The Efficacy of Andexanet Alfa for the Reversal of Factor Xa Inhibitors Is Not Influenced by Hemodilution with Different Volume Expanders
Abