2371. [Google Scholar] [CrossRef] [PubMed]
- Sud, A.; Jones, M.; Broggio, J.; Loveday, C.; Torr, B.; Garrett, A.; Nicol, D.; Jhanji, S.; Boyce, S.A.; Gronthoud, F.; et al. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020, 31, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, Y.; Zhao, X.; Xu, F.; Li, J.; Huang, T.; Sun, P.; Li, L.; Ai, X.; Xiao, H.; et al. Factors Influencing Delayed Treatment in Patients with Breast Cancer During COVID-19 Pandemic. Front. Public Health 2022, 10, 808873. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.; Fasano, G.; An, A.; Mount, L.; Bayard, S.; Rosenberg, S.; Taiwo, E.; Loeb-Zeitlin, S.; Marti, J.; Ashamalla, H.; et al. Psychosocial well-being during the COVID-19 pandemic among women with and without breast cancer. Psychooncology 2023, 32, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Soriano, E.C.; Perndorfer, C.; Otto, A.K.; Fenech, A.L.; Siegel, S.D.; Dickson-Witmer, D.; Clements, L.; Laurenceau, J.P. Psychosocial Impact of Cancer Care Disruptions in Women with Breast Cancer During the COVID-19 Pandemic. Front. Psychol. 2021, 12, 662339. [Google Scholar] [CrossRef]
- Tsapatsaris, A.; Babagbemi, K.; Reichman, M.B. Barriers to breast cancer screening are worsened amidst COVID-19 pandemic: A review. Clin. Imaging 2022, 82, 224–227. [Google Scholar] [CrossRef]
- Du, S.; Carfang, L.; Restrepo, E.; Benjamin, C.; Epstein, M.M.; Fairley, R.; Roudebush, L.; Hertz, C.; Eshraghi, L.; Warner, E.T. Patient-Reported Experiences of Breast Cancer Screening, Diagnosis, and Treatment Delay, and Telemedicine Adoption during COVID-19. Curr. Oncol. 2022, 29, 5919–5932. [Google Scholar] [CrossRef]
- Teng, H.; Dang, W.; Curpen, B. Impact of COVID-19 and Socioeconomic Factors on Delays in High-Risk MRI Breast Cancer Screening. Tomography 2022, 8, 2171–2181. [Google Scholar] [CrossRef]
- Akın, M. Rate and reasons of missed screening mammography in the COVID-19 pandemic from Turkey. J. Health Sci. Med. 2022, 5, 1068–1072. [Google Scholar] [CrossRef]
- Ayhan Baser, D.; Acar, F.; Bayer, S.; Cakir Altınyaprak, E.; Aksoy, H.; Fidanci, I.; Cankurtaran, M. The Impact of COVID-19 Pandemic on Cancer Screening Program Applications: A Descriptive Study. J. Mol. Virol. Immunol. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Bulbul, H.; Hürsoy, N.; Tasci, F.; Bedir, R.; Bulbul, O.; Aydın, E.; Pergel, A. Impact of COVID-19 Pandemic on Breast Cancer Screening and Diagnosis Process. Ank. Eğitim Araştırma Hastan. Tıp Derg. 2023, 56, 75–79. [Google Scholar] [CrossRef]
- Calpbinici, P.; Uzunkaya Öztoprak, P. The Effect of Fear of COVID-19 on Women’s Attitudes toward Cancer Screening and Healthy Lifestyle Behaviors: A Cross-Sectional Study. Indian. J. Gynecol. Oncol. 2023, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Koca, B.; Yildirim, M. Delay in breast cancer diagnosis and its clinical consequences during the coronavirus disease pandemic. J. Surg. Oncol. 2021, 124, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Miller, M.; Meneveau, M.; Rochman, C.; Schroen, A.; Lattimore, C.; Gaspard, P.; Cubbage, R.; Showalter, S. Impact of the COVID-19 pandemic on breast cancer screening volumes and patient screening behaviors. Breast Cancer Res. Treat. 2021, 189, 237–246. [Google Scholar] [CrossRef]
- DeGroff, A.; Miller, J.; Sharma, K.; Sun, J.; Helsel, W.; Kammerer, W.; Rockwell, T.; Sheu, A.; Melillo, S.; Uhd, J.; et al. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January-June 2020, in the United States. Prev. Med. 2021, 151, 106559. [Google Scholar] [CrossRef]
- Nogueira, L.; Palis, B.; Boffa, D.; Lum, S.; Yabroff, K.; Nelson, H. Evaluation of the Impact of the COVID-19 Pandemic on Reliability of Cancer Surveillance Data in the National Cancer Database. Ann. Surg. Oncol. 2023, 30, 2087–2093. [Google Scholar] [CrossRef]
- Toss, A.; Isca, C.; Venturelli, M.; Nasso, C.; Ficarra, G.; Bellelli, V.; Armocida, C.; Barbieri, E.; Cortesi, L.; Moscetti, L.; et al. Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open 2021, 6, 100055. [Google Scholar] [CrossRef]
- Vanni, G.; Tazzioli, G.; Pellicciaro, M.; Materazzo, M.; Paolo, O.; Cattadori, F.; Combi, F.; Papi, S.; Pistolese, C.A.; Cotesta, M.; et al. Delay in Breast Cancer Treatments During the First COVID-19 Lockdown. A Multicentric Analysis of 432 Patients. Anticancer Res. 2020, 40, 7119–7125. [Google Scholar] [CrossRef]
- Tonneson, J.; Hoskin, T.; Day, C.; Durgan, D.; Dilaveri, C.; Boughey, J. Impact of the COVID-19 Pandemic on Breast Cancer Stage at Diagnosis, Presentation, and Patient Management. Ann. Surg. Oncol. 2022, 29, 2231–2239. [Google Scholar] [CrossRef]
- Mason, H.; Friedrich, A.K.; Niakan, S.; Jacobbe, D.; Casaubon, J.; Pérez Coulter, A. The Influence of Screening Mammography Cessation and Resumption on Breast Cancer Presentation and Treatment: A Multi-Hospital Health System Experience during the Early COVID-19 Pandemic. Eur. J. Breast Health 2022, 18, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Bonadio, R.C.; Messias, A.P.; Moreira, O.A.; Leis, L.V.; Orsi, B.Z.; Testa, L.; Estevez-Diz, M.D.P. Impact of the COVID-19 pandemic on breast and cervical cancer stage at diagnosis in Brazil. Ecancermedicalscience 2021, 15, 1299. [Google Scholar] [CrossRef]
- Wilke, L.G.; Nguyen, T.T.; Yang, Q.; Hanlon, B.M.; Wagner, K.A.; Strickland, P.; Brown, E.; Dietz, J.R.; Boughey, J.C. Analysis of the Impact of the COVID-19 Pandemic on the Multidisciplinary Management of Breast Cancer: Review from the American Society of Breast Surgeons COVID-19 and Mastery Registries. Ann. Surg. Oncol. 2021, 28, 5535–5543. [Google Scholar] [CrossRef] [PubMed]
- Hawrot, K.; Shulman, L.; Bleiweiss, I.; Wilkie, E.; Frosch, Z.; Jankowitz, R.; Laughlin, A. Time to Treatment Initiation for Breast Cancer During the 2020 COVID-19 Pandemic. JCO Oncol. Pract. 2021, 17, 534–540. [Google Scholar] [CrossRef]
- Vanni, G.; Pellicciaro, M.; Materazzo, M.; Pedini, D.; Portarena, I.; Buonomo, C.; Perretta, T.; Rizza, S.; Pistolese, C.A.; Buonomo, O.C. Advanced Stages and Increased Need for Adjuvant Treatments in Breast Cancer Patients: The Effect of the One-year COVID-19 Pandemic. Anticancer Res. 2021, 41, 2689–2696. [Google Scholar] [CrossRef]
- Sezer, A.; Cicin, İ.; Karadeniz Çakmak, G.; Özkan Gürdal, S.; Başaran, G.; Oyan, B.; Eralp, Y.; Güllüoğlu, B.M. Turkish national consensus on breast cancer management during temporary state of emergency due to COVID-19 outbreak. Turk. J. Surg. 2020, 36, 147–163. [Google Scholar] [CrossRef]
- Petropoulou, Z.; Arkadopoulos, N.; Michalopoulos, N.V. Breast Cancer and COVID-19: Challenges in Surgical Management. Cancers 2022, 14, 5360. [Google Scholar] [CrossRef]
- Rocco, N.; Montagna, G.; Di Micco, R.; Benson, J.; Criscitiello, C.; Chen, L.; Di Pace, B.; Esgueva Colmenarejo, A.J.; Harder, Y.; Karakatsanis, A.; et al. The Impact of the COVID-19 Pandemic on Surgical Management of Breast Cancer: Global Trends and Future Perspectives. Oncologist 2021, 26, e66–e77. [Google Scholar] [CrossRef]
- Ilgun, A.; Ozmen, V. The Impact of the COVID-19 Pandemic on Breast Cancer Patients. Eur. J. Breast Health 2022, 18, 85–90. [Google Scholar] [CrossRef]
- Arikan, A.; Kara, H.l.; Dulgeroglu, O.; Uras, C. Breast Surgery can be Performed Safely During the COVID-19 Pandemic: A Retrospective Single-Center Analysis. İstanbul Med. J. 2022, 23, 45–50. [Google Scholar] [CrossRef]
- Borella, F.; Bertero, L.; Di Giovanni, F.; Witel, G.; Orlando, G.; Ricci, A.A.; Pittaro, A.; Castellano, I.; Cassoni, P. COVID-19 and Breast Cancer: Analysis of Surgical Management of a Large Referral Center during the 2020–2021 Pandemic Period. Curr. Oncol. 2023, 30, 4767–4778. [Google Scholar] [CrossRef] [PubMed]
- Di Cosimo, S.; Ljevar, S.; Trama, A.; Bernasconi, A.; Lasalvia, P.; De Santis, M.C.; Cappelletti, V.; Miceli, R.; Apolone, G. Direct and indirect effects of COVID-19 on short-term mortality of breast cancer patients. Breast 2023, 71, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Romics, L.; Doughty, J.; Stallard, S.; Mansell, J.; Blackhall, V.; Lannigan, A.; Elgammal, S.; Reid, J.; McGuigan, M.C.; Savioli, F.; et al. A prospective cohort study of the safety of breast cancer surgery during COVID-19 pandemic in the West of Scotland. Breast 2021, 55, 1–6. [Google Scholar] [CrossRef]
- Jansen, L.; Hermann, S.; Bergbold, S.; Arndt, V. Changes in breast cancer incidence and surgical treatment in Baden-Württemberg (Germany) during the COVID-19 pandemic. Sci. Rep. 2024, 14, 24912. [Google Scholar] [CrossRef]
- Gentile, D.; Martorana, F.; Karakatsanis, A.; Caruso, F.; Caruso, M.; Castiglione, G.; Di Grazia, A.; Pane, F.; Rizzo, A.; Vigneri, P.; et al. Predictors of mastectomy in breast cancer patients with complete remission of primary tumor after neoadjuvant therapy: A retrospective study. Eur. J. Surg. Oncol. 2024, 50, 108732. [Google Scholar] [CrossRef]
- Caplan, L. Delay in breast cancer: Implications for stage at diagnosis and survival. Front. Public Health 2014, 2, 87. [Google Scholar] [CrossRef]
