Peripheral and Organ Perfusion’s Role in Prognosis of Disease Severity and Mortality in Severe COVID-19 Patients: Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Oxygenation Ratio—PaO2/FiO2
2.2. Capillary Refill Time (CRT)
2.3. Pulse Oximetry
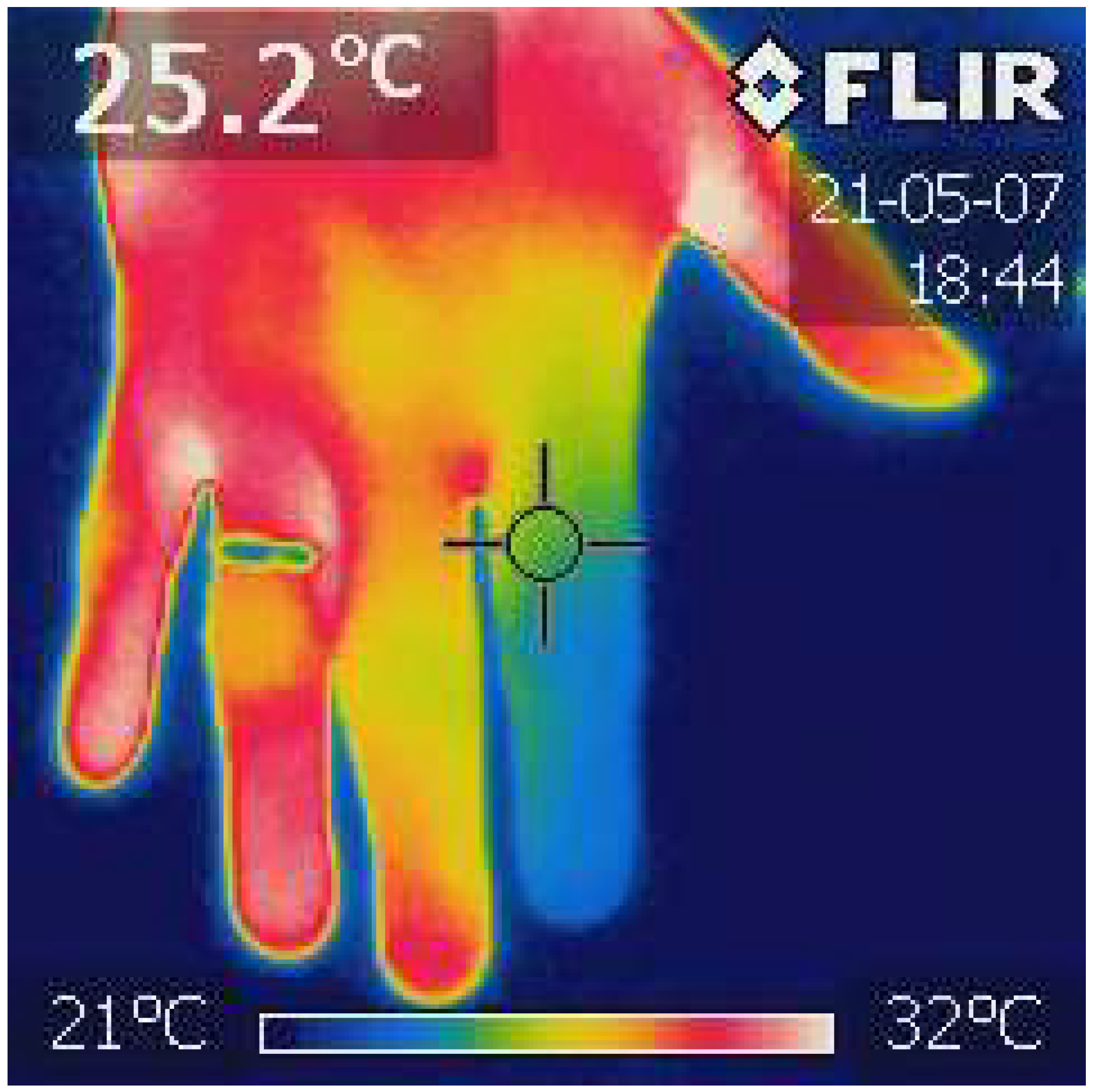
2.4. Finger Infrared Thermography
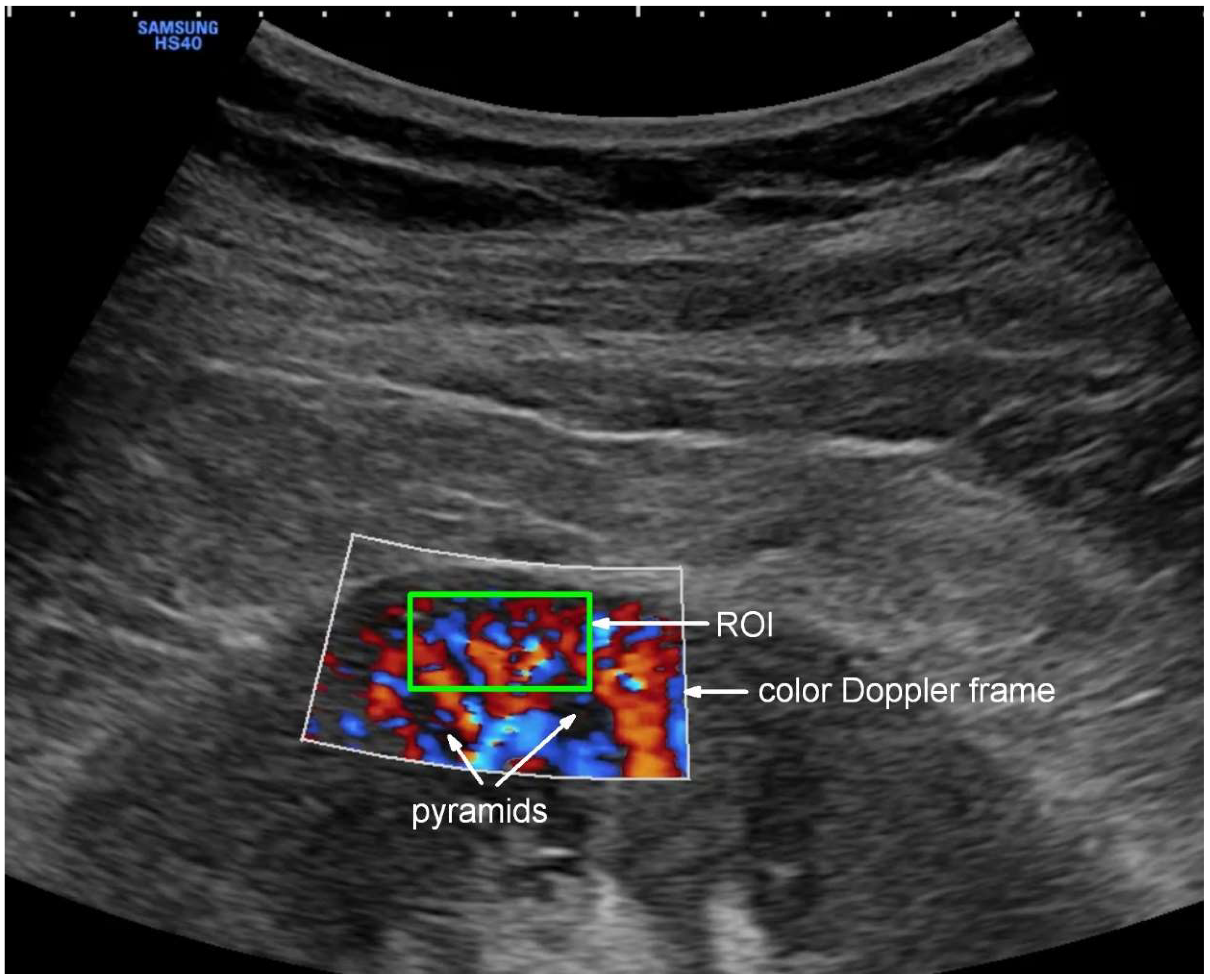
2.5. Ultrasound Examination
2.6. Statistical Analysis
3. Results
3.1. Descriptive Data: Demographics, Comorbidities, and ARDS Severity Based on the Oxygenation Ratio
3.2. Risk Factors for Clinical Deterioration and Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570371/ (accessed on 1 December 2023).
- Kanoore Edul, V.S.; Caminos Eguillor, J.F.; Ferrara, G.; Estenssoro, E.; Siles, D.S.P.; Cesio, C.E.; Dubin, A. Microcirculation Alterations in Severe COVID-19 Pneumonia. J. Crit. Care 2021, 61, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.P.R.; Dyson, A.; Singer, M.; Fraser, J. Microcirculatory Dysfunction and Resuscitation: Why, When, and How. Br. J. Anaesth. 2015, 115, 366–375. [Google Scholar] [CrossRef]
- Ince, C. The Microcirculation Is the Motor of Sepsis. Crit. Care 2005, 9 (Suppl. S4), S13–S19. [Google Scholar] [CrossRef]
- Fernández-Sarmiento, J.; Schlapbach, L.J.; Acevedo, L.; Santana, C.R.; Acosta, Y.; Diana, A.; Monsalve, M.; Carcillo, J.A. Endothelial Damage in Sepsis: The Importance of Systems Biology. Front. Pediatr. 2022, 10, 828968. [Google Scholar] [CrossRef] [PubMed]
- Spronk, P.E.; Zandstra, D.F.; Ince, C. Bench-to-Bedside Review: Sepsis Is a Disease of the Microcirculation. Crit. Care 2004, 8, 462. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Maistre, E.; Savard, P.; Guinot, P.-G. COVID-19 and the Concept of Thrombo-Inflammation: Review of the Relationship between Immune Response, Endothelium and Coagulation. J. Clin. Med. 2023, 12, 7245. [Google Scholar] [CrossRef]
- Nicolai, L.; Leunig, A.; Brambs, S.; Kaiser, R.; Joppich, M.; Hoffknecht, M.; Gold, C.; Engel, A.; Polewka, V.; Muenchhoff, M.; et al. Vascular Neutrophilic Inflammation and Immunothrombosis Distinguish Severe COVID-19 from Influenza Pneumonia. J. Thromb. Haemost. 2021, 19, 574–581. [Google Scholar] [CrossRef]
- Nicolai, L.; Leunig, A.; Brambs, S.; Kaiser, R.; Weinberger, T.; Weigand, M.; Muenchhoff, M.; Hellmuth, J.C.; Ledderose, S.; Schulz, H.; et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation 2020, 142, 1176–1189. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary Manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial Cell Infection and Endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Hariri, G.; Joffre, J.; Leblanc, G.; Bonsey, M.; Lavillegrand, J.-R.; Urbina, T.; Guidet, B.; Maury, E.; Bakker, J.; Ait-Oufella, H. Narrative Review: Clinical Assessment of Peripheral Tissue Perfusion in Septic Shock. Ann. Intensive Care 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, L.J.; McPartland, K.; Santora, T.A.; Trooskin, S.Z. Start with a Subjective Assessment of Skin Temperature to Identify Hypoperfusion in Intensive Care Unit Patients. J. Trauma Inj. Infect. Crit. Care 2001, 50, 620–627, discussion 627. [Google Scholar] [CrossRef]
