Study on Pharmacological Treatment of Impulse Control Disorders in Parkinson’s Disease
Abstract
1. Introduction
- −
- Pathological gambling (PG), code F63.0 in ICD-10—turned into gambling disorder (GD), code 6C50 in ICD-11;
- −
- Hypersexuality (HS), code F52.8 in ICD-10—turned into compulsive sexual behavior disorder (CSBD), code 6C72 in ICD-11;
- −
- Binge eating disorder (BED), code F50.81 in ICD-10—keeps to binge eating disorder (BED), code 6B82 in ICD-11;
- −
- Compulsive shopping (CS), code F63.8 in ICD-10—turned into compulsive buying-shopping disorder (CBSD), code 6C7Y in ICD-11.
2. Materials and Methods
3. Results
- Depression could be considered a cause for pathological gambling (PG);
- Anxiety disorder could be the background for compulsive shopping (CS) and for binge eating disorder (BED);
- Behavior disorder could be a cause for hypersexuality (HS).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, R.P.; Tumas, V.; Pedroso, J.L.; Silveira-Moriyama, L. The clinical diagnosis of Parkinson’s disease. Arq. Neuropsiquiatr. 2024, 82, s00431777775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moreau, C.; Rouaud, T.; Grabli, D.; Benatru, I.; Remy, P.; Marques, A.-R.; Drapier, S.; Mariani, L.-L.; Roze, E.; Devos, D.; et al. Overview on wearable sensors for the management of Parkinson’s disease. NPJ Park. Dis. 2023, 9, 153. [Google Scholar] [CrossRef]
- Sigcha, L.; Pavón, I.; Costa, N.; Costa, S.; Gago, M.; Arezes, P.; López, J.M.; De Arcas, G. Automatic Resting Tremor Assessment in Parkinson’s Disease Using Smartwatches and Multitask Convolutional Neural Networks. Sensors 2021, 21, 291. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- El Otmani, H.; Mouni, F.Z.; Abdulhakeem, Z.; Attar, Z.; Rashad, L.; Saali, I.; El Moutawakil, B.; Rafai, M.A.; Slassi, I.; Nadifi, S. Impulse Control Disorders in Parkinson Disease: A Cross-Sectional Study in Morocco. Rev. Neurol. 2019, 175, 233–237. [Google Scholar] [CrossRef]
- Latella, D.; Maggio, M.G.; Maresca, G.; Saporoso, A.F.; Le Cause, M.; Manuli, A.; Milardi, D.; Bramanti, P.; De Luca, R.; Calabrò, R.S. Impulse Control Disorders in Parkinson’s Disease: A Systematic Review on Risk Factors and Pathophysiology. J. Neurol. Sci. 2019, 398, 101–106. [Google Scholar] [CrossRef]
- Tanwani, P.; Fernie, B.A.; Nikčević, A.V.; Spada, M.M. A Systematic Review of Treatments for Impulse Control Disorders and Related Behaviours in Parkinson’s Disease. Psychiatry Res. 2015, 225, 402–406. [Google Scholar] [CrossRef]
- Dawson, A.; Michael, J.; Dilkes-Frayne, E.; Hall, W.; Dissanayaka, N.N.; Carter, A. Capacity, Control and Responsibility in Parkinson’s Disease Patients with Impulse Control Disorders: Views of Neurological and Psychiatric Experts. Int. J. Law Psychiatry 2019, 65, 101343. [Google Scholar] [CrossRef]
- Vijiaratnam, N.; Simuni, T.; Bandmann, O.; Morris, H.R.; Foltynie, T. Progress towards Therapies for Disease Modification in Parkinson’s Disease. Lancet Neurol. 2021, 20, 559–572. [Google Scholar] [CrossRef]
