Abstract
Endometriosis is an inflammatory chronic condition associated with nociceptive, neuropathic, and nociplastic pain. Central sensitization (CS) is the primary nociplastic pain mechanism. However, there are currently no standardized methods for detecting CS or nociplastic pain. This review aims to identify available tools for characterizing CS/nociplastic pain in endometriosis-related chronic pelvic pain. Following the PRISMA-P protocol, MEDLINE, Embase, Scopus, and PsychINFO databases were searched on 23 April 2024, for the terms “endometriosis”, “central sensitization”, “nociplastic pain”, “widespread pain”, and “assessment tools”. Publications were selected if they mentioned tool(s) for detecting nociplastic pain or CS in endometriosis patients. Information was extracted on study demographics, assessment types, and the tools used for detection. Of the 379 citations retrieved, 30 papers met the inclusion criteria. When working to identify CS and nociplastic pain, fourteen studies exclusively used patient-reported questionnaires, six used quantitative sensory testing (QST), two used clinical assessments, and eight used multiple approaches combining patient-reported questionnaires and clinical assessment. This review illustrates the diversity of tools currently used to identify CS and nociplastic pain in endometriosis patients. Further research is needed to evaluate their validity and to standardize methods in order to improve the accuracy of nociplastic pain identification and guide treatment.
1. Introduction
The goal of this scoping review is to assess the state of the literature regarding nociplastic pain in endometriosis. In 2021, the International Association for the Study of Pain (IASP) updated their definitions and clinical criteria for musculoskeletal pain [1]. One of the aims of this update was to provide more precise definitions of 3 pain types: nociceptive, neuropathic, and nociplastic pain [2]. According to the IASP, nociceptive pain refers to “pain that arises from actual or threatened damage to non-neural tissue and is due to the activation of nociceptors” [1]. This differs from neuropathic pain, which is described as “pain caused by a lesion or disease of the somatosensory nervous system” [1,2]. Nociplastic pain is a descriptive term used to characterize the pain phenotype that could not be exclusively defined as nociceptive or neuropathic [3]. In the 2021 definition, the IASP defines nociplastic pain as “[p]ain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain” [1,2]. Clinically, nociplastic pain is defined by four patient-reported requirements:
- (1)
- A pain duration of at least 3 months;
- (2)
- A regional pain distribution (i.e., not discrete);
- (3)
- Pain that is not entirely explained by nociceptive or neuropathic mechanisms;
- (4)
- Signs of pain hypersensitivity [2].
If a patient meets all four of these criteria, they are considered to have “possible nociplastic pain” [1,2]. However, if a patient meets these criteria, has a history of hypersensitivity in the region of pain, and one or more of the following comorbidities—increased sensitivity to sound, light, and/or odors, sleep disturbance, fatigue, or cognitive problems—they are considered to have “probable nociplastic pain” [2].
Notably, there is a lack of clinical criteria for definite nociplastic pain. This uncertainty in the definition of nociplastic pain has resulted in variability in its clinical interpretation and identification. Patients with conditions such as fibromyalgia, irritable bowel syndrome, and bladder pain syndrome are often characterized as having nociplastic pain. This can be attributed to heightened pain perception, which results in symptoms of chronic abdominopelvic pain and widespread musculoskeletal pain [3,4,5]. Chronic pain conditions tend to be highly heterogenous, with some patients experiencing more than one of these three types of pain [6]. To add further complexity, nociceptive inputs from peripheral tissues and neuropathic pain can also influence the development and persistence of central nervous system (CNS) sensitization [7,8].
Central nervous system sensitization (central sensitization or CS) describes the “amplification of neural signaling within the CNS that elicits pain hypersensitivity” and is the primary underlying mechanism in nociplastic pain [3,9]. CS can develop from different underlying causes. In some cases, peripheral damage can lead to the sensitization of the CNS in a way that persists over time but can be resolved with interventions that treat the peripheral cause. In other cases, CS is driven by a central component that is independent of peripheral damage, and thus does not respond to treatments that target this damage [7]. The peripheral sensitization of local nociceptors, which describes increased responsiveness at the primary site of pain (called primary hyperalgesia), can also occur, and this may lead to the development of, or exist alongside, CS [10,11]. However, while peripheral sensitization is a local pathology, one indicator of CS is an increased sensitivity to stimuli outside of the area where the primary injury or damage occurred (secondary hyperalgesia) [7,11]. Other clinical manifestations of CS include regional allodynia, defined as pain due to a normally non-painful stimulus [1,12]. CS has also been linked to psychological factors such as anxiety, panic attacks, and depression [13]. While some conditions, such as fibromyalgia, have been studied in relation to CS and treatment outcomes, others are still lacking research [7].
Endometriosis is an inflammatory chronic pain condition characterized by the growth of endometrial-like lesions outside of the uterus [14]. These lesions are predominantly found in the abdominopelvic organs and peritoneal cavity [15,16]. These endometrial-like tissues are hormonally regulated (estrogen-dependent), with a component of local inflammation contributing to nociceptive pain [15]. Increasing evidence also supports the role of multifactorial contributors to pain such as abdominal wall pain, pelvic floor myalgia, and higher scores on the pain catastrophizing scale in individuals with endometriosis [17]. Endometriosis-related pain can take several forms, including dysmenorrhea (painful menstruation), dyschezia (painful bowel movements), dysuria (painful urination), dyspareunia (painful sexual activity), and cyclical and noncyclic pelvic pain [18]. Endometriosis is known to be heterogenous, and patients often report a variety of outcomes for the same treatment [19]. Nociplastic pain may provide an explanation for some of this heterogeneity. However, there are currently no known standardized methods for characterizing nociplastic pain in endometriosis-specific populations. This gap presents a limitation in treating endometriosis-related chronic pelvic pain. As such, this review intends to elucidate the current tools that are being used to identify nociplastic pain in endometriosis pain populations and assess the quality of evidence in support of their use.
Historically, endometriosis-related pain was considered a result of local inflammation and irritation from lesions or nodules and was thus conceptualized as predominantly nociceptive [20]. Recent studies suggest nociceptive, neuropathic, and nociplastic mechanisms could all be associated with endometriosis-related pain [18]. Endometriosis-related neuropathic pain results from damage to the nerves surrounding endometriosis lesions or nodules [19]. CS and nociplastic pain are clinically relevant, as studies show that endometriosis patients with more symptoms of CS are more likely to experience worse pelvic pain symptoms, independent of endometriosis type or previous surgery [21]. In fact, endometriosis patients with clinical indicators or comorbidities commonly associated with CS have a worse quality of life and higher pain scores for chronic pelvic pain, deep dyspareunia, dyschezia, and back pain following surgical treatment for endometriosis [10,22,23]. Thus, the ability to clinically assess for nociplastic pain characteristics and the presence of CS is imperative. Moreover, the improved classification of nociplastic pain and CS in clinical settings could enable the implementation of more personalized pain treatments, targeting specific pain phenotypes and their multifactorial influences in people with endometriosis-related pain [7].
We conducted a scoping review to determine the scope of available tools and resources for characterizing CS in endometriosis patients and assess their quality.
2. Scoping Review: Identifying Nociplastic Pain in Endometriosis
2.1. Rationale
Identifying patients with endometriosis who present with symptoms of CS, and consequently, nociplastic pain, is paramount to improving their quality of life and pain outcomes. While there are various questionnaires and assessments clinically used to detect nociceptive and neuropathic pain in endometriosis populations, there are currently no standardized methods of detecting nociplastic pain. This discrepancy may stem from the relative novelty of the nociplastic pain phenotype in the research community [9]. Nociplastic pain and CS exemplify neuroplasticity, occurring at cellular and functional levels, and often present unclear clinical features [2,8,24]. Combined with the subjective nature of pain and the frequently heterogeneous clinical presentations that overlap with psychological factors such as anxiety and depression, capturing instances of CS in a clinical setting is challenging [25,26]. Fortunately, there are an increasing number of methods for detecting and quantifying the degree of CS that may act as a proxy for nociplastic pain in chronic pain populations. For example, the Central Sensitization Inventory (CSI) has been validated and used to assess CS in endometriosis populations [27]. It provides an estimate of the impact of CS symptoms on a scale from 0 to 100. The CSI is able to distinguish between patients with chronic overlapping pain conditions (COPCs) and those with chronic pain conditions without a central pain component, as well as between patients with COPCs and healthy controls [28,29]. Additionally, in an endometriosis population, a 40-point cutoff has shown clinical significance [27], which the CSI correlating with worse pain outcomes following endometriosis surgery [23]. However, the CSI is only a proxy and may primarily be associated with psychological factors such as stress, depression, and anxiety [26]. Nonetheless, having a standardized and widely accepted clinical tool that can efficiently and effectively identify these patients is essential for treating endometriosis and managing pain.