- Kothari, A.; Fentiman, I. Diagnostic delays in breast cancer and impact on survival. Int. J. Clin. Pract. 2003, 57, 200–203. [Google Scholar] [CrossRef]
- Bleicher, R. Timing and Delays in Breast Cancer Evaluation and Treatment. Ann. Surg. Oncol. 2018, 25, 2829–2838. [Google Scholar] [CrossRef]
- Hanna, T.; King, W.; Thibodeau, S.; Jalink, M.; Paulin, G.; Harvey-Jones, E.; O’Sullivan, D.; Booth, C.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef]
- Ng, J.; Hamilton, D. Assessing the impact of the COVID-19 pandemic on breast cancer screening and diagnosis rates: A rapid review and meta-analysis. J. Med. Screen. 2022, 29, 209–218. [Google Scholar] [CrossRef]
- Li, T.; Nickel, B.; Ngo, P.; McFadden, K.; Brennan, M.; Marinovich, M.L.; Houssami, N. A systematic review of the impact of the COVID-19 pandemic on breast cancer screening and diagnosis. Breast 2023, 67, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Patel, B. A systematic review on management of breast cancer patients during the covid 19 pandemic: To assess if there was a clinical delay in treatment and patient perception on delayed treatment. Literature review of the current covid 19 management guidelines for breast cancer patients. Eur. J. Cancer 2022, 175, S65. [Google Scholar] [CrossRef]
- Budiarta, M.; Brennan, M. The Impact of COVID-19 on Breast Cancer Treatment: A Systematic Review: Breast cancer treatment during pandemic. Arch. Breast Cancer 2022, 9, 421–438. [Google Scholar] [CrossRef]
- Biagioli, V.; Belloni, S.; Albanesi, B.; Piredda, A.; Caruso, R. Comment on “The experience on coronavirus disease 2019 and cancer from an oncology hub institution in Milan, Lombardy Region” and reflections from the Italian Association of Oncology Nurses. Eur. J. Cancer 2020, 135, 8–10. [Google Scholar] [CrossRef]
- Belsky, J.A.; Tullius, B.P.; Lamb, M.G.; Sayegh, R.; Stanek, J.R.; Auletta, J.J. COVID-19 in immunocompromised patients: A systematic review of cancer, hematopoietic cell and solid organ transplant patients. J. Infect. 2021, 82, 329–338. [Google Scholar] [CrossRef]
- Yildiz, F.; Oksuzoglu, B. Teleoncology or telemedicine for oncology patients during the COVID-19 pandemic: The new normal for breast cancer survivors? Future Oncol. 2020, 16, 2191–2195. [Google Scholar] [CrossRef]
- Sonagli, M.; Cagnacci Neto, R.; Leite, F.P.M.; Makdissi, F.B.A. The use of telemedicine to maintain breast cancer follow-up and surveillance during the COVID-19 pandemic. J. Surg. Oncol. 2021, 123, 371–374. [Google Scholar] [CrossRef]
- Subramanian, M.J.; Ravi, R.M. Phone-Based Breasts Self-Examination as an Intervention in Breast Cancer Control during the COVID-19 Pandemic. Indian. J. Gynecol. Oncol. 2022, 20, 28. [Google Scholar] [CrossRef]
- Uchikov, P.; Khalid, U.; Dedaj-Salad, G.; Ghale, D.; Rajadurai, H.; Kraeva, M.; Kraev, K.; Hristov, B.; Doykov, M.; Mitova, V.; et al. Artificial Intelligence in Breast Cancer Diagnosis and Treatment: Advances in Imaging, Pathology, and Personalized Care. Life 2024, 14, 1451. [Google Scholar] [CrossRef]