- Silva, B.; Canas-Simião, H.; Cavanna, A.E. Neuropsychiatric Aspects of Impulse Control Disorders. Psychiatr. Clin. N. Am. 2020, 43, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, F.; Valente, E.M.; Arena, G. Mechanisms of Neurodegeneration in Parkinson’s Disease: Keep Neurons in the PINK1. Mech. Ageing Dev. 2020, 189, 111277. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.R.; Hinman, J.M.; Johnson, T.E. Chapter 20-Impulse-Control Disorders. In Functional Analysis in Clinical Treatment (Second Edition); Sturmey, P., Ed.; Academic Press: San Diego, CA, USA, 2020; pp. 479–500. ISBN 18730450. [Google Scholar]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Biars, J.W.; Johnson, N.L.; Nespeca, M.; Busch, R.M.; Kubu, C.S.; Floden, D.P. Iowa Gambling Task Performance in Parkinson Disease Patients with Impulse Control Disorders. Arch. Clin. Neuropsychol. 2019, 34, 310–318. [Google Scholar] [CrossRef]
- Codling, D.; Shaw, P.; David, A.S. Hypersexuality in Parkinson’s Disease: Systematic Review and Report of 7 New Cases. Mov. Disord. Clin. Pract. 2015, 2, 116–126. [Google Scholar] [CrossRef]
- de Chazeron, I.; Durif, F.; Lambert, C.; Chereau-Boudet, I.; Fantini, M.L.; Marques, A.; Derost, P.; Debilly, B.; Brousse, G.; Boirie, Y.; et al. A Case–Control Study Investigating Food Addiction in Parkinson Patients. Sci. Rep. 2021, 11, 10934. [Google Scholar] [CrossRef]
- Morgante, F.; Fasano, A.; Ginevrino, M.; Petrucci, S.; Ricciardi, L.; Bove, F.; Criscuolo, C.; Moccia, M.; De Rosa, A.; Sorbera, C.; et al. Impulsive-Compulsive Behaviors in Parkin -Associated Parkinson Disease. Neurology 2016, 87, 1436–1441. [Google Scholar] [CrossRef]
- ICD-11 2024 Release. Available online: https://www.who.int/news/item/08-02-2024-icd-11-2024-release (accessed on 21 October 2024).
- Zhang, Q.; Chen, X.; Chen, F.; Wen, S.; Zhou, C. Dopamine Agonists versus Levodopa Monotherapy in Early Parkinson’s Disease for the Potential Risks of Motor Complications: A Network Meta-Analysis. Eur. J. Pharmacol. 2023, 954, 175884. [Google Scholar] [CrossRef]
- Ceravolo, R.; Frosini, D.; Rossi, C.; Bonuccelli, U. Impulse Control Disorders in Parkinson’s Disease: Definition, Epidemiology, Risk Factors, Neurobiology and Management. Park. Relat. Disord. 2009, 15 (Suppl. S4), S111–S115. [Google Scholar] [CrossRef]
- Lizarraga, K.J.; Fox, S.H.; Strafella, A.P.; Lang, A.E. Hallucinations, Delusions and Impulse Control Disorders in Parkinson Disease. Clin. Geriatr. Med. 2020, 36, 105–118. [Google Scholar] [CrossRef]
- Phu, A.L.; Xu, Z.; Brakoulias, V.; Mahant, N.; Fung, V.S.C.; Moore, G.D.; Martin, A.; Starcevic, V.; Krause, M. Effect of Impulse Control Disorders on Disability and Quality of Life in Parkinson’s Disease Patients. J. Clin. Neurosci. 2014, 21, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Previziune: Numarul Bolnavilor de Parkinson se va Dubla pana in Anul 2030. Available online: https://arpim.ro/previziune-numarul-bolnavilor-de-parkinson-se-va-dubla-pana-in-anul-2030/ (accessed on 24 October 2024).
- Garcia-Ruiz, P.J. Impulse Control Disorders and Dopamine-Related Creativity: Pathogenesis and Mechanism, Short Review, and Hypothesis. Front. Neurol. 2018, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, G.P.; Tomescu, D.R.; Pleș, L.; Panaitescu, A.M.; Dragosloveanu, Ș.; Scheau, C.; Sima, R.M.; Coman, I.S.; Grigorean, V.T.; Cochior, D. Implications of Using Artificial Intelligence in the Diagnosis of Sepsis/Sepsis Shock. GERMS 2024, 14, 77–84. [Google Scholar] [CrossRef] [PubMed]

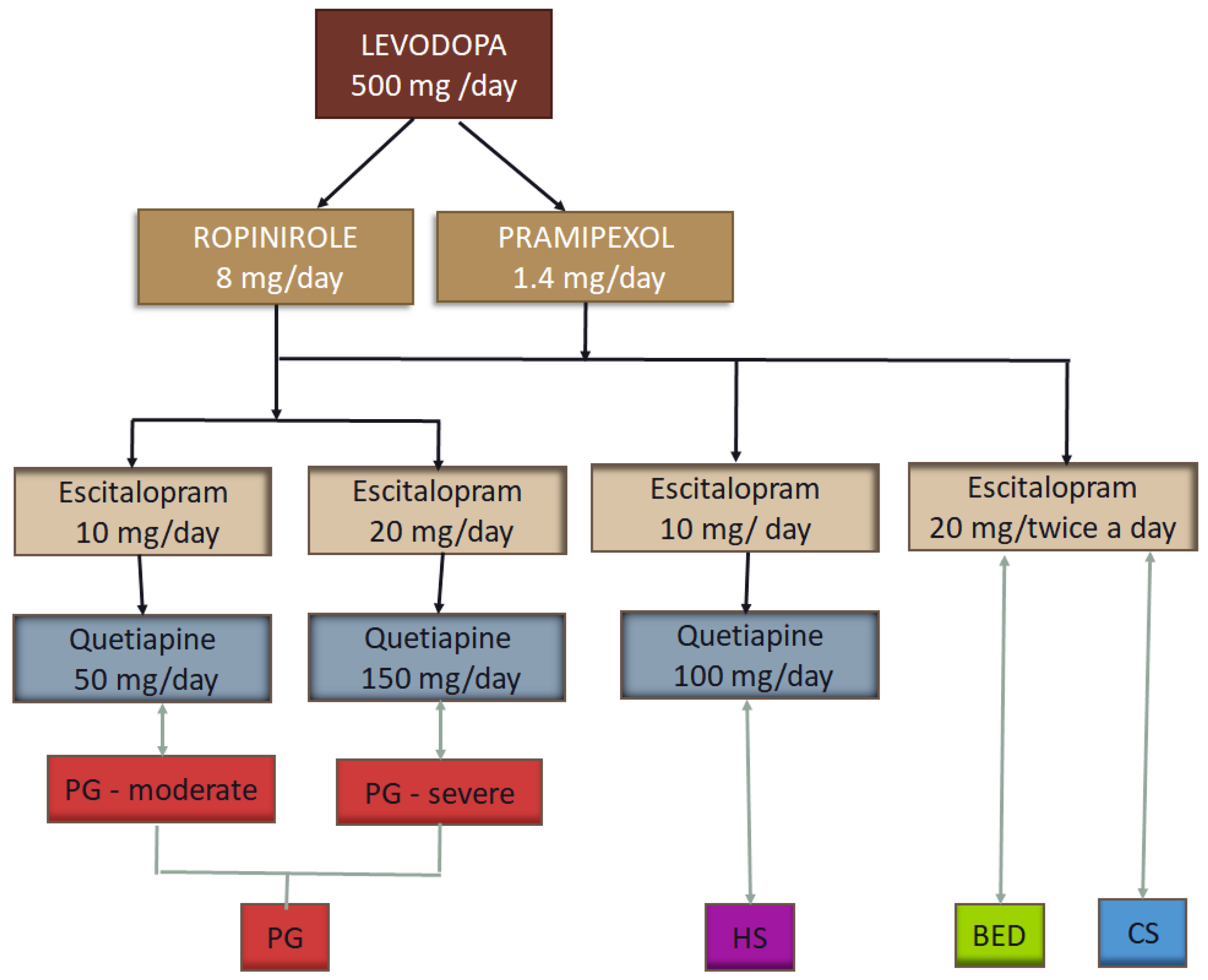
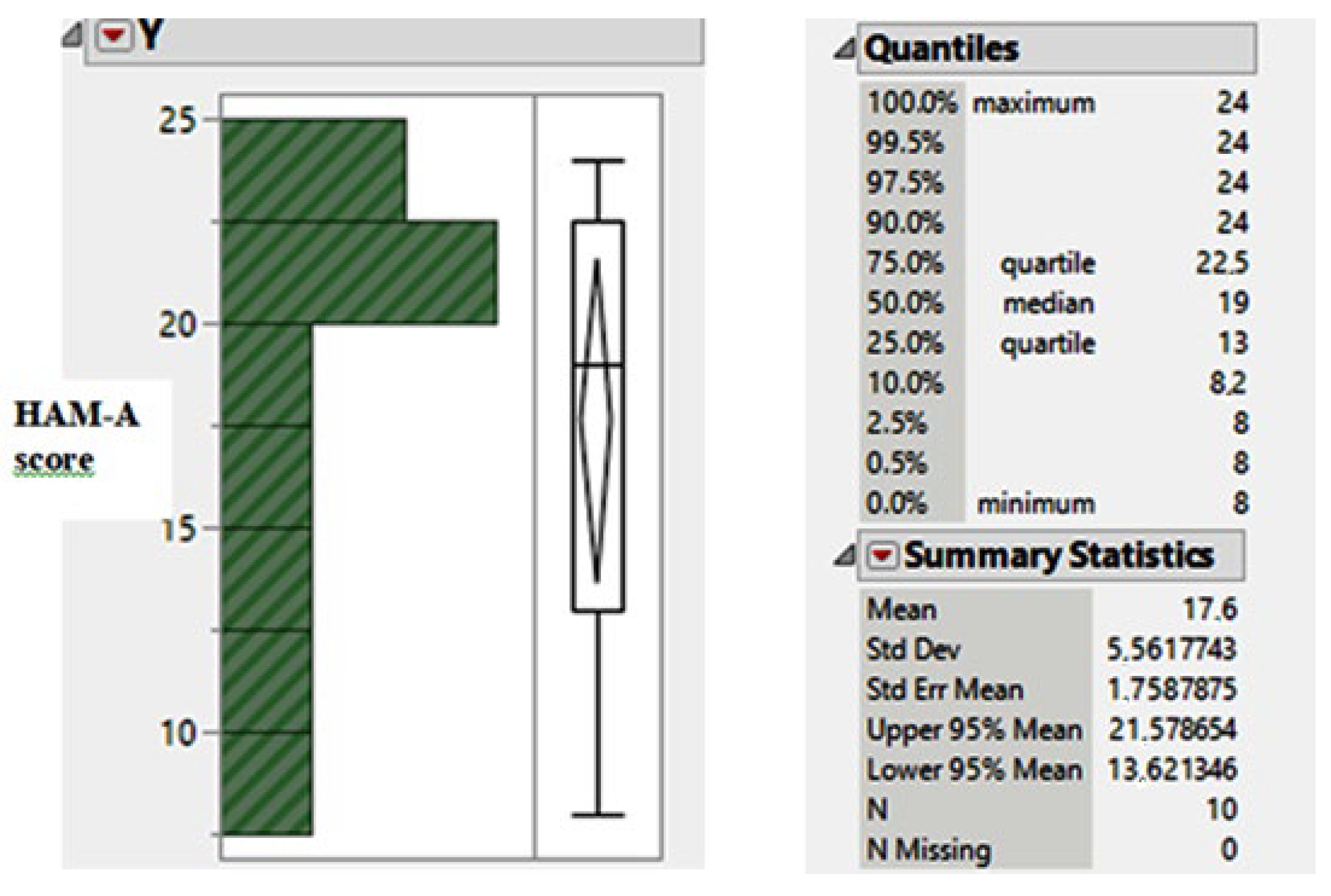
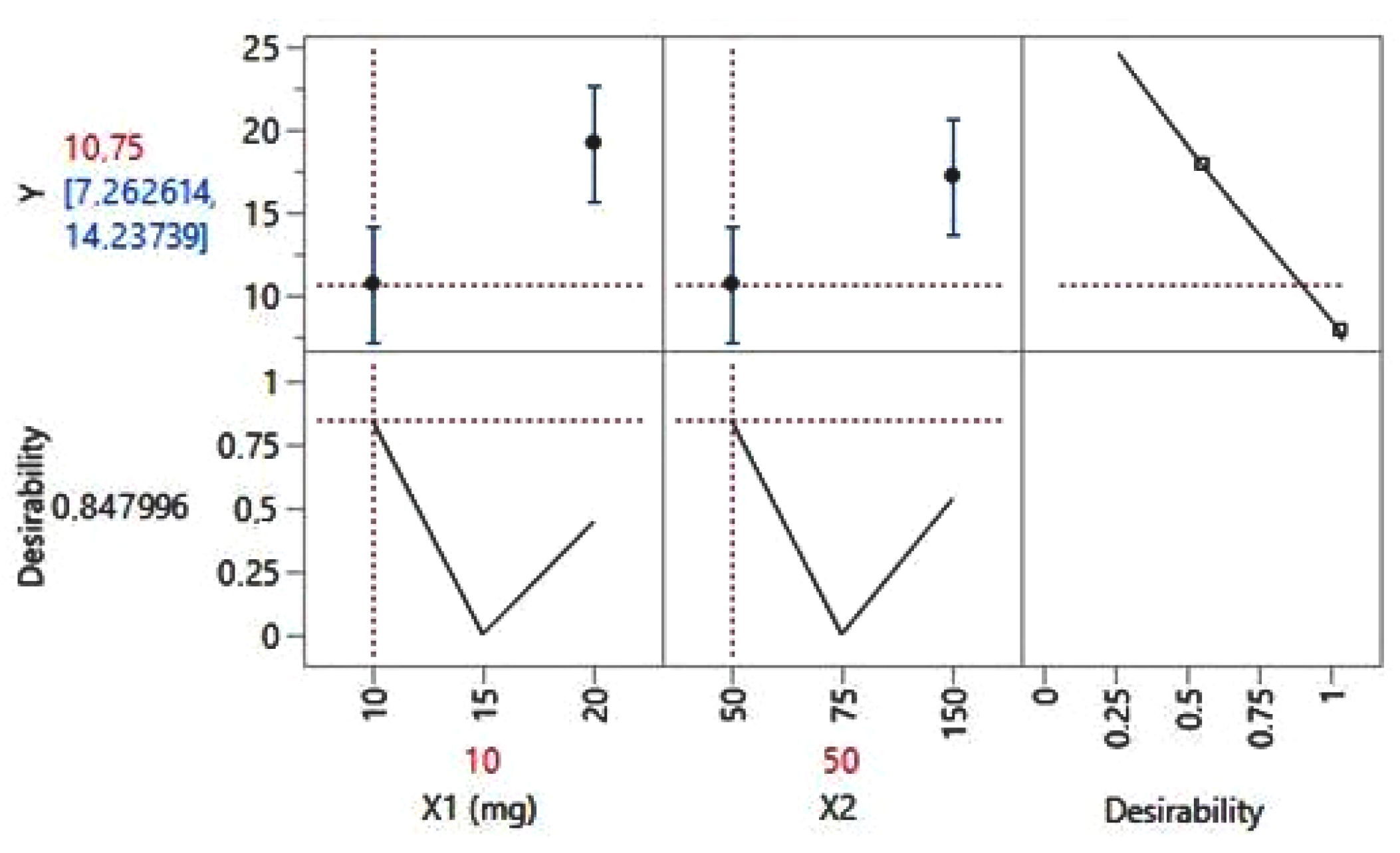
| Patient Code | Number of Patients | Age | Gender | Socio-Economic Status | Marital Status |
|---|---|---|---|---|---|
| 1 | 4 | 42 | Female | High income | Married |
| 2 | 6 | 63 | Male | Low income | Single |
| 3 | 2 | 39 | Female | Low income | Single |
| 4 | 4 | 49 | Male | Low income | Divorced |
| 5 | 4 | 52 | Male | High income | Married |