Quantitative sensory testing (QST) is a semi-objective test that is frequently used to detect modulations in somatosensory processing in people with CS through a battery of sensory and pain threshold tests [30]. Another method includes using functional magnetic resonance imaging (fMRI) to detect changes in gray matter in areas of the brain related to pain sensation [31]. However, both these methods are resource-extensive and not feasible for use in clinical settings. Furthermore, ongoing research is needed to further validate these methods as an indicator of CS in endometriosis populations. As a result, there may be a subset of people living with endometriosis who also experience CS and nociplastic pain that remains unrecognized and untreated. In fact, within 5 years of standard endometriosis treatment, over 40% of patients will experience persistent or recurrent pain [32]. This scoping review investigates the range of questionnaires, tools, and clinical assessments being used to detect CS or nociplastic pain in populations with endometriosis-related pain. This review will provide an overview of current tools and methods of assessment being used to detect CS and nociplastic pain in an endometriosis pain population, along with supporting evidence. The findings from this review will inform future research seeking to determines the optimal clinical approach for identifying CS and nociplastic pain in endometriosis.
2.2. Methods
This scoping review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis extension for Scoping Review (PRISMA-ScR) (Table S2 in Supplementary Materials) [33]. Additionally, the protocol was created using the Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (PRISMA-P) and was revised by the research team. The final protocol was registered on the Open Science Framework (OSF) (https://www.cos.io/ accessed on 12 April 2024) on 12 April 2024 and can be found at (https://doi.org/10.17605/OSF.IO/S358T) [34,35].
With the aid of a health science librarian, we conducted a systematic search of MEDLINE, Embase, Scopus, and PsychINFO on 23 April 2024. We used the following key terms: “endometriosis”, “central sensitization”, “nociplastic pain”, “widespread pain”, and “assessment tools”. The final search strategy used for MEDLINE can be found in Table S1 in the Supplementary Materials. Given the novelty of nociplastic pain in IASP’s characterization of pain, we did not apply any time limits. The final search was uploaded to Veritas Health Innovation (Melbourne, Australia) Covidence systematic review software (www.covidence.org accessed on 23 April 2024), an online platform that facilitates the production of systematic and other literature reviews [36]. We excluded published abstracts, editorial letters, preclinical studies, and studies that were not written in English.
Abstract and full-text text screening were conducted in duplicate by two independent reviewers (AG and NM) according to eligibility criteria outlined in the review protocol. All publications that explicitly mentioned the use of a tool to detect nociplastic pain or central sensitization in people with endometriosis-related pain were included. All discrepancies were resolved by a third party (EG) or discussion. Before screening, AG, NM, and EG reviewed five publications together to ensure a clear understanding of the eligibility criteria. The data collection form was jointly created by members of the team in order to determine variables that were meaningful for extraction. Data extraction was conducted by AG and NM, who used information on study demographics (i.e., country of origin, author, study type), assessment type (i.e., patient-reported, clinician-reported, etc.), the tool used for detecting nociplastic pain or central sensitization, and the study’s reasoning or psychometric properties if they were available.
We conducted a narrative synthesis of all included studies, which were grouped according to assessment type and the tool used. When we encountered a systematic review, we noted the type of assessment used to identify central sensitization or nociplastic pain in each study.
2.3. Results
A total of 379 citations were retrieved from the databases. After removing 148 duplicates, 231 citations underwent title and abstract screening. Of these, 67 proceeded to full-text screening and were assessed for eligibility. All told, 37 studies were excluded for the following reasons: 1 was a letter to the editor, 11 were published abstracts, 3 did not have the full text available, 1 did not have an endometriosis population, 9 did not mention an assessment tool or questionnaire, and 12 did not focus on central sensitization (CS), nociplastic pain, or widespread pain. In total, 30 papers were included in the final review and underwent data extraction. The PRISMA flow diagram for study inclusion is shown in Figure 1. Of the 30 papers, 21 (70%) were primary research articles including cross-sectional (n = 12), cohort (n = 7), case–control (n = 1), and cross-over (n = 1) studies, where the method used to identify sample endometriosis participants with CS or nociplastic pain was clearly stated. The remaining 9 papers were reviews that explicitly stated a tool or assessment method used to identify CS, nociplastic pain, or widespread pain.
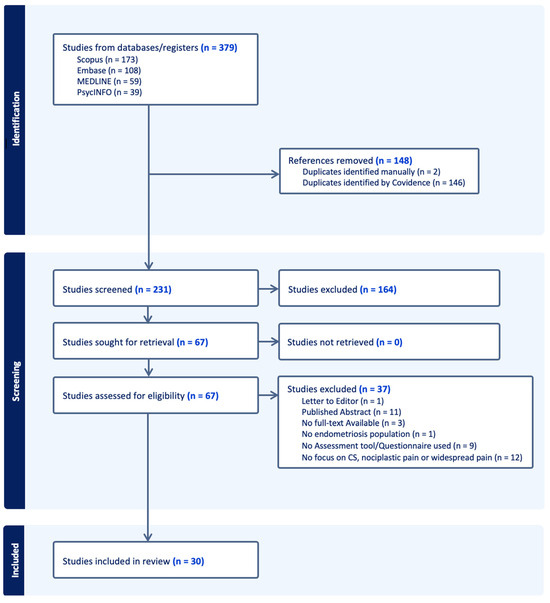
Figure 1.
PRISMA flow diagram.
Most studies were from the United States (n = 8), Italy (n = 6), and Canada (n = 4). The remaining countries, each with fewer than 4 studies, were Australia, Brazil, China, Denmark, France, New Zealand, Spain, Sweden, and the United Kingdom. All studies referenced an adult population experiencing endometriosis-related chronic pelvic pain. A complete list of included papers and their general demographics is shown in Table 1. Approximately, half (14/30) of the studies reported the exclusive use of patient-reported questionnaires to identify CS and nociplastic pain. Conversely, 6 papers exclusively referenced the use of semi-objective assessments like QST to identify CS and nociplastic pain, with the pressure pain threshold (PPT) consistently used across all studies. Only 2 studies exclusively mentioned clinical assessments as a method of detecting CS: one focused on identifying abdominopelvic trigger points, while the other used the convergence pelvic and perineal (PP) criteria. Lastly, 27% (8/30) of the papers used mixed methods of identification. This involved the collective use of patient-reported questionnaires, including the PainDETECT questionnaire, the McGill questionnaire, the brief pain inventory, and the visual analogue scale (VAS), along with QST modalities and clinical assessments. A summary of the assessments identified in all 30 papers is shown in Table 2.

Table 1.
Study characteristics.

Table 2.
Summary of assessments/tools for nociplastic pain in endometriosis.
2.4. Discussion
2.4.1. Patient-Reported Questionnaires
Given the complex pathophysiology of nociplastic pain and the mechanisms underlying CS development, detection through subjective assessments, such as questionnaires, is challenging. However, with increasing amounts of research identifying conditions with shared symptomatology, such as in chronic overlapping pain conditions (COPCs), questionnaire tools designed to differentiate based on CS-related symptoms are becoming more prevalent in clinical practice [61].
Most studies in this review exclusively used independent patient-reported questionnaires as proxies of centralized pain. The American College of Rheumatology (ACR) 2011 fibromyalgia survey score (FSS) was referenced as the sole indicator of CS [21,46,47,54], or was used alongside the CSI [56,59]. The fibromyalgia questionnaire assesses widespread pain using a 19-point body map and a 12-point symptom severity scale to give a total score out of 31. A score of ≥13/31 indicates fibromyalgia; however, recent studies suggest using the score as a continuous measure to assess the degree of CS present [46]. As-Sanie et al. (2021) [46] found a 27% increase in the odds of post-hysterectomy persistent pelvic pain with every 1-point increase in the pre-operative FSS. Endometriosis and fibromyalgia are both on the National Institutes of Health Pain Consortium list of COPCs. These conditions frequently co-occur and are postulated to share CS as their underlying mechanism of persistent pain [46,61]. Therefore, using the FSS as an indicator of CS—and by extension, the nociplastic pain phenotype—is promising in clinical settings involving both endometriosis and fibromyalgia. However, there are limited studies focusing exclusively on the use of the FSS in an endometriosis population.