- Khalid, A.; Mehmood, A.; Alabrah, A.; Alkhamees, B.F.; Amin, F.; AlSalman, H.; Choi, G.S. Breast Cancer Detection and Prevention Using Machine Learning. Diagnostics 2023, 13, 3113. [Google Scholar] [CrossRef]
- Al Masry, Z.; Zerhouni, N.; Gay, C.; Meraghni, S.; Lodi, M.; Mathelin, C.; Devalland, C. Connected bras for breast cancer detection in 2021: Analysis and perspectives. Gynecol. Obstet. Fertil. Senol. 2021, 49, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Sirisha, P.; Radhika, B.; Manasa, G.; Divya, K.; Sree, C. Review on–Smart Bra Device for Detecting Breast Cancer Using Machine Learning. IJMRSET 2023, 6, 61–65. [Google Scholar] [CrossRef]
- Moreno, M.V.; Herrera, E. Evaluation on Phantoms of the Feasibility of a Smart Bra to Detect Breast Cancer in Young Adults. Sensors 2019, 19, 5491. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Categories | Number (%) |
|---|---|---|
| Group | Pre-COVID-19 | 240 (58.0) |
| COVID-19 Era | 174 (42.0) | |
| Gender | Female | 410 (99.0) |
| Male | 4 (1.0) | |
| Comorbidity | No | 254 (61.3) |
| Yes | 160 (38.7) | |
| ASA scores | 1 | 83 (20.05) |
| 2 | 287 (69.3) | |
| 3 | 44 (10.6) | |
| Preoperative PET-CT | No | 63 (15.2) |
| Yes | 351 (84.8) | |
| Neoadjuvant CT | No | 303 (73.2) |
| Yes | 111 (26.8) | |
| Adjuvant CRT | No | 53 (12.8) |
| Yes | 360 (87.2) | |
| Type of surgery | Mastectomy | 314 (75.9) |
| BCS | 100 (24.1) | |
| Which breast | Right | 197 (47.6) |
| Left | 217 (52.4) | |
| Which quadrant | Lower outer | 80 (19.3) |
| Lower inner | 47 (11.4) | |
| Upper outer | 185 (44.7) | |
| Upper inner | 88 (21.3) | |
| Retroareolar | 14 (3.4) | |
| Multicentricity | No | 363 (87.7) |
| Yes | 51 (12.3) | |
| Multifocality | No | 360 (87.0) |
| Yes | 54 (13.04) | |
| Histopathological diagnosis | Apocrine carcinoma | 2 (0.5) |
| DCIS | 16 (3.9) | |
| IDC + ILC | 10 (2.4) | |
| IDC | 290 (70.1) | |
| ILC | 29 (7.0) | |
| Micropapillary carcinoma | 10 (2.4) | |
| Mucinous carcinoma | 17 (4.1) | |
| Tubular carcinoma | 2 (0.5) | |
| LCIS | 2 (0.5) | |
| Malignant phyllodes tumor | 1 (0.2) | |
| Medullary carcinoma | 10 (2.4) | |
| Metaplastic carcinoma | 5 (1.2) | |
| Microinvasive carcinoma | 2 (0.5) | |
| NET (poorly differentiated) | 7 (1.7) | |
| NET (well differentiated) | 2 (0.5) | |
| Triple-negative breast carcinoma | 9 (2.2) | |
| MBR grade | 1 | 39 (11.3) |
| 2 | 132 (38.4) | |
| 3 | 173 (50.3) | |
| Skin involvement | No | 377 (91.1) |
| Yes | 37 (8.9) | |
| Nipple involvement | No | 389 (94.0) |
| Yes | 25 (6.0) | |
| Lymphovascular invasion | No | 161 (39.0) |
| Yes | 252 (61.0) | |
| Perineural invasion | No | 317 (76.8) |
| Yes | 96 (23.2) | |
| Comedo necrosis | No | 319 (77.1) |
| Yes | 95 (23.0) | |
| ER | Negative | 67 (16.6) |
| Positive | 336 (83.4) | |
| PR | Negative | 109 (27.5) |
| Positive | 287 (72.5) | |
| c-erbB2 | Negative | 273 (68.9) |
| Positive | 123 (31.1) | |
| E-cadherin | Negative | 34 (13.6) |
| Positive | 216 (86.4) | |
| TNM classification | 0 | 18 (4.35) |
| IA | 80 (19.3) | |
| IIA | 97 (23.4) | |
| IIB | 55 (13.3) | |
| IIIA | 65 (15.7) | |
| IIIB | 34 (8.2) | |
| IIIC | 48 (11.6) | |
| IV | 17 (4.1) | |
| Recurrence | No | 401 (97.8) |
| Yes | 9 (2.2) | |
| Metastasis | No | 373 (91.0) |
| Yes | 37 (9.0) | |
| Outcomes | Alive | 393 (94.9) |
| Mortality | 21 (5.1) |
| Parameters [Median (95%CI)] | Pre-COVID-19 (n = 240) | COVID-19 Era (n = 174) | p |
|---|---|---|---|
| Age (years) | 51 (50-54) | 50 (49-54) | 0.857 |
| From diagnosis to surgery (days) | 25 (21-31) | 28.5 (26-35) | 0.121 |
| From surgery to pathology report (days) | 28 (28-30) | 23 (22-26) | <0.001 |
| Specimen weight (gram) | 876 (792-946) | 939 (830-988) | 0.125 |
| Tubule formation | 3 (3-3) | 3 (3-3) | 0.156 |
| Nuclear pleomorphism | 3 (3-3) | 3 (3-3) | 0.230 |
| Mitotic Count | 2 (2-3) | 2(2-3) | 0.681 |
| Tumor size (mm) | 24 (22-26) | 24 (20-28) | 0.501 |
| Total LAP | 8.5 (5-10) | 10.5 (8-12) | 0.116 |
| Positive LAP | 0 (0-0) | 0 (0-0) | 0.793 |
| Ki-67 (%) | 20 (20-30) | 20 (20-25) | 0.059 |
| Parameters | Categories | Pre-COVID-19 (n = 240) | COVID-19 Era (n = 174) | p |
|---|---|---|---|---|
| Gender | Female | 237 (98.8) | 173 (99.4) | 0.642 * |
| Male | 3 (1.3) | 1 (0.6) | ||
| Comorbidity | No | 132 (55.0) | 122 (70.1) | 0.002 ** |
| Yes | 108 (45.0) | 52 (29.9) | ||
| ASA scores | 1 | 47 (19.6) | 36 (20.7) | 0.716 ** |
| 2 | 165 (68.8) | 122 (70.1) | ||
| 3 | 28 (11.7) | 16 (9.2) | ||
| Preoperative PET-CT | No | 47 (19.6) | 16 (9.2) | 0.006 *** |
| Yes | 193 (80.4) | 158 (90.8) | ||
| Neoadjuvant CT | No | 189 (78.8) | 114 (65.5) | 0.003 ** |
| Yes | 51 (21.3) | 60 (34.5) | ||
| Adjuvant CRT | No | 29 (12.1) | 24 (13.8) | 0.727 *** |
| Yes | 210 (87.9) | 150 (86.2) | ||
| Type of surgery | Mastectomy | 173 (72.1) | 141 (81.0) | 0.036 |
| BCS | 67 (27.9) | 33 (19.0) | ||
| Which breast | Right | 120 (50.0) | 77 (44.3) | 0.248 ** |
| Left | 120 (50.0) | 97 (55.8) | ||
| Which quadrant | Lower outer | 46 (19.2) | 34 (19.5) | 0.091 ** |
| Lower inner | 21 (8.8) | 26 (14.9) | ||
| Upper outer | 116 (48.3) | 69 (39.7) | ||
| Upper inner | 52 (21.7) | 36 (20.7) | ||
| Retroareolar | 5 (2.1) | 9 (5.2) | ||
| Multicentricity | No | 214 (89.2) | 149 (85.6) | 0.280 ** |
| Yes | 26 (10.8) | 25 (14.4) | ||
| Multifocality | No | 204 (85.0) | 156 (89.7) | 0.215 *** |
| Yes | 36 (15.0) | 18 (10.3) | ||
| MBR grade | 1 | 25(12.3) | 14 (9.9) | 0.613 *** |
| 2 | 74 (36.5) | 58 (41.1) | ||
| 3 | 104 (51.2) | 69 (48.9) | ||
| Skin involvement | No | 220 (91.7) | 157 (90.2) | 0.740 *** |
| Yes | 20 (8.3) | 17 (9.8) | ||
| Nipple involvement | No | 228 (95.0) | 161 (92.5) | 0.405 *** |
| Yes | 12 (5.0) | 13 (7.5) | ||
| Lymphovascular invasion | No | 84 (35.2) | 77 (44.3) | 0.061 ** |
| Yes | 155 (64.9) | 97 (55.8) | ||
| Perineural invasion | No | 184 (77.0) | 133 (76.4) | 0.896 ** |
| Yes | 55 (23.0) | 41 (23.6) | ||
| Comedo necrosis | No | 186 (77.5) | 133 (76.4) | 0.800 ** |