- Chien, L.-C.; Lu, K.J.Q.; Wo, C.C.J.; Shoemaker, W.C. Hemodynamic Patterns Preceding Circulatory Deterioration and Death after Trauma. J. Trauma Inj. Infect. Crit. Care 2007, 62, 928–932. [Google Scholar] [CrossRef]
- McGee, S.; Abernethy, W.B.; Simel, D.L. The Rational Clinical Examination. Is This Patient Hypovolemic? J. Am. Med. Assoc. 1999, 281, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Dosal, A.; Kolosovas-Machuca, E.S.; Rivera-Vega, R.; Simón, J.; González, F.J. Use of Infrared Thermography in Children with Shock: A Case Series. SAGE Open Med. Case Rep. 2014, 2, 2050313X14561779. [Google Scholar] [CrossRef]
- Ammer, K. The Glamorgan Protocol for Recording and Evaluation of Thermal Images of the Human Body. Thermol. Int. 2008, 18, 125–144. [Google Scholar]
- Scholbach, T.; Dimos, I.; Scholbach, J. A New Method of Color Doppler Perfusion Measurement via Dynamic Sonographic Signal Quantification in Renal Parenchyma. Nephron Physiol. 2004, 96, 99–104. [Google Scholar] [CrossRef]
- Scholbach, T. Dynamic Tissue Perfusion Measurement—Basics and Applications. In Sonography; Thoirs, K., Ed.; InTech: London, UK, 2012; ISBN 978-953-307-947-9. [Google Scholar]
- Scholbach, T.M.; Vogel, C.; Bergner, N. Color Doppler Sonographic Dynamic Tissue Perfusion Measurement Demonstrates Significantly Reduced Cortical Perfusion in Children with Diabetes Mellitus Type 1 without Microalbuminuria and Apparently Healthy Kidneys. Ultraschall Der Med. 2014, 35, 445–450. [Google Scholar] [CrossRef]
- Lubas, A.; Zegadło, A.; Frankowska, E.; Klimkiewicz, J.; Jędrych, E.; Niemczyk, S. Ultrasound Doppler Flow Parameters Are Independently Associated with Renal Cortex Contrast-Enhanced Multidetector Computed Tomography Perfusion and Kidney Function. J. Clin. Med. 2023, 12, 2111. [Google Scholar] [CrossRef]
- Harrois, A.; Grillot, N.; Figueiredo, S.; Duranteau, J. Acute Kidney Injury Is Associated with a Decrease in Cortical Renal Perfusion during Septic Shock. Crit. Care 2018, 22, 161. [Google Scholar] [CrossRef] [PubMed]
- Lubas, A.; Kade, G.; Saracyn, M.; Niemczyk, S.; Dyrla, P. Dynamic Tissue Perfusion Assessment Reflects Associations between Antihypertensive Treatment and Renal Cortical Perfusion in Patients with Chronic Kidney Disease and Hypertension. Int. Urol. Nephrol. 2018, 50, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The Immunology and Immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- Salton, F.; Confalonieri, P.; Campisciano, G.; Cifaldi, R.; Rizzardi, C.; Generali, D.; Pozzan, R.; Tavano, S.; Bozzi, C.; Lapadula, G.; et al. Cytokine Profiles as Potential Prognostic and Therapeutic Markers in SARS-CoV-2-Induced ARDS. J. Clin. Med. 2022, 11, 2951. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine Elevation in Severe and Critical COVID-19: A Rapid Systematic Review, Meta-Analysis, and Comparison with Other Inflammatory Syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Agresti, N.; Lalezari, J.P.; Amodeo, P.P.; Mody, K.; Mosher, S.F.; Seethamraju, H.; Kelly, S.A.; Pourhassan, N.Z.; Sudduth, C.D.; Bovinet, C.; et al. Disruption of CCR5 Signaling to Treat COVID-19-Associated Cytokine Storm: Case Series of Four Critically Ill Patients Treated with Leronlimab. J. Transl. Autoimmun. 2021, 4, 100083. [Google Scholar] [CrossRef]
- Baratella, E.; Bussani, R.; Zanconati, F.; Marrocchio, C.; Fabiola, G.; Braga, L.; Maiocchi, S.; Berlot, G.; Volpe, M.C.; Moro, E.; et al. Radiological-Pathological Signatures of Patients with COVID-19-Related Pneumomediastinum: Is There a Role for the Sonic Hedgehog and Wnt5a Pathways? ERJ Open Res. 2021, 7, 00346–02021. [Google Scholar] [CrossRef]
- Sutkowska, E.; Stanek, A.; Madziarska, K.; Jakubiak, G.K.; Sokołowski, J.; Madziarski, M.; Sutkowska-Stępień, K.; Biernat, K.; Mazurek, J.; Borowkow-Bulek, A.; et al. Physical Activity Modifies the Severity of COVID-19 in Hospitalized Patients—Observational Study. J. Clin. Med. 2023, 12, 4046. [Google Scholar] [CrossRef]
- Soltanieh, S.; Salavatizadeh, M.; Ghazanfari, T.; Jahromi, S.R.; Yari, Z.; Mansournia, M.A.; Nazemipour, M.; Kheradmand, J.A.; Ardestani, S.K.; Karimi, S.; et al. Plant-Based Diet and COVID-19 Severity: Results from a Cross-Sectional Study. BMJ Nutr. Prev. Health 2023, 6, 182–187. [Google Scholar] [CrossRef]
- Zacarias, L.C.; Torres, D.M.; Magalhães, S.C.; Sobreira-Neto, M.A.; Leite, C.F. Is Obstructive Sleep Apnea Associated with Higher COVID-19 Severity? Sleep Sci. 2024, 17, e304–e309. [Google Scholar] [CrossRef]
- Matthay, M.A.; Arabi, Y.; Arroliga, A.C.; Bernard, G.; Bersten, A.D.; Brochard, L.J.; Calfee, C.S.; Combes, A.; Daniel, B.M.; Ferguson, N.D.; et al. A New Global Definition of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2024, 209, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Ospina-Tascón, G.A.; Damiani, L.P.; Estenssoro, E.; Dubin, A.; Hurtado, J.; Friedman, G.; Castro, R.; Alegría, L.; Teboul, J.-L.; et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019, 321, 654–664. [Google Scholar] [CrossRef]
- Rustecki, B.; Rustecka, A.; Kalicki, B.; Ring, E.F.J.; Kwasiborski, P.; Truszczyński, A.; Jung, A. A Study of Heat Loss in Patients Undergoing General Anesthesia Warmed with a Heated Mattress with Esophageal Temperature Monitoring Compared to Facial Infrared Thermography. J. Med. Imaging Health Inform. 2016, 6, 141–145. [Google Scholar] [CrossRef]
- Unal, I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput. Math. Methods Med. 2017, 2017, e3762651. [Google Scholar] [CrossRef]
- Kattan, E.; Hernández, G.; Ospina-Tascón, G.; Valenzuela, E.D.; Bakker, J.; Castro, R.; ANDROMEDA-SHOCK Study Investigators and the Latin America Intensive Care Network (LIVEN). A Lactate-Targeted Resuscitation Strategy May Be Associated with Higher Mortality in Patients with Septic Shock and Normal Capillary Refill Time: A Post Hoc Analysis of the ANDROMEDA-SHOCK Study. Ann. Intensive Care 2020, 10, 114. [Google Scholar] [CrossRef]
- Castro, R.; Kattan, E.; Ferri, G.; Pairumani, R.; Valenzuela, E.D.; Alegría, L.; Oviedo, V.; Pavez, N.; Soto, D.; Vera, M.; et al. Effects of Capillary Refill Time-vs. Lactate-Targeted Fluid Resuscitation on Regional, Microcirculatory and Hypoxia-Related Perfusion Parameters in Septic Shock: A Randomized Controlled Trial. Ann. Intensive Care 2020, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Hernandez, G. Can Peripheral Skin Perfusion Be Used to Assess Organ Perfusion and Guide Resuscitation Interventions? Front. Med. 2020, 7, 291. [Google Scholar] [CrossRef] [PubMed]
- Ait-Oufella, H.; Bige, N.; Boelle, P.Y.; Pichereau, C.; Alves, M.; Bertinchamp, R.; Baudel, J.L.; Galbois, A.; Maury, E.; Guidet, B. Capillary Refill Time Exploration during Septic Shock. Intensive Care Med. 2014, 40, 958–964. [Google Scholar] [CrossRef]
- Lima, A.; Jansen, T.C.; van Bommel, J.; Ince, C.; Bakker, J. The Prognostic Value of the Subjective Assessment of Peripheral Perfusion in Critically Ill Patients. Crit. Care Med. 2009, 37, 934–938. [Google Scholar] [CrossRef]
- Hernández, G.; Castro, R.; Bakker, J. Capillary Refill Time: The Missing Link between Macrocirculation and Microcirculation in Septic Shock? J. Thorac. Dis. 2020, 12, 1127–1129. [Google Scholar] [CrossRef]
- Brunauer, A.; Koköfer, A.; Bataar, O.; Gradwohl-Matis, I.; Dankl, D.; Bakker, J.; Dünser, M.W. Changes in Peripheral Perfusion Relate to Visceral Organ Perfusion in Early Septic Shock: A Pilot Study. J. Crit. Care 2016, 35, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, F.G. Physiopathology of Shock. J. Emergencies Trauma Shock 2011, 4, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moore, F.A.; Bellomo, R. Acute Kidney Injury, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 809–817. [Google Scholar]
- Bouchard, J.; Acharya, A.; Cerda, J.; Maccariello, E.R.; Madarasu, R.C.; Tolwani, A.J.; Liang, X.; Fu, P.; Liu, Z.-H.; Mehta, R.L. A Prospective International Multicenter Study of AKI in the Intensive Care Unit. Clin. J. Am. Soc. Nephrol. 2015, 10, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Lubas, A.; Kade, G. Imaging Methods in Quantitative Assessment of Renal Perfusion. Lekarz Wojskowy, Tom 88, Nr 4, 403-407; 2010.—Szukaj w Google. Available online: https://lekarzwojskowy.wim.mil.pl/Numer-4-2010,10661 (accessed on 8 December 2024).
- Post, E.H.; Kellum, J.A.; Bellomo, R.; Vincent, J.-L. Renal Perfusion in Sepsis: From Macro- to Microcirculation. Kidney Int. 2017, 91, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, T.; Liu, Z. Sepsis-Associated Acute Kidney Injury. Intensive Care Res. 2023, 3, 251–258. [Google Scholar] [CrossRef]
- Dyson, A.; Bezemer, R.; Legrand, M.; Balestra, G.; Singer, M.; Ince, C. Microvascular and Interstitial Oxygen Tension in the Renal Cortex and Medulla Studied in a 4-h Rat Model of LPS-Induced Endotoxemia. Shock 2011, 36, 83–89. [Google Scholar] [CrossRef]
- Petersen, L.J.; Petersen, J.R.; Talleruphuus, U.; Ladefoged, S.D.; Mehlsen, J.; Jensen, H.A. The Pulsatility Index and the Resistive Index in Renal Arteries. Associations with Long-Term Progression in Chronic Renal Failure. Nephrol. Dial. Transplant. 1997, 12, 1376–1380. [Google Scholar] [CrossRef]
- Gutowski, M.; Klimkiewicz, J.; Rustecki, B.; Michałowski, A.; Paryż, K.; Lubas, A. Effect of Respiratory Failure on Peripheral and Organ Perfusion Markers in Severe COVID-19: A Prospective Cohort Study. J. Clin. Med. 2024, 13, 469. [Google Scholar] [CrossRef]
- Lubas, A.; Ryczek, R.; Kade, G.; Smoszna, J.; Niemczyk, S. Impact of Cardiovascular Organ Damage on Cortical Renal Perfusion in Patients with Chronic Renal Failure. BioMed Res. Int. 2013, 2013, 137868. [Google Scholar] [CrossRef]
- Lubas, A.; Ryczek, R.; Maliborski, A.; Dyrla, P.; Niemczyk, L.; Niemczyk, S. Left Ventricular Strain and Relaxation Are Independently Associated with Renal Cortical Perfusion in Hypertensive Patients. Adv. Exp. Med. Biol. 2019, 1133, 1–8. [Google Scholar] [CrossRef]
- Saracyn, M.; Durma, A.D.; Bober, B.; Lubas, A.; Kołodziej, M.; Kapusta, W.; Dmochowska, B.; Kamiński, G. Renal Disturbances during and after Radioligand Therapy of Neuroendocrine Tumors—Extended Analysis of Potential Acute and Chronic Complications. Int. J. Mol. Sci. 2023, 24, 7508. [Google Scholar] [CrossRef] [PubMed]
- Lubas, A.; Grzywacz, A.; Niemczyk, S.; Kamiński, G.; Saracyn, M. Renal Cortical Perfusion Estimated in Color Doppler Dynamic Tissue Perfusion Measurement in Patients Treated with Levothyroxine Following Total Thyroidectomy for Resectable Thyroid Cancer Is Independently Associated with Free Thyroxine: A Single-Center Prospective Study. Med. Sci. Monit. 2021, 27, e932096. [Google Scholar] [CrossRef] [PubMed]
- Joly, H.R.; Weil, M.H. Temperature of the Great Toe as an Indication of the Severity of Shock. Circulation 1969, 39, 131–138. [Google Scholar] [CrossRef]
- Bourcier, S.; Pichereau, C.; Boelle, P.-Y.; Nemlaghi, S.; Dubée, V.; Lejour, G.; Baudel, J.-L.; Galbois, A.; Lavillegrand, J.-R.; Bigé, N.; et al. Toe-to-Room Temperature Gradient Correlates with Tissue Perfusion and Predicts Outcome in Selected Critically Ill Patients with Severe Infections. Ann. Intensive Care 2016, 6, 63. [Google Scholar] [CrossRef]
- Houwink, A.P.I.; Rijkenberg, S.; Bosman, R.J.; van der Voort, P.H.J. The Association between Lactate, Mean Arterial Pressure, Central Venous Oxygen Saturation and Peripheral Temperature and Mortality in Severe Sepsis: A Retrospective Cohort Analysis. Crit. Care 2016, 20, 56. [Google Scholar] [CrossRef]
- Boerma, E.C.; Kuiper, M.A.; Kingma, W.P.; Egbers, P.H.; Gerritsen, R.T.; Ince, C. Disparity between Skin Perfusion and Sublingual Microcirculatory Alterations in Severe Sepsis and Septic Shock: A Prospective Observational Study. Intensive Care Med. 2008, 34, 1294–1298. [Google Scholar] [CrossRef][Green Version]
- Hiemstra, B.; Koster, G.; Wiersema, R.; Hummel, Y.M.; van der Harst, P.; Snieder, H.; Eck, R.J.; Kaufmann, T.; Scheeren, T.W.L.; Perner, A.; et al. The Diagnostic Accuracy of Clinical Examination for Estimating Cardiac Index in Critically Ill Patients: The Simple Intensive Care Studies-I. Intensive Care Med. 2019, 45, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Lara, B.; Enberg, L.; Ortega, M.; Leon, P.; Kripper, C.; Aguilera, P.; Kattan, E.; Castro, R.; Bakker, J.; Hernandez, G. Capillary Refill Time during Fluid Resuscitation in Patients with Sepsis-Related Hyperlactatemia at the Emergency Department Is Related to Mortality. PLoS ONE 2017, 12, e0188548. [Google Scholar] [CrossRef]
- Fernández-Sarmiento, J.; Lamprea, S.; Barrera, S.; Acevedo, L.; Duque, C.; Trujillo, M.; Aguirre, V.; Jimenez, C. The Association between Prolonged Capillary Refill Time and Microcirculation Changes in Children with Sepsis. BMC Pediatr. 2024, 24, 68. [Google Scholar] [CrossRef]
- Watchorn, J.; Huang, D.Y.; Joslin, J.; Bramham, K.; Hutchings, S.D. Critically Ill COVID-19 Patients with Acute Kidney Injury Have Reduced Renal Blood Flow and Perfusion Despite Preserved Cardiac Function: A Case-Control Study Using Contrast-Enhanced Ultrasound. Shock 2021, 55, 479–487. [Google Scholar] [CrossRef]
- Watchorn, J.; Huang, D.; Bramham, K.; Hutchings, S. Decreased Renal Cortical Perfusion, Independent of Changes in Renal Blood Flow and Sublingual Microcirculatory Impairment, Is Associated with the Severity of Acute Kidney Injury in Patients with Septic Shock. Crit. Care 2022, 26, 261. [Google Scholar] [CrossRef] [PubMed]



| Variable | n Median (Mean) | % IQR (±SD) | |
|---|---|---|---|
| Gender | Male | 62 | 60.8 |
| Female | 40 | 39.2 | |
| Ward Type | Intensive Care Unit | 34 | 33.3 |
| High-Dependency Unit | 68 | 66.7 | |
| Deterioration | 40 | 39.2 | |
| HFNO/NIV | 17 | 16.7 | |
| Invasive Mechanical Ventilation | 33 | 32.4 | |
| Death | 24 | 23.5 | |
| Comorbidity | Malignancy | 11 | 10.8 |
| Obesity | 14 | 13.7 | |
| Chronic Kidney Disease | 5 | 4.9 | |
| Coronary Artery Disease | 7 | 6.9 | |
| Heart Failure | 6 | 5.9 | |
| Myocardial infarction | 2 | 2.0 | |
| Atrial fibrillation | 7 | 6.9 | |
| Hypertension | 32 | 31.4 | |
| Diabetes | 19 | 18.6 | |
| Asthma | 7 | 6.9 | |
| Chronic Obstructive Pulmonary Disease | 5 | 4.9 | |
| Smoker | 3 | 2.9 | |
| ARDS | No ARDS (PaO2/FiO2 > 300) | 31 | 30.4 |
| MILD (PaO2/FiO2 300 to 200) | 22 | 21.6 | |
| MODERATE (PaO2/FiO2 200 to 100) | 25 | 24.5 | |
| SEVERE (PaO2/FiO2 < 100) | 24 | 23.5 | |
| Age [years] | 61 | 21 | |
| WBC [1 × 109/L] | 7.62 | 6.13 | |
| Hgb [g/dL] | 13.6 | 3.2 | |
| PLT [1 × 109/L] | 186 | 111 | |
| CRP [mg/dL] | 7.4 | 10.9 | |
| Creatinine [mg/dL] | 0.9 | 0.4 | |
| Urea [mg/dL] | 36 | 27 | |
| AST [U/L] | 45 | 35 | |
| ALT [U/L] | 35 | 25.5 | |
| CK [U/L] | 168 | 386 | |
| LDH [U/L] | 394 | 251 | |
| SBP [mmHg] | 129 | 21.5 | |
| DBP [mmHg] | (79) | (12) | |
| Oxygenation Ratio | 211.5 | 221 | |
| Saturation [%] | 95 | 5 | |
| CRT [s] | 3 | 1.75 | |
| FIT [°C] | 32.35 | 4 | |
| RCP [cm/s] | 0.148 | 0.221 | |
| Non-Deterioration n= 62 | Deterioration n = 40 | p-Value | Survived n = 88 | Deceased n = 24 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (MEAN) | IQR (±SD) | Median (MEAN) | IQR (±SD) | Median (MEAN) | IQR (±SD) | Median (MEAN) | IQR (±SD) | |||
| Age [years] | 58.0 | 13.4 | 63.5 | 17.5 | 0.359 | 57.5 | 13.5 | 65.0 | 13.0 | 0.099 |
| WBC [1 × 109/L] | 6.05 | 5.76 | 8.61 | 5.61 | 0.005 | 6.48 | 5.21 | 9.37 | 7.15 | 0.124 |
| Hgb [g/dL] | 13.6 | 3.5 | (13.2) | (1.96) | 0.704 | 13.9 | 3.3 | 12.9 | 2 | 0.044 |
| PLT [1 × 109/L] | 177.5 | 123.0 | 191.0 | 85.5 | 0.550 | 186 | 121 | 185 | 94 | 0.940 |
| CRP [mg/dL] | 5.3 | 8.6 | 11.6 | 11.2 | 0.002 | 6.4 | 0.3 | (11.6) | (7.6) | 0.057 |
| Creatinine [mg/dL] | 0.8 | 0.3 | 1.0 | 0.6 | 0.195 | 0.9 | 0.4 | 0.95 | 0.07 | 0.829 |
| Urea [mg/dL] | 33.0 | 22.5 | 42.0 | 32.5 | 0.055 | 35 | 21 | 44.5 | 44.5 | 0.086 |
| AST [U/L] | 36.5 | 29.5 | 58 | 37 | 0.005 | 40 | 37 | 48.5 | 32.0 | 0.781 |
| ALT [U/L] | 31.5 | 25.0 | 38 | 24 | 0.176 | 33 | 26 | 39.5 | 22.0 | 0.564 |
| CK [U/L] | 140 | 355 | 243 | 475 | 0.482 | 182.0 | 447.5 | 166.0 | 345.0 | 0.466 |
| LDH [U/L] | (350.8) | (147.0) | 547.5 | 194 | <0.001 | 367 | 214 | (526.2) | (166.7) | 0.002 |
| SBP [mmHg] | 129 | 27.5 | (127) | (13) | 0.438 | 130 | 25 | 127 | 11 | 0.866 |
| DBP [mmHg] | (80) | (14) | (77) | (10) | 0.242 | (79) | (13) | (77) | (11) | 0.469 |
| Oxygenation Ratio | (272.8) | (98.4) | 100 | 58 | <0.001 | 269 | 212 | 92.5 | 53 | <0.001 |
| Saturation [%] | (94) | (3) | (93) | (3) | 0.198 | 95 | 4 | (93) | (3) | 0.421 |