The CSI was another questionnaire that was frequently used to identify CS in clinical settings [13,23,27,45,50,51,52,56,57,58,59,60]. This questionnaire has 25 items and focuses on the psychophysiological symptoms of daily living that indicate CS, such as sensitivity to light and restlessness, as well as more localized pain in areas such as the pelvis [62]. It scores patients from 0 to 100, with higher scores indicating a greater degree of CS [62]. Unlike the FSS, the CSI has a validated cut-off point (≥40/100) that indicates CS symptoms in an endometriosis pain population [27]. Orr et al. (2022, 2023) [23,27] demonstrated that endometriosis patients who scored above the 40-point cut-off on the CSI also have poorer patient-reported pain outcomes, indicating its potential use as a screening tool for pain outcomes. Similarly, using the CSI, Raimondo et al. (2023) [13] found that individuals with CS also had higher rates of first-line hormonal treatment failure compared to those without CS. Furthermore, in people with endometriosis, the CSI score was significantly associated with a number of COPCs, which in this paper were part of a wider category of termed central sensitization syndromes and pelvic pain-related comorbidities [23]. These syndromes/comorbidities have been shown to be independent predictors of quality of life following endometriosis surgery [22].
However, there has been speculation as to whether the CSI measures symptoms related to CS or simply the presence of a hypervigilant psychological state related to stress [59]. This theory is further supported by the strong association between CSI scores and psychological markers, like pain catastrophizing, depression, and anxiety [26]. Alternatively, the CSI has also shown significant correlations with QST measures like the pressure pain threshold (PPT) (r = −0.25 [95% CI: −0.28 to −0.21]) in chronic pain populations [63]. Therefore, there is a need for more research that explicitly looks at the relationship between the CSI and semi-objective assessments of somatosensory changes, like QST, in an endometriosis population.
Some studies noted the use of the PainDETECT questionnaire, a 7-item self-reported form asking patients to rate the quality, severity, and characteristics of their pain in order to assign a total score from −1 to 38 [64,65]. This questionnaire was initially developed in association with the German Research Network on Neuropathic Pain (DFNS) (Munich, Germany) to aid clinicians in detecting neuropathic-like pain in chronic lower back pain patients. It was found to have sensitivity and specificity values of 84%, with a cut-off point of 19/31 indicating a likely “neuropathic-like” component [64,66]. Since then, it has been used in other chronic-pain related studies including a cross-sectional study looking at women with endometriosis-associated pain, where the PainDETECT cut-off scores are used to group participants into those with nociceptive (≤12), mixed nociceptive and neuropathic pain (13–18), and neuropathic-like (≥19) pain; 40% were classified as neuropathic, 35% as mixed, and 25% as nociceptive [67]. They also showed that women classified as having “neuropathic-like” pain also reported more psychological distress (i.e., anxiety, depression, etc.) and higher pain intensity scores [67]. Moreover, there have been strong correlations between PainDETECT scores and FSSs (rho = 0.441, p < 0.001) [67]. Since fibromyalgia is considered a nociplastic pain condition, the strong association between the PainDETECT and FSS indicates that the PainDETECT questionnaire may have the ability to capture those with nociplastic pain. Within the same study, the Pain Sensitivity Questionnaire (PSQ) was also correlated with the FSS but was not found to have clinical significance despite previously showing positive correlations with experimental pain intensity rating in people with chronic pain [68] and endometriosis [69]. Whether the PSQ has clinical applications as a CS proxy in endometriosis populations is still to be determined.
Additionally, in a sample of women with endometriosis, bladder pain syndrome, and pelvic pain, Coxon et al. (2023) correlated the seven sensory-based questions from the PainDETECT questionnaire to QST modalities that measured the same stimulus–response relationship. The heat pain threshold (HPT) correlated significantly with the question “is cold or heat (bath water) in this area occasionally painful?” (r = 0.32, p = 0.032), the pressure pain threshold (PPT) correlated significantly with the question “does slight pressure in this area, e.g., with a finger, trigger pain?” (r =0.47, p < 0.001), and mechanical pain sensitivity (MPS) correlated significantly with the question “is light touching (clothing, a blanket) in this area painful?” (r = 0.38, p = 0.009) [4]. They also found that women in the endometriosis group described “painful attacks” as their most common sensory symptom [4].
Given its association with HPT, PPT, and MPS, as well as its moderate association with the FSS, the PainDETECT questionnaire may be a promising tool for classifying CS and the presence of nociplastic pain in endometriosis populations with mixed pain phenotypes. However, there are limited studies exclusively examining the neuropathic-like pain subgroup, which may include a subset with concurrent nociplastic pain and CS, using PainDETECT [67]. Therefore, additional investigation in an endometriosis pain population is needed to determine if the PainDETECT can distinguish those in the neuropathic pain group who exclusively have neuropathic pain from those with nociplastic pain features.
Lastly, Phan et al. (2021) [48] used a cut-off >9/75 painful sites on the brief pain inventory (BPI) body map as an identifier of widespread pain. The BPI was created by the Pain Research Group of the World Health Organization (WHO) Collaborating Centre for Symptom Evaluation in Cancer Care to assess pain intensity and daily interference with life in cancer patients [70]. Phan et al. (2021) [48] only noted the use of the BPI body map in collaboration with the PPT and myofascial trigger points. Its short form has been used in endometriosis-associated pain assessments and has indicated minimal clinically important differences (MCIDs) after treatment [71]. However, like the visual analogue scale (VAS) and Numerical Scale Rating (NRS), which have also shown MCID in endometriosis pain populations [71], there are limited studies specifically looking at the BPI map or score, and any association with CS and nociplastic pain.
It is important to note that the presence of CS in and of itself indicates altered CNS processing, and therefore, outside of their use as patient-reported outcome measures, simple NRS and VAS questionnaires asking patients to rate their general pain may not be reliable indicators of CS [9]. However, some studies have used a VAS in association with other questionnaires such as the McGill pain questionnaire to help identify widespread hyperalgesia [37].
2.4.2. Semi-Objective Assessments
Quantitative Sensory Testing (QST) is an objective measure of altered somatosensation and CS. Standardized by the German Research Network on Neuropathic Pain (DFNS) in 2006, QST assesses sensory loss or gain and identifies patterns of large- and small-nerve fiber dysfunction through 13 different modalities [72]. Although the DFNS recommends using the full QST protocol, most studies in this review did not. Only Coxon et al. (2023) [4] used all 13 DFNS modalities, along with the PainDETECT questionnaire, in their “Translational Research in Pelvic Pain” study. The DFNS modalities include 1) thermal tests such as heat and cold detection and pain thresholds (HPT, HDT, CPT, CDT), thermal sensory limen (TSL), and paradoxical heat sensation (PHS), and 2) mechanical tests such as mechanical detection threshold (MDT), mechanical pain threshold (MPT), stimulus–response function including mechanical pain sensitivity (MPS) and dynamic mechanical allodynia (DMA), the wind-up ratio (WUR), vibrational detection threshold (VDT), and pressure pain threshold (PPT) [4,30].
Furthermore, when all 13 modalities are assessed together, the scores from each modality can be entered into a non-deterministic clustering algorithm and used to group patients into different sensory profiles: (1) sensory loss; (2) mechanism hyperalgesia; (3) thermal hyperalgesia; or (4) healthy [73]. A key feature of distinguishing CS from peripheral sensitization with QST is the use of a primary site, usually the site of local pain for detecting peripheral sensitization, and the use of a heterotopic site away from the primary noxious stimulus for CS [30]. Additionally, the assessment of sensory changes must be compared using reference values for the body site, age, and sex to ensure proper comparisons can be made [30,72]. The assessment of whether reported QST was properly standardized using reference data was outside the scope of this review; however, this may be a further research interest for studies investigating the validity of QST results in endometriosis populations.
However, other methods of pain stimulation and assessment were used in the papers reviewed, such as the electrical pain threshold test (EPT), which involved the application of electrical pulses using the PainMatcher (Cefar Medical AB, Lund, Sweden), and ischemic pain tests (IPTs), which measure the pain response following a reduction in blood flow to a limb [39,74]. Although there may be similarities between these tests and the 13 DFNS tests, it is important to note that they measure different mechanisms of pain. For instance, the PPT, which was the most commonly referenced semi-objective assessment, can detect CS in deep tissue as a result of pressure application, while the ischemic pain test measures the pain threshold as a result of reduced blood flow and lactic acid build-up in the muscles [4,39,72]. While there is sufficient evidence supporting the connection between PPT and CS in women with endometriosis, studies tying ischemic pain to CS in women with endometriosis are limited [4,69,75,76].
Despite the standardization of the QST methodology by the DFNS, many studies describe alternative ways to record and measure modalities. For example, some use a pressure cuff [40] instead of an algometer [37,38,43,48,49,69] for PPT determination. Another example is using the 1st, 5th, and 10th mechanical stimulus ratings to determine moderately painful stimuli for WUR as an alternative to the DFNS recommendation of 256 mN [40,72]. Therefore, it appears that even in an endometriosis pain sensitivity research setting, there is no standard method or equipment used to conduct QST or any of the associated modalities, as many deviate from the DFNS recommendations. This makes it difficult to compare results across studies and to reproduce findings.
2.4.3. Clinician Assessments
Various physician-led clinical assessments were used to detect the presence of endometriosis-related sensitization. For instance, Aredo et al. (2017) [42] and Phan et al. (2021) [48] noted the use of a digital exam to identify active myofascial trigger points in the pelvic region as possible indicators of abdominopelvic tenderness and allodynia, which are proxies of sensitization. Somatosensory signaling from visceral and somatic organs sometimes converges on the same sensory neuron within the CNS [77] (pp. 175–177). This process is known as viscerosomatic convergence and provides an explanation for the pain experienced by some women with endometriosis [19,77]. However, this viscerosomatic neural interaction also explains how chronic visceral pain signals can sensitize afferent neurons in the spinal cord, resulting in symptoms of widespread hyperalgesia and allodynia [8,78]. Additionally, prolonged viscerosomatic convergence from pain signaling can lead to hypertrophy in skeletal muscles in the referred area, which can present itself as viscerosomatic reflexes like myofascial trigger points [79]. Aredo et al. (2017) [42] proposed examining the number and location of abdominopelvic myofascial trigger points, as well as the intensity rating of the pain elicited, as indicators of CS. Furthermore, some studies have linked the presence of active myofascial trigger points to sustained nociception and decreased pain thresholds following the removal of the initial noxious stimulus [8,79].
Following the examination, Aredo et al. (2017) [42] also conducted a semi-objective neuromusculoskeletal pain assessment of dermatome allodynia and hyperalgesia using a pinch-and-roll technique and a Wartenburg pinwheel, respectively. They also assessed myotome myofascial trigger points in regional and general muscles. However, although myofascial trigger points are frequently found in endometriosis patients and in those with other COPCs like irritable bowel syndrome, bladder pain syndrome, and vulvodynia, there is limited evidence supporting the belief that all endometriosis patients with these trigger points also have CS [12,80,81].
Alternatively, Cardaillac et al. (2023) used the convergence pelvic and perineal (PP) pain criteria, which were developed by an international panel of specialists to help facilitate CS detection in people with chronic pelvic pain [55,82]. The convergence PP criteria prompt clinicians to assess pelvic sensitivity and pain diffusion in the lower urinary and digestive tract, the genito-sexual tract, the mucocutaneous area, and the muscular system using a checklist of 10 items [82]. This clinical method has shown excellent specificity (87%) and sensitivity (95%), with a score ≥5/10 in people with pelvic perineal pain when compared to expert diagnosis, and has been used to identify sensitivity in various chronic pelvic pain populations [83].
3. Application to Endometriosis Clinical Care
In endometriosis, nociceptive pain arises from the endometriosis lesions themselves, which can be locally invasive, due in part to somatic driver mutations [84], and/or be associated with peripheral (local) neuroproliferation in the microenvironment. This is associated with the expression of inflammatory and neurotrophic factors by endometriosis-related and other cell types [84,85,86,87,88,89,90]. Although this suggests that there may be a correlation between these peripheral factors and pain symptoms, more sufficiently powered studies to investigate their influence on nociceptive pain are needed. As endometriosis is also an estrogen-dependent disease, nociceptive pain in endometriosis could be treated by hormonal suppression of the endometrial-like lesions themselves as well as the hypothalamic–pituitary–ovarian axis. In addition, the surgical excision of these endometrial-like lesions would be expected to improve nociceptive pain. Deeply invasive endometrial-like lesions that infiltrate peripheral nerves (e.g., sciatic) may also contribute to neuropathic pain in patients, which can be treated by hormonal suppression or surgical excision of the endometrial-like lesions themselves.
Unlike neuropathic and nociceptive pain, CS and nociplastic pain persist despite unclear evidence regarding tissue damage in the somatosensory system [9]. Consequently, other measures such as those reviewed in this article are needed to identify nociplastic pain in endometriosis patients. Furthermore, treating the central contributors of pain in endometriosis largely depends on identifying the mechanism of sensitization development: is it ‘bottom-up,’ where peripheral influences and nociception drive CNS neuroplasticity, ultimately resulting in nociplastic pain, or is it ‘top-down,’ where pain amplification in the CNS exists independently of peripheral influences [91]? Nonetheless, endometriosis patients with a significant nociplastic pain component may not respond to hormonal or surgical treatments alone, and an interdisciplinary approach that addresses multiple pain contributors, whether local, central or psychosocial, may be more appropriate for pain management [92,93]. In a randomized controlled trial comparing an interdisciplinary pain treatment plan to standard peripheral pain treatments in women with chronic pelvic pain, 75% of patients in the multidisciplinary pain program reported improvements in their overall pain after one year, compared to 41% in the standard treatment group (p < 0.01) [94]. Additionally, interdisciplinary care models have historically shown significant effects in terms of improving patient-reported pain outcomes in people with chronic lower back pain, a known COPC, compared to standard monotherapies [95,96,97,98].
Many of these interdisciplinary approaches encompass pain neuroscience education, which includes pain beliefs and pain catastrophizing, validates pain despite the absence of positive test results, and explains the influences of psychological and social aspects on pain perception. It has been attributed to reduced pain and disability, and improved function and movement [99,100]. Health behavior changes, such as the adoption of diets that target comorbidities, such as irritable bowel syndrome and painful bladder syndrome [17,101,102], and the consumption of whole foods [103,104] have also been found to be effective in reducing pain. Psychological treatments such as cognitive behavioral therapy (CBT), mindfulness, meditation, and acceptance and commitment therapy can lead to improved mood, function, self-efficacy, and reduced pain and catastrophizing in people with rheumatoid arthritis [105]. CBT can also be effective in treating the adverse effects of chronic pain, such as insomnia and sexual dysfunction [106]. Additionally, site specific manual physiotherapy has been proven to significantly improve dysmenorrhea and dyspareunia in women with endometriosis [107]. It has also shown a significant effect on global response assessment, pain intensity, quality of life, fear avoidance, and disability in women with CPP [108,109,110,111]. There may also be a role for myofascial trigger point injections, treatments targeted to functional bladder and bowel pain, and medications that modulate the nervous system [112].
4. Limitations
This scoping review was limited by the novelty of the nociplastic pain phenotype within the research and clinical communities. The IASP only recently published definitions and guidelines for ‘possible’ and ‘probable’ nociplastic pain clinical identification [2]. Clinical terminology is still evolving as clinicians and researchers work to better identify nociplastic pain and CS [6,9]. As a result, we may have missed some studies related to endometriosis pain that investigated nociplastic pain using different terms. Additionally, although recent evidence supports the use of biomarker identification in endometriosis pathology [113], we did not capture biomarker measures of nociplastic pain in this review. Another limitation was the inclusion criteria, which allowed the use of all study types, including review articles. The inclusion of review articles may have hindered comparisons across the different articles. Furthermore, we utilized a narrative analysis to identify tools and methods of assessments. While this allows for a comprehensive qualitative synthesis and interpretation of the available literature, it also inherently introduces a degree of selection and interpretation bias, modulated by reviewer expertise. Additionally, many studies were of mixed pelvic pain groups, that included endometriosis patients, which prevented further analysis of population characteristics across studies.
5. Conclusions
This review provided a glimpse into the breadth of assessments used to identify evidence of CS and the presence of the nociplastic pain phenotype in individuals with endometriosis-related pain. Multiple tools were used, including patient-reported questionnaires such as the CSI, FSS, and PainDETECT, as well as clinician assessments of trigger points in the abdominopelvic area and QST. However, the ability of these tools to accurately identify CS and nociplastic pain within an endometriosis population is still under investigation. More primary research is needed to elucidate the mechanisms of CS in endometriosis and provide a core set of validated clinically and feasible methods of identifying both CS and nociplastic pain in endometriosis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13247521/s1, Table S1: Ovid MEDLINE (R) ALL <1946 to 22 April 2024>; Table S2: Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist.