| Yes | 54 (22.5) | 41 (23.6) | ||
| ER | Negative | 38 (16.3) | 29 (17.1) | 0.842 ** |
| Positive | 195 (83.7) | 141 (82.9) | ||
| PR | Negative | 63 (27.9) | 46 (27.1) | 0.857 ** |
| Positive | 163 (72.1) | 124 (72.9) | ||
| c-erbB2 | Negative | 149 (65.4) | 124 (73.8) | 0.072 ** |
| Positive | 79 (34.7) | 44 (26.2) | ||
| E-cadherin | Negative | 19 (14.6) | 15 (12.5) | 0.762 *** |
| Positive | 111 (85.4) | 105 (87.5) | ||
| TNM classification | 0 | 14 (5.8) | 4 (2.3) | 0.448 ** |
| 1A | 50 (20.8) | 30 (17.2) | ||
| 2A | 57 (23.8) | 40 (23.0) | ||
| 2B | 32 (13.3) | 23 (13.2) | ||
| 3A | 32 (13.3) | 33 (19.0) | ||
| 3B | 17 (7.1) | 17 (9.8) | ||
| 3C | 29 (12.1) | 19 (10.9) | ||
| 4 | 9 (3.8) | 8 (4.6) | ||
| Recurrence | No | 229 (97.0) | 172 (98.9) | 0.312 * |
| Yes | 7 (3.0) | 2 (1.15) | ||
| Metastasis | No | 209 (88.6) | 164 (94.3) | 0.070 *** |
| Yes | 27 (11.4) | 10 (5.8) | ||
| Outcomes | Alive | 223 (92.9) | 170 (97.7) | 0.049 *** |
| Dead | 17 (7.1) | 4 (2.3) |
| Parameters [Median (95%CI)] | Alive (n = 393) | Mortality (n = 21) | p |
|---|---|---|---|
| Age (years) | 50 (49-52) | 54 (51-65) | 0.069 |
| From diagnosis to surgery (days) | 26 (22-29) | 148 (68-229) | 0.001 |
| From surgery to pathology report (days) | 26 (25-28) | 33 (28-41) | 0.018 |
| Specimen weight (gram) | 882 (824–940) | 1414 (947-1876) | 0.001 |
| Tubule formation | 3 (3-3) | 3 (3-3) | 0.142 |
| Nuclear pleomorphism | 3 (3-3) | 3 (3-3) | 0.033 |
| Mitotic count | 2 (2-3) | 3 (3-3) | 0.020 |
| Tumor size (mm) | 23 (22-25) | 42 (36-85) | 0.000 |
| Total LAP | 10 (9-12) | 6 (3-15) | 0.609 |
| Positive LAP | 0 (0-0) | 2 (2-11) | 0.032 |
| Ki-67 (%) | 20 (20-25) | 40 (40-70) | 0.009 |
| Parameters | Categories | Alive (n = 393) | Mortality (n = 21) | p |
|---|---|---|---|---|
| Group | Pre-COVID-19 | 223 (56.7) | 17 (81.0) | 0.049 *** |
| COVID-19 Era | 170 (43.3) | 4 (19.1) | ||
| Gender | Female | 389 (99.0) | 21 (100.0) | 0.999 * |
| Male | 4 (1.0) | 0 (0.0) | ||
| Comorbidity | No | 244 (62.1) | 10 (47.6) | 0.273 *** |
| Yes | 149 (37.9) | 11 (52.4) | ||
| ASA scores | 1 | 79 (20.1) | 4 (19.1) | 0.022 ** |
| 2 | 276 (70.2) | 1 (52.4) | ||
| 3 | 38 (9.7) | 6 (28.6) | ||
| Preoperative PET-CT | No | 61 (15.5) | 2 (9.5) | 0.754 * |
| Yes | 332 (84.5) | 19 (90.5) | ||
| Neoadjuvant CT | No | 295 (75.1) | 8 (38.1) | 0.001 *** |
| Yes | 98 (24.9) | 13 (61.9) | ||
| Adjuvant CRT | No | 46 (11.7) | 7 (33.3) | 0.011 * |
| Yes | 346 (88.3) | 14 (66.7) | ||
| Type of surgery | Mastectomy | 293 (74.6) | 21 (100.0) | 0.017 *** |
| BCS | 100 (25.5) | 0 (0.0) | ||
| Which breast | Right | 184 (46.8) | 13 (61.9) | 0.261 *** |
| Left | 209 (53.2) | 8 (38.1) | ||
| Which quadrant | Lower outer | 75 (19.1) | 5 (23.8) | 0.890 ** |
| Lower inner | 45 (11.5) | 2 (9.5) | ||
| Upper outer | 176 (44.78) | 9 (42.9) | ||
| Upper inner | 83 (21.1) | 5 (23.8) | ||
| Retroareolar | 14 (3.6) | 1 (0.0) | ||
| Multicentricity | No | 353 (89.8) | 10 (47.6) | <0.001 * |