| CRT [s] | 3.0 | 1.25 | (4.0) | (1.4) | 0.005 | 3.0 | 1.5 | (4.3) | (1.5) | 0.003 |
| FIT [°C] | 31.8 | 3.9 | 33.0 | 4.9 | 0.350 | 31.9 | 4.0 | 33.2 | 5.2 | 0.806 |
| RCP [cm/s] | 0.187 | 0.281 | 0.061 | 0.136 | <0.001 | 0.181 | 0.259 | 0.055 | 0.113 | <0.001 |
| Deterioration | Death | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | ||||||||
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | |
| Saturation [%] | 0.863 | 0.743–1.003 | 0.055 | - | - | - | 0.888 | 0.752–1.049 | 0.161 | - | - | - |
| CRT [s] | 1.697 | 1.201–2.398 | 0.0027 | 1.825 | 1.227–2.714 | 0.003 | 1.814 | 1.244–2.644 | 0.002 | 1.910 | 1.249–2.921 | 0.003 |
| FIT [°C] | 1.000 | 0.884–1.132 | 0.997 | - | - | - | 0.971 | 0.845–1.116 | 0.677 | - | - | - |
| RCP [cm/s] | 0.002 | 0.000–0.080 | 0.0007 | 0.002 | 0.000–0.067 | 0.001 | 0.001 | 0.000–0.110 | 0.004 | 0.001 | 0.000–0.108 | 0.004 |
| Prediction Target | RCP Cut-Off Value [cm/s] | Method | Sensitivity [%] | Specificity [%] |
|---|---|---|---|---|
| Deterioration | 0.127 | EH | 70.0 | 70.5 |
| 0.149 | Youden | 75.0 | 65.6 | |
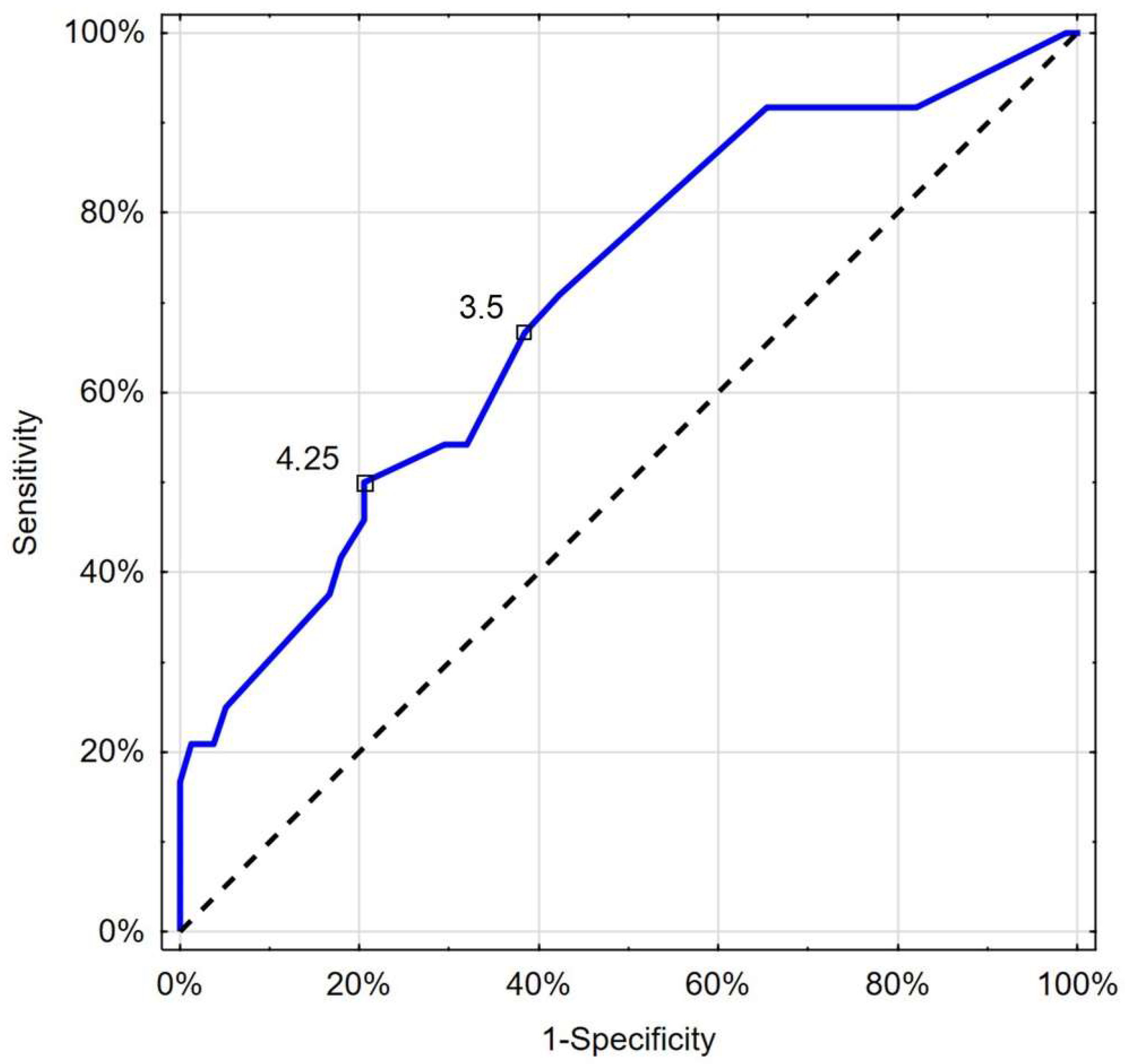
| Mortality | 0.112 | EH | 66.7 | 65.4 |
| 0.163 | Youden | 87.5 | 55.1 |
| Prediction Target | CRT Cut-Off Value [s] | Method | Sensitivity [%] | Specificity [%] |
|---|---|---|---|---|
| Deterioration | 3.25 | Youden, EH | 65.0 | 62.3 |
| Mortality | 3.5 | EH | 66.7 | 61.5 |
| 4.25 | Youden | 50 | 79.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutowski, M.; Klimkiewicz, J.; Rustecki, B.; Michałowski, A.; Skalec, T.; Lubas, A. Peripheral and Organ Perfusion’s Role in Prognosis of Disease Severity and Mortality in Severe COVID-19 Patients: Prospective Cohort Study. J. Clin. Med. 2024, 13, 7520. https://doi.org/10.3390/jcm13247520
Gutowski M, Klimkiewicz J, Rustecki B, Michałowski A, Skalec T, Lubas A. Peripheral and Organ Perfusion’s Role in Prognosis of Disease Severity and Mortality in Severe COVID-19 Patients: Prospective Cohort Study. Journal of Clinical Medicine. 2024; 13(24):7520. https://doi.org/10.3390/jcm13247520
Chicago/Turabian StyleGutowski, Mateusz, Jakub Klimkiewicz, Bartosz Rustecki, Andrzej Michałowski, Tomasz Skalec, and Arkadiusz Lubas. 2024. "Peripheral and Organ Perfusion’s Role in Prognosis of Disease Severity and Mortality in Severe COVID-19 Patients: Prospective Cohort Study" Journal of Clinical Medicine 13, no. 24: 7520. https://doi.org/10.3390/jcm13247520
APA StyleGutowski, M., Klimkiewicz, J., Rustecki, B., Michałowski, A., Skalec, T., & Lubas, A. (2024). Peripheral and Organ Perfusion’s Role in Prognosis of Disease Severity and Mortality in Severe COVID-19 Patients: Prospective Cohort Study. Journal of Clinical Medicine, 13(24), 7520. https://doi.org/10.3390/jcm13247520