Author Contributions
Conceptualization, A.G. and P.J.Y.; methodology, A.G. and P.J.Y.; formal analysis, A.G., N.M., and E.G.; investigation, A.G., and N.M.; writing—original draft preparation, A.G., E.G., Y.B., N.M. and P.J.Y.; writing—review and editing, A.G., E.G., Y.B., N.M. and P.J.Y.; visualization, A.G.; supervision, P.J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Canadian Institutes for Health Research (PJT-156084). PJY is supported by the Canada Research Chairs program.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IASP. Terminology|International Association for the Study of Pain; International Association for the Study of Pain|IASP: Washington, DC, USA, 1994. [Google Scholar]
- Nijs, J.; Lahousse, A.; Kapreli, E.; Bilika, P.; Saraçoğlu, İ.; Malfliet, A.; Coppieters, I.; De Baets, L.; Leysen, L.; Roose, E.; et al. Nociplastic Pain Criteria or Recognition of Central Sensitization? Pain Phenotyping in the Past, Present and Future. J. Clin. Med. 2021, 10, 3203. [Google Scholar] [CrossRef] [PubMed]
- Kosek, E.; Cohen, M.; Baron, R.; Gebhart, G.F.; Mico, J.-A.; Rice, A.S.C.; Rief, W.; Sluka, A.K. Do We Need a Third Mechanistic Descriptor for Chronic Pain States? Pain 2016, 157, 1382. [Google Scholar] [CrossRef] [PubMed]
- Coxon, L.; Vollert, J.; Perro, D.; Lunde, C.E.; Ferreira-Gomes, J.; Charrua, A.; Abreu-Mendes, P.; Krassowski, M.; Birch, J.; Meijlink, J.; et al. Comprehensive Quantitative Sensory Testing Shows Altered Sensory Function in Women with Chronic Pelvic Pain: Results from the Translational Research in Pelvic Pain (TRiPP) Study. Pain 2023, 164, 2528–2539. [Google Scholar] [CrossRef]
- Mayer, E.A.; Ryu, H.J.; Bhatt, R.R. The Neurobiology of Irritable Bowel Syndrome. Mol. Psychiatry 2023, 28, 1451–1465. [Google Scholar] [CrossRef]
- Kosek, E.; Clauw, D.; Nijs, J.; Baron, R.; Gilron, I.; Harris, R.E.; Mico, J.-A.; Rice, A.S.C.; Sterling, M. Chronic Nociplastic Pain Affecting the Musculoskeletal System: Clinical Criteria and Grading System. Pain 2021, 162, 2629. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; George, S.Z.; Clauw, D.J.; Fernández-de-las-Peñas, C.; Kosek, E.; Ickmans, K.; Fernández-Carnero, J.; Polli, A.; Kapreli, E.; Huysmans, E.; et al. Central Sensitisation in Chronic Pain Conditions: Latest Discoveries and Their Potential for Precision Medicine. Lancet Rheumatol. 2021, 3, e383–e392. [Google Scholar] [CrossRef]
- Woolf, C.J. Central Sensitization: Implications for the Diagnosis and Treatment of Pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Nijs, J.; Malfliet, A.; Nishigami, T. Nociplastic Pain and Central Sensitization in Patients with Chronic Pain Conditions: A Terminology Update for Clinicians. Braz. J. Phys. Ther. 2023, 27, 100518. [Google Scholar] [CrossRef]
- Song, S.Y.; Jung, Y.W.; Shin, W.; Park, M.; Lee, G.W.; Jeong, S.; An, S.; Kim, K.; Ko, Y.B.; Lee, K.H.; et al. Endometriosis-Related Chronic Pelvic Pain. Biomedicines 2023, 11, 2868. [Google Scholar] [CrossRef]
- Nijs, J.; Van Houdenhove, B.; Oostendorp, R.A.B. Recognition of Central Sensitization in Patients with Musculoskeletal Pain: Application of Pain Neurophysiology in Manual Therapy Practice. Man. Ther. 2010, 15, 135–141. [Google Scholar] [CrossRef]
- Stratton, P.; Khachikyan, I.; Sinaii, N.; Ortiz, R.; Shah, J. Association of Chronic Pelvic Pain and Endometriosis with Signs of Sensitization and Myofascial Pain. Obstet. Gynecol. 2015, 125, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, D.; Raffone, A.; Renzulli, F.; Sanna, G.; Raspollini, A.; Bertoldo, L.; Maletta, M.; Lenzi, J.; Rovero, G.; Travaglino, A.; et al. Prevalence and Risk Factors of Central Sensitization in Women with Endometriosis. J. Minim. Invasive Gynecol. 2023, 30, 73–80.e1. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, N.; Abrao, M.S.; Einarsson, J.I.; Horne, A.W.; Johnson, N.P.; Lee, T.T.M.; Missmer, S.; Petrozza, J.; Tomassetti, C.; Zondervan, K.T.; et al. Endometriosis Classification, Staging and Reporting Systems: A Review on the Road to a Universally Accepted Endometriosis Classification. J. Minim. Invasive Gynecol. 2021, 28, 1822–1848. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zheng, H.; Nie, H.; Li, C.-F.; Zhang, W.; Wang, J.-J. Endometriosis of the Lung: A Case Report and Review of Literature. World J. Clin. Cases 2023, 11, 4326. [Google Scholar] [CrossRef]
- Yosef, A.; Allaire, C.; Williams, C.; Ahmed, A.G.; Al-Hussaini, T.; Abdellah, M.S.; Wong, F.; Lisonkova, S.; Yong, P.J. Multifactorial Contributors to the Severity of Chronic Pelvic Pain in Women. Am. J. Obstet. Gynecol. 2016, 215, 760.e1. [Google Scholar] [CrossRef]
- Coxon, L.; Evans, E.; Vincent, K. Endometriosis—A Painful Disease. Curr. Opin. Anesthesiol. 2023, 36, 595. [Google Scholar] [CrossRef]
- Yong, P.J.; Williams, C.; Bedaiwy, M.A.; Allaire, C. A Proposed Platform for Phenotyping Endometriosis-Associated Pain: Unifying Peripheral and Central Pain Mechanisms. Curr. Obstet. Gynecol. Rep. 2020, 9, 89–97. [Google Scholar] [CrossRef]
- Coxon, L.; Horne, A.W.; Vincent, K. Pathophysiology of Endometriosis-Associated Pain: A Review of Pelvic and Central Nervous System Mechanisms. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 53–67. [Google Scholar] [CrossRef]
- Till, S.R.; Schrepf, A.; Clauw, D.J.; Harte, S.E.; Williams, D.A.; As-Sanie, S. Association Between Nociplastic Pain and Pain Severity and Impact in Women with Chronic Pelvic Pain. J. Pain 2023, 24, 1406–1414. [Google Scholar] [CrossRef]
- Tucker, D.R.; Noga, H.L.; Lee, C.; Chiu, D.S.; Bedaiwy, M.A.; Williams, C.; Allaire, C.; Talhouk, A.; Yong, P.J. Pelvic Pain Comorbidities Associated with Quality of Life after Endometriosis Surgery. Am. J. Obstet. Gynecol. 2023, 229, 147.e1–147.e20. [Google Scholar] [CrossRef] [PubMed]
- Orr, N.L.; Huang, A.J.; Liu, Y.D.; Noga, H.; Bedaiwy, M.A.; Williams, C.; Allaire, C.; Yong, P.J. Association of Central Sensitization Inventory Scores with Pain Outcomes After Endometriosis Surgery. JAMA Netw. Open 2023, 6, e230780. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Salter, M.W. Neuronal Plasticity: Increasing the Gain in Pain. Science 2000, 288, 1765–1768. [Google Scholar] [CrossRef]
- Yunus, M.B. Central Sensitivity Syndromes: A New Paradigm and Group Nosology for Fibromyalgia and Overlapping Conditions, and the Related Issue of Disease versus Illness. Semin. Arthritis Rheum. 2008, 37, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.R.; Gandhi, W.; Harrison, R.; van Reekum, C.M.; Wood-Anderson, D.; Gilron, I.; Salomons, T.V. Do “Central Sensitization” Questionnaires Reflect Measures of Nociceptive Sensitization or Psychological Constructs? A Systematic Review and Meta-Analyses. Pain 2023, 164, 1222–1239. [Google Scholar] [CrossRef]
- Orr, N.L.; Wahl, K.J.; Lisonek, M.; Joannou, A.; Noga, H.; Albert, A.; Bedaiwy, M.A.; Williams, C.; Allaire, C.; Yong, P.J. Central Sensitization Inventory in Endometriosis. Pain 2022, 163, E234–E245. [Google Scholar] [CrossRef]
- Neblett, R.; Cohen, H.; Choi, Y.; Hartzell, M.; Williams, M.; Mayer, T.G.; Gatchel, R.J. The Central Sensitization Inventory (CSI): Establishing Clinically-Significant Values For Identifying Central Sensitivity Syndromes In An Outpatient Chronic Pain Sample. J. Pain 2013, 14, 438–445. [Google Scholar] [CrossRef]
- Cuesta-Vargas, A.I.; Neblett, R.; Chiarotto, A.; Kregel, J.; Nijs, J.; van Wilgen, C.P.; Pitance, L.; Knezevic, A.; Gatchel, R.J.; Mayer, T.G.; et al. Dimensionality and Reliability of the Central Sensitization Inventory in a Pooled Multicountry Sample. J. Pain 2018, 19, 317–329. [Google Scholar] [CrossRef]
- Rolke, R.; Baron, R.; Maier, C.; Tölle, T.R.; Treede, R.D.; Beyer, A.; Binder, A.; Birbaumer, N.; Birklein, F.; Bötefür, I.C.; et al. Quantitative Sensory Testing in the German Research Network on Neuropathic Pain (DFNS): Standardized Protocol and Reference Values. Pain 2006, 123, 231–243. [Google Scholar] [CrossRef]
- As-Sanie, S.; Harris, R.E.; Napadow, V.; Kim, J.; Neshewat, G.; Kairys, A.; Williams, D.; Clauw, D.J.; Schmidt-Wilcke, T. Changes in Regional Gray Matter Volume in Women with Chronic Pelvic Pain: A Voxel-Based Morphometry Study. Pain 2012, 153, 1006–1014. [Google Scholar] [CrossRef]
- Selçuk, İ.; Bozdağ, G. Recurrence of Endometriosis; Risk Factors, Mechanisms and Biomarkers; Review of the Literature. J. Turk. Ger. Gynecol. Assoc. 2013, 14, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Gentles, A.; Marshall, N.; Goodwin, E.; Yong, P. Clinical Tools and Approaches for Nociplastic Pain Phenotyping in People with Endometriosis-Related Pain: A Scoping Review. Gen. Syst. Rev. Regist. 2024. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Covidence—Better Systematic Review Management. Available online: https://www.covidence.org/ (accessed on 7 August 2024).