| 6 | 6 | 52 | Female | Average income | Married |
| 7 | 6 | 66 | Male | Retired | Married |
| 8 | 4 | 63 | Male | Average income | Widow |
| 9 | 2 | 59 | Male | High income | Single |
| 10 | 4 | 50 | Female | Unemployed | Married |
| 11 | 2 | 61 | Male | High income | Divorced |
| 12 | 4 | 49 | Male | Low income | Widow |
| Patient Code | Period Since PD Diagnosis [years] | History of Psychiatric Illness | Smoking Habit/Alcohol Use | Medication | ICD | Hoehn and Yahr Score |
|---|---|---|---|---|---|---|
| 1 | >3 | Anxiety disorder | Smoker | Pramipexol 2.1 mg daily | CS | 3 |
| 2 | >3 | Depression | Smoker and heavy drinker | Levodopa 750 mg + Ropinirole 12 mg daily | PG | 3 |
| 3 | 2 | Anxiety disorder | None | Pramipexol 2.1 mg daily | BED | 2 |
| 4 | 2 | Anxiety disorder | Smoker and moderate drinker | Pramipexol 2.8 mg daily | BED | 2 |
| 5 | >3 | Behavior disorder | Smoker and social drinker | Levodopa 750 mg + Pramipexol 2.1 mg daily | HS | 3 |