| Yes | 40 (10.2) | 11 (52.4) | ||
| Multifocality | No | 343 (87.3) | 17 (81.0) | 0.500 * |
| Yes | 50 (12.7) | 4 (19.1) | ||
| MBR grade | 1 | 38 (11.7) | 1 (5.3) | 0.110 ** |
| 2 | 128 (39.4) | 4 (21.1) | ||
| 3 | 159 (48.9) | 14 (73.7) | ||
| Skin involvement | No | 363 (92.4) | 14 (66.7) | 0.001 * |
| Yes | 30 (7.6) | 7 (33.3) | ||
| Nipple involvement | No | 373 (94.9) | 16 (76.2) | 0.006 * |
| Yes | 20 (5.1) | 5 (23.8) | ||
| Lymphovascular invasion | No | 159 (40.6) | 2 (9.5) | 0.009 *** |
| Yes | 233 (59.4) | 19 (90.5) | ||
| Perineural invasion | No | 306 (78.1) | 11 (52.4) | 0.014 * |
| Yes | 86 (21.9) | 10 (47.6) | ||
| Comedo necrosis | No | 303 (77.1) | 16 (76.2) | 0.999 * |
| Yes | 90 (22.9) | 5 (23.8) | ||
| ER | Negative | 61 (16.0) | 6 (28.6) | 0.136 * |
| Positive | 321 (84.0) | 15 (71.4) | ||
| PR | Negative | 99 (26.3) | 10 (52.6) | 0.025 *** |
| Positive | 278 (73.7) | 9 (47.4) | ||
| c-erbB2 | Negative | 261 (69.6) | 12 (57.1) | 0.338 *** |
| Positive | 114 (30.4) | 9 (42.9) | ||
| E-cadherin | Negative | 32 (13.6) | 2 (13.3) | 0.999 * |
| Positive | 203 (86.4) | 13 (86.7) | ||
| TNM classification | 0 | 18 (4.6) | 0 (0.00) | <0.001 ** |
| IA | 80 (20.4) | 0 (0.0) | ||
| IIA | 92 (23.4) | 5 (23.8) | ||
| IIB | 55 (14.0) | 0 (0.0) | ||
| IIIA | 63 (16.0) | 2 (9.5) | ||
| IIIB | 32 (8.1) | 2 (9.5) | ||
| IIIC | 44 (11.2) | 4 (19.1) | ||
| IV | 9 (2.3) | 8 (38.1) | ||
| Recurrence | No | 385 (98.7) | 16 (80.0) | <0.001 * |
| Yes | 5 (1.3) | 4 (20.0) | ||
| Metastasis | No | 370 (94.9) | 3 (15.0) | <0.001 * |
| Yes | 20 (5.1) | 17 (85.0) |
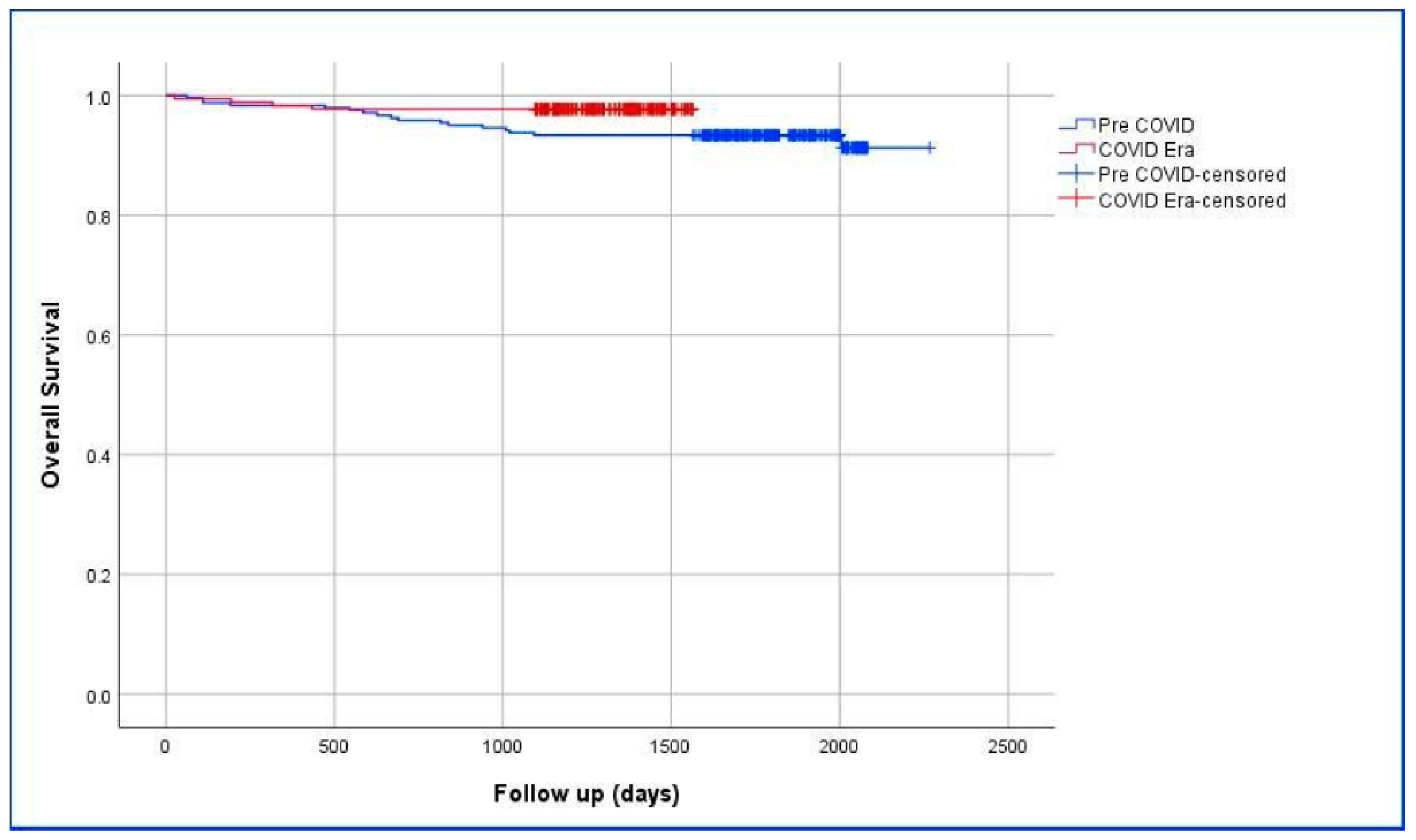
| Groups | Mean (Days) | p | |||
|---|---|---|---|---|---|
| Estimate | Std. Error | 95% CI | |||
| Lower Bound | Upper Bound | ||||
| Pre-COVID-19 | 2151.051 | 27.690 | 2096.778 | 2205.324 | 0.044 |
| COVID-19 Era | 1533.609 | 15.120 | 1503.973 | 1563.245 | |
| Overall | 2177.773 | 19.359 | 2139.828 | 2215.717 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalda, Y.; Akbulut, S.; Kucukakcali, Z.; Ogut, Z.; Dalda, O.; Alan, S.; Isik, B. Effect of the COVID-19 Pandemic on the Management of Breast Cancer Patients. J. Clin. Med. 2024, 13, 7673. https://doi.org/10.3390/jcm13247673
Dalda Y, Akbulut S, Kucukakcali Z, Ogut Z, Dalda O, Alan S, Isik B. Effect of the COVID-19 Pandemic on the Management of Breast Cancer Patients. Journal of Clinical Medicine. 2024; 13(24):7673. https://doi.org/10.3390/jcm13247673
Chicago/Turabian StyleDalda, Yasin, Sami Akbulut, Zeynep Kucukakcali, Zeki Ogut, Ozlem Dalda, Saadet Alan, and Burak Isik. 2024. "Effect of the COVID-19 Pandemic on the Management of Breast Cancer Patients" Journal of Clinical Medicine 13, no. 24: 7673. https://doi.org/10.3390/jcm13247673
APA StyleDalda, Y., Akbulut, S., Kucukakcali, Z., Ogut, Z., Dalda, O., Alan, S., & Isik, B. (2024). Effect of the COVID-19 Pandemic on the Management of Breast Cancer Patients. Journal of Clinical Medicine, 13(24), 7673. https://doi.org/10.3390/jcm13247673