- Bajaj, P. Endometriosis Is Associated with Central Sensitization: A Psychophysical Controlled Study. J. Pain 2003, 4, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Laursen, B.S.; Bajaj, P.; Olesen, A.S.; Delmar, C.; Arendt-Nielsen, L. Health Related Quality of Life and Quantitative Pain Measurement in Females with Chronic Non-malignant Pain. Eur. J. Pain 2005, 9, 267. [Google Scholar] [CrossRef]
- He, W.; Liu, X.; Zhang, Y.; Guo, S.W. Generalized Hyperalgesia in Women with Endometriosis and Its Resolution Following a Successful Surgery. Reprod. Sci. 2010, 17, 1099–1111. [Google Scholar] [CrossRef]
- Napadow, V.; Edwards, R.R.; Cahalan, C.M.; Mensing, G.; Greenbaum, S.; Valovska, A.; Li, A.; Kim, J.; Maeda, Y.; Park, K.; et al. Evoked Pain Analgesia in Chronic Pelvic Pain Patients Using Respiratory-Gated Auricular Vagal Afferent Nerve Stimulation. Pain Med. 2012, 13, 777–789. [Google Scholar] [CrossRef]
- Giamberardino, M.A.; Tana, C.; Costantini, R. Pain Thresholds in Women with Chronic Pelvic Pain. Curr. Opin. Obstet. Gynecol. 2014, 26, 253–259. [Google Scholar] [CrossRef]
- Aredo, J.; Heyrana, K.; Karp, B.; Shah, J.; Stratton, P. Relating Chronic Pelvic Pain and Endometriosis to Signs of Sensitization and Myofascial Pain and Dysfunction. Semin. Reprod. Med. 2017, 35, 88–97. [Google Scholar] [CrossRef]
- Costantini, R.; Affaitati, G.; Wesselmann, U.; Czakanski, P.; Giamberardino, M.A. Visceral Pain as a Triggering Factor for Fibromyalgia Symptoms in Comorbid Patients. Pain 2017, 158, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
- Grundström, H.; Gerdle, B.; Alehagen, S.; Berterö, C.; Arendt-Nielsen, L.; Kjølhede, P. Reduced Pain Thresholds and Signs of Sensitization in Women with Persistent Pelvic Pain and Suspected Endometriosis. Acta Obstet. Et Gynecol. Scand. 2019, 98, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Orr, N.L.; Wahl, K.J.; Noga, H.; Allaire, C.; Williams, C.; Bedaiwy, M.A.; Albert, A.; Smith, K.B.; Yong, P.J. Phenotyping Sexual Pain in Endometriosis Using the Central Sensitization Inventory. J. Sex. Med. 2020, 17, 761–770. [Google Scholar] [CrossRef] [PubMed]
- As-Sanie, S.; Till, S.R.; Schrepf, A.D.; Griffith, K.C.; Tsodikov, A.; Missmer, S.A.; Clauw, D.J.; Brummett, C.M. Incidence and Predictors of Persistent Pelvic Pain Following Hysterectomy in Women with Chronic Pelvic Pain. Am. J. Obstet. Gynecol. 2021, 225, 568.e1–568.e11. [Google Scholar] [CrossRef]
- Evans, S.; Mikocka-Walus, A.; Olive, L.; Seidman, L.C.; Druitt, M.; Payne, L.A. Phenotypes of Women with and Without Endometriosis and Relationship with Functional Pain Disability. Pain Med. Malden Mass 2021, 22, 1511–1521. [Google Scholar] [CrossRef]
- Phan, V.T.; Stratton, P.; Tandon, H.K.; Sinaii, N.; Aredo, J.V.; Karp, B.I.; Merideth, M.A.; Shah, J.P. Widespread Myofascial Dysfunction and Sensitisation in Women with Endometriosis-associated Chronic Pelvic Pain: A Cross-sectional Study. Eur. J. Pain 2021, 25, 831–840. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Martel, E.; Missmer, S.A.; Clauw, D.J.; Harte, S.E.; As-Sanie, S.; Sieberg, C.B. Pelvic Floor, Abdominal and Uterine Tenderness in Relation to Pressure Pain Sensitivity among Women with Endometriosis and Chronic Pelvic Pain. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 247–253. [Google Scholar] [CrossRef]
- de Arruda, G.T.; Driusso, P.; Rodrigues, J.C.; de Godoy, A.G.; Degani, A.; Danna-dos-Santos, A.; Avila, M.A. Are Menstrual Symptoms Associated with Central Sensitization Inventory? A Cross-Sectional Study. Eur. J. Pain 2022, 26, 1759–1767. [Google Scholar] [CrossRef]
- Green, I.C.; Burnett, T.; Famuyide, A. Persistent Pelvic Pain in Patients with Endometriosis. Clin. Obstet. Gynecol. 2022, 65, 775–785. [Google Scholar] [CrossRef]
- Ryan, A.; Healey, M.; Cheng, C.; Dior, U.; Reddington, C. Central Sensitisation in Pelvic Pain: A Cohort Study. Aust. N. Z. J. Obstet. Gynaecol. 2022, 62, 868–874. [Google Scholar] [CrossRef]
- Simpson, G.; Philip, M.; Lucky, T.; Ang, C.; Kathurusinghe, S. A Systematic Review of the Efficacy and Availability of Targeted Treatments for Central Sensitization in Women with Endometriosis. Clin. J. Pain 2022, 38, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Till, S.R.; Nakamura, R.; Schrepf, A.; As-Sanie, S. Approach to Diagnosis and Management of Chronic Pelvic Pain in Women: Incorporating Chronic Overlapping Pain Conditions in Assessment and Management. Obstet. Gynecol. Clin. N. Am. 2022, 49, 219–239. [Google Scholar] [CrossRef]
- Cardaillac, C.; Levesque, A.; Riant, T.; Mortier, A.; Neunlist, M.; Perrouin-Verbe, M.-A.; Volteau, C.; Thubert, T.; Brochard, C.; Ploteau, S. Evaluation of a Scoring System for the Detection of Central Sensitization among Women with Chronic Pelvic Pain. Am. J. Obstet. Gynecol. 2023, 229, 530.e1–530.e17. [Google Scholar] [CrossRef]
- Cetera, G.E.; Merli, C.E.M.; Facchin, F.; Viganò, P.; Pesce, E.; Caprara, F.; Vercellini, P. Non-Response to First-Line Hormonal Treatment for Symptomatic Endometriosis: Overcoming Tunnel Vision. A Narrative Review. BMC Womens Health 2023, 23, 347. [Google Scholar] [CrossRef]
- Cetera, G.E.; Merli, C.E.M.; Boero, V.; Caia, C.; Facchin, F.; Barbara, G.; Monti, E.; Vercellini, P. Central Sensitization in Vulvodynia and Endometriosis: What Have We Been Overlooking So Far? Obstet. Gynecol. Surv. 2023, 78, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Quintas-Marquès, L.; Martínez-Zamora, M.-Á.; Camacho, M.; Gràcia, M.; Rius, M.; Ros, C.; Carrión, A.; Carmona, F. Central Sensitization in Patients with Deep Endometriosis. Pain Med. 2023, 24, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Cetera, G.E.; Merli, C.E.M.; Barbara, G.; Caia, C.; Vercellini, P. Questionnaires for the Assessment of Central Sensitization in Endometriosis: What Is the Available Evidence? A Systematic Review with a Narrative Synthesis. Reprod. Sci. 2023, 31, 633–644. [Google Scholar] [CrossRef]
- Liu, Y.D.; Noga, H.; Allaire, C.; Bedaiwy, M.A.; Lee, C.E.; Williams, C.; Booth, A.; Galea, L.A.M.; Kaida, A.; Ogilvie, G.S.; et al. Mental Health Outcomes of Endometriosis Patients during the COVID-19 Pandemic: Impact of Pre-Pandemic Central Nervous System Sensitization. J. Pain 2024, 25, 104481. [Google Scholar] [CrossRef]
- Maixner, W.; Fillingim, R.B.; Williams, D.A.; Smith, S.B.; Slade, G.D. Overlapping Chronic Pain Conditions: Implications for Diagnosis and Classification. J. Pain Off. J. Am. Pain Soc. 2016, 17, T93. [Google Scholar] [CrossRef]