| 6 | >3 | Anxiety disorder | Smoker | Levodopa 750 mg + Pramipexol 2.1 mg daily | CS | 3 |
| 7 | >3 | Depression | Smoker and heavy drinker | Levodopa 750 mg + Ropinirole 16 mg daily | PG | 3 |
| 8 | >3 | Depression | Smoker and heavy drinker | Levodopa 750 mg + Pramipexol 2.1 mg daily | PG | 3 |
| 9 | >3 | Behavior disorder | Smoker | Levodopa 750 mg + Pramipexol 2.1 mg daily | HS | 3 |
| 10 | 2 | Anxiety disorder | None | Pramipexol 2.1 mg daily | CS | 2 |
| 11 | >3 | Depression | Smoker and moderate drinker | Levodopa 750 mg + Pramipexol 2.1 mg daily | PG | 3 |
| 12 | >3 | Depression | Smoker | Levodopa 750 mg + Pramipexol 2.8 mg daily | PG | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furdu-Lunguț, E.; Antal, C.; Turcu, S.; Costea, D.-G.; Mitran, M.; Mitran, L.; Diaconescu, A.-S.; Novac, M.-B.; Gorecki, G.-P. Study on Pharmacological Treatment of Impulse Control Disorders in Parkinson’s Disease. J. Clin. Med. 2024, 13, 6708. https://doi.org/10.3390/jcm13226708