- Mayer, T.G.; Neblett, R.; Cohen, H.; Howard, K.J.; Choi, Y.H.; Williams, M.J.; Perez, Y.; Gatchel, R.J. The Development and Psychometric Validation of the Central Sensitization Inventory. Pain Pract. 2012, 12, 276–285. [Google Scholar] [CrossRef]
- Neblett, R.; Sanabria-Mazo, J.P.; Luciano, J.V.; Mirčić, M.; Čolović, P.; Bojanić, M.; Jeremić-Knežević, M.; Aleksandrić, T.; Knežević, A. Is the Central Sensitization Inventory (CSI) Associated with Quantitative Sensory Testing (QST)? A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2024, 161, 105612. [Google Scholar] [CrossRef] [PubMed]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. painDETECT: A New Screening Questionnaire to Identify Neuropathic Components in Patients with Back Pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, S.; Lin, C. painDETECT Questionnaire. J. Physiother. 2013, 59, 211. [Google Scholar] [CrossRef] [PubMed]
- Freynhagen, R.; Tölle, T.R.; Gockel, U.; Baron, R. The painDETECT Project—Far More than a Screening Tool on Neuropathic Pain. Curr. Med. Res. Opin. 2016, 32, 1033–1057. [Google Scholar] [CrossRef]
- Coxon, L.; Wiech, K.; Vincent, K. Is There a Neuropathic-Like Component to Endometriosis-Associated Pain? Results From a Large Cohort Questionnaire Study. Front. Pain Res. 2021, 2, 743812. [Google Scholar] [CrossRef] [PubMed]
- Ruscheweyh, R.; Verneuer, B.; Dany, K.; Marziniak, M.; Wolowski, A.; Çolak-Ekici, R.; Schulte, T.L.; Bullmann, V.; Grewe, S.; Gralow, I.; et al. Validation of the Pain Sensitivity Questionnaire in Chronic Pain Patients. Pain 2012, 153, 1210–1218. [Google Scholar] [CrossRef]
- Grundström, H.; Larsson, B.; Arendt-Nielsen, L.; Gerdle, B.; Kjølhede, P. Associations between Pain Thresholds for Heat, Cold and Pressure, and Pain Sensitivity Questionnaire Scores in Healthy Women and in Women with Persistent Pelvic Pain. Eur. J. Pain 2019, 23, 1631–1639. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Ryan, K.M. Pain Assessment: Global Use of the Brief Pain Inventory. Ann. Acad. Med. 1994, 23, 129–138. [Google Scholar]
- Bourdel, N.; Alves, J.; Pickering, G.; Ramilo, I.; Roman, H.; Canis, M. Systematic Review of Endometriosis Pain Assessment: How to Choose a Scale? Hum. Reprod. Update 2015, 21, 136–152. [Google Scholar] [CrossRef]
- Rolke, R.; Magerl, W.; Andrews Campbell, K.; Schalber, C.; Caspari, S.; Birklein, F.; Treede, R.-D. Quantitative Sensory Testing: A Comprehensive Protocol for Clinical Trials. Eur. J. Pain 2005, 10, 77–88. [Google Scholar] [CrossRef]
- Vollert, J.; Maier, C.; Attal, N.; Bennett, D.L.H.; Bouhassira, D.; Enax-Krumova, E.K.; Finnerup, N.B.; Freynhagen, R.; Gierthmühlen, J.; Haanpää, M.; et al. Stratifying Patients with Peripheral Neuropathic Pain Based on Sensory Profiles: Algorithm and Sample Size Recommendations. Pain 2017, 158, 1446. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Kowalski, J.; Lundeberg, T. A New Highly Reliable Instrument for the Assessment of Pre- and Postoperative Gynecological Pain. Anesth. Analg. 2002, 95, 151–157, table of contents. [Google Scholar] [CrossRef] [PubMed]
- den Boer, C.; Terluin, B.; van der Wouden, J.C.; Blankenstein, A.H.; van der Horst, H.E. Tests for Central Sensitization in General Practice: A Delphi Study. BMC Fam. Pract. 2021, 22, 206. [Google Scholar] [CrossRef]
- As-Sanie, S.; Harris, R.E.; Harte, S.E.; Tu, F.F.; Neshewat, G.; Clauw, D.J. Increased Pressure Pain Sensitivity in Women with Chronic Pelvic Pain. Obstet. Gynecol. 2013, 122, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.S.; Gebhart, G.F. Visceral Pain. In Behavioral Neurobiology of Chronic Pain; Taylor, B.K., Finn, D.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 171–197. ISBN 978-3-662-45094-9. [Google Scholar]
- Hoffman, D. Understanding Multisymptom Presentations in Chronic Pelvic Pain: The Inter-Relationships between the Viscera and Myofascial Pelvic Floor Dysfunction. Curr. Pain Headache Rep. 2011, 15, 343–346. [Google Scholar] [CrossRef]
- Shah, J.P.; Thaker, N.; Heimur, J.; Aredo, J.V.; Sikdar, S.; Gerber, L.H. Myofascial Trigger Points Then and Now: A Historical and Scientific Perspective. PM&R 2015, 7, 746–761. [Google Scholar] [CrossRef]
- Gerwin, R.D. Myofascial and Visceral Pain Syndromes: Visceral-Somatic Pain Representations. In The Clinical Neurobiology of Fibromyalgia and Myofascial Pain; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-1-00-306381-0. [Google Scholar]
- Pastore, E.A.; Katzman, W.B. Recognizing Myofascial Pelvic Pain in the Female Patient with Chronic Pelvic Pain. J. Obstet. Gynecol. Neonatal Nurs. 2012, 41, 680–691. [Google Scholar] [CrossRef]
- Levesque, A.; Riant, T.; Ploteau, S.; Rigaud, J.; Labat, J.-J. Convergences PP Network Clinical Criteria of Central Sensitization in Chronic Pelvic and Perineal Pain (Convergences PP Criteria): Elaboration of a Clinical Evaluation Tool Based on Formal Expert Consensus. Pain Med. Malden Mass 2018, 19, 2009–2015. [Google Scholar] [CrossRef]
- Quistrebert-Davanne, V.; Hardouin, J.-B.; Riant, T.; Ploteau, S.; Rigaud, J.; Levesque, A. Validation Psychométrique Des Critères de Convergences PP. Prog. Urol. 2021, 31, 1192–1200. [Google Scholar] [CrossRef]
- Orr, N.L.; Albert, A.; Liu, Y.D.; Lum, A.; Hong, J.; Ionescu, C.L.; Senz, J.; Nazeran, T.M.; Lee, A.F.; Noga, H.; et al. KRAS Mutations and Endometriosis Burden of Disease. J. Pathol. Clin. Res. 2023, 9, 302–312. [Google Scholar] [CrossRef]
- Sreya, M.; Tucker, D.R.; Yi, J.; Alotaibi, F.T.; Lee, A.F.; Noga, H.; Yong, P.J. Nerve Bundle Density and Expression of NGF and IL-1β Are Intra-Individually Heterogenous in Subtypes of Endometriosis. Biomolecules 2024, 14, 583. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Alotaibi, F.T.; Sediqi, S.; Bedaiwy, M.A.; Yong, P.J. Role of Interleukin-1β in Nerve Growth Factor Expression, Neurogenesis and Deep Dyspareunia in Endometriosis. Hum. Reprod. 2020, 35, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Zhan, H.; Alotaibi, F.; Alkusayer, G.M.; Bedaiwy, M.A.; Yong, P.J. Nerve Growth Factor Is Associated with Sexual Pain in Women with Endometriosis. Reprod. Sci. 2018, 25, 540–549. [Google Scholar] [CrossRef]
- Zoet, G.; Tucker, D.R.; Orr, N.L.; Alotaibi, F.T.; Liu, Y.D.; Noga, H.; Köbel, M.; Yong, P.J. Standardized Protocol for Quantification of Nerve Bundle Density as a Biomarker for Endometriosis. Front. Reprod. Health 2023, 5, 1297986. [Google Scholar] [CrossRef]
- Williams, C.; Hoang, L.; Yosef, A.; Alotaibi, F.; Allaire, C.; Brotto, L.; Fraser, I.S.; Bedaiwy, M.A.; Ng, T.L.; Lee, A.F.; et al. Nerve Bundles and Deep Dyspareunia in Endometriosis. Reprod. Sci. 2016, 23, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Mwaura, A.N.; Marshall, N.; Anglesio, M.S.; Yong, P.J. Neuroproliferative Dyspareunia in Endometriosis and Vestibulodynia. Sex. Med. Rev. 2023, 11, 323–332. [Google Scholar] [CrossRef]
- Eller-Smith, O.C.; Nicol, A.L.; Christianson, J.A. Potential Mechanisms Underlying Centralized Pain and Emerging Therapeutic Interventions. Front. Cell. Neurosci. 2018, 12, 35. [Google Scholar] [CrossRef]
- Allaire, C.; Bedaiwy, M.A.; Yong, P.J. Diagnosis and Management of Endometriosis. CMAJ 2023, 195, E363–E371. [Google Scholar] [CrossRef] [PubMed]
- Allaire, C.; Aksoy, T.; Bedaiwy, M.; Britnell, S.; Noga, H.L.; Yager, H.; Yong, P.J. An Interdisciplinary Approach to Endometriosis-Associated Persistent Pelvic Pain. J. Endometr. Pelvic Pain Disord. 2017, 9, 77–86. [Google Scholar] [CrossRef]
- Peters, A.A.; van Dorst, E.; Jellis, B.; van Zuuren, E.; Hermans, J.; Trimbos, J.B. A Randomized Clinical Trial to Compare Two Different Approaches in Women with Chronic Pelvic Pain. Obstet. Gynecol. 1991, 77, 740–744. [Google Scholar] [CrossRef]
- Guzmán, J.; Esmail, R.; Karjalainen, K.; Malmivaara, A.; Irvin, E.; Bombardier, C. Multidisciplinary Bio-Psycho-Social Rehabilitation for Chronic Low Back Pain. Cochrane Database Syst. Rev. 2002, 350, CD000963. [Google Scholar] [CrossRef]
- Davin, S.; Lapin, B.; Mijatovic, D.; Fox, R.; Benzel, E.; Stilphen, M.; Machado, A.; Katzan, I.L. Comparative Effectiveness of an Interdisciplinary Pain Program for Chronic Low Back Pain, Compared to Physical Therapy Alone. Spine 2019, 44, 1715–1722. [Google Scholar] [CrossRef]
- Gregg, C.D.; Hoffman, C.W.; Hall, H.; McIntosh, G.; Robertson, P.A. Outcomes of an Interdisciplinary Rehabilitation Programme for the Management of Chronic Low Back Pain. J. Prim. Health Care 2011, 3, 222–227. [Google Scholar] [CrossRef]
- Kamper, S.J.; Apeldoorn, A.T.; Chiarotto, A.; Smeets, R.J.E.M.; Ostelo, R.W.J.G.; Guzman, J.; Tulder, M.W. van Multidisciplinary Biopsychosocial Rehabilitation for Chronic Low Back Pain: Cochrane Systematic Review and Meta-Analysis. BMJ 2015, 350, h444. [Google Scholar] [CrossRef]
- Louw, A.; Zimney, K.; Puentedura, E.J.; Diener, I. The Efficacy of Pain Neuroscience Education on Musculoskeletal Pain: A Systematic Review of the Literature. Physiother. Theory Pract. 2016, 32, 332–355. [Google Scholar] [CrossRef]
- Richter, M.; Rauscher, C.; Kluttig, A.; Mallwitz, J.; Delank, K.-S. Effect of Additional Pain Neuroscience Education in Interdisciplinary Multimodal Pain Therapy on Current Pain. A Non-Randomized, Controlled Intervention Study. J. Pain Res. 2020, 13, 2947–2957. [Google Scholar] [CrossRef] [PubMed]
- Cox, A. Management of Interstitial Cystitis/Bladder Pain Syndrome. Can. Urol. Assoc. J. 2018, 12, S157–S160. [Google Scholar] [CrossRef]
- Moayyedi, P.; Andrews, C.N.; MacQueen, G.; Korownyk, C.; Marsiglio, M.; Graff, L.; Kvern, B.; Lazarescu, A.; Liu, L.; Paterson, W.G.; et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Irritable Bowel Syndrome (IBS). J. Can. Assoc. Gastroenterol. 2019, 2, 6–29. [Google Scholar] [CrossRef] [PubMed]
- Field, R.; Pourkazemi, F.; Turton, J.; Rooney, K. Dietary Interventions Are Beneficial for Patients with Chronic Pain: A Systematic Review with Meta-Analysis. Pain Med. 2021, 22, 694–714. [Google Scholar] [CrossRef]
- Elma, Ö.; Yilmaz, S.T.; Deliens, T.; Coppieters, I.; Clarys, P.; Nijs, J.; Malfliet, A. Do Nutritional Factors Interact with Chronic Musculoskeletal Pain? A Systematic Review. J. Clin. Med. 2020, 9, 702. [Google Scholar] [CrossRef]
- Davis, M.C.; Zautra, A.J.; Wolf, L.D.; Tennen, H.; Yeung, E.W. Mindfulness and Cognitive-Behavioral Interventions for Chronic Pain: Differential Effects on Daily Pain Reactivity and Stress Reactivity. J. Consult. Clin. Psychol. 2015, 83, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Broderick, J.E.; Keefe, F.J.; Schneider, S.; Junghaenel, D.U.; Bruckenthal, P.; Schwartz, J.E.; Kaell, A.T.; Caldwell, D.S.; McKee, D.; Gould, E. Cognitive Behavioral Therapy for Chronic Pain Is Effective, but for Whom? Pain 2016, 157, 2115–2123. [Google Scholar] [CrossRef]
- Wurn, B.F.; Wurn, L.J.; Patterson, K.; King, C.R.; Scharf, E.S. Decreasing Dyspareunia and Dysmenorrhea in Women with Endometriosis via a Manual Physical Therapy: Results from Two Independent Studies. J. Endometr. 2011, 3, 188–196. [Google Scholar] [CrossRef]
- FitzGerald, M.P.; Payne, C.K.; Lukacz, E.S.; Yang, C.C.; Peters, K.M.; Chai, T.C.; Nickel, J.C.; Hanno, P.M.; Kreder, K.J.; Burks, D.A.; et al. Randomized Multicenter Clinical Trial of Myofascial Physical Therapy in Women with Interstitial Cystitis/Painful Bladder Syndrome and Pelvic Floor Tenderness. J. Urol. 2012, 187, 2113–2118. [Google Scholar] [CrossRef]
- Oyama, I.A.; Rejba, A.; Lukban, J.C.; Fletcher, E.; Kellogg-Spadt, S.; Holzberg, A.S.; Whitmore, K.E. Modified Thiele Massage as Therapeutic Intervention for Female Patients with Interstitial Cystitis and High-Tone Pelvic Floor Dysfunction. Urology 2004, 64, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, F.; Bastani, P.; Hajebrahimi, S.; Jafarabadi, M.A.; Berghmans, B. Pelvic Floor Rehabilitation in the Treatment of Women with Dyspareunia: A Randomized Controlled Clinical Trial. Int. Urogynecology J. 2019, 30, 1849–1855. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Patterson, B.; Mahajan, S. Prevalence of Myofascial Chronic Pelvic Pain and the Effectiveness of Pelvic Floor Physical Therapy. J. Reprod. Med. 2013, 58, 504–510. [Google Scholar] [PubMed]
- Allaire, C.; Yong, P.J.; Bajzak, K.; Jarrell, J.; Lemos, N.; Miller, C.; Morin, M.; Nasr-Esfahani, M.; Singh, S.S.; Chen, I. Guideline No. 445: Management of Chronic Pelvic Pain. J. Obstet. Gynaecol. Can. 2024, 46, 102283. [Google Scholar] [CrossRef]
- Brulport, A.; Bourdon, M.; Vaiman, D.; Drouet, C.; Pocate-Cheriet, K.; Bouzid, K.; Marcellin, L.; Santulli, P.; Abo, C.; Jeljeli, M.; et al. An Integrated Multi-Tissue Approach for Endometriosis Candidate Biomarkers: A Systematic Review. Reprod. Biol. Endocrinol. 2024, 22, 21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).