Furdu-Lunguț E, Antal C, Turcu S, Costea D-G, Mitran M, Mitran L, Diaconescu A-S, Novac M-B, Gorecki G-P. Study on Pharmacological Treatment of Impulse Control Disorders in Parkinson’s Disease. Journal of Clinical Medicine. 2024; 13(22):6708. https://doi.org/10.3390/jcm13226708
Chicago/Turabian StyleFurdu-Lunguț, Emilia, Claudia Antal, Suzana Turcu, Dan-Gabriel Costea, Mihai Mitran, Loredana Mitran, Andrei-Sebastian Diaconescu, Marius-Bogdan Novac, and Gabriel-Petre Gorecki. 2024. "Study on Pharmacological Treatment of Impulse Control Disorders in Parkinson’s Disease" Journal of Clinical Medicine 13, no. 22: 6708. https://doi.org/10.3390/jcm13226708
APA StyleFurdu-Lunguț, E., Antal, C., Turcu, S., Costea, D.-G., Mitran, M., Mitran, L., Diaconescu, A.-S., Novac, M.-B., & Gorecki, G.-P. (2024). Study on Pharmacological Treatment of Impulse Control Disorders in Parkinson’s Disease. Journal of Clinical Medicine, 13(22), 6708. https://doi.org/10.3390/jcm13226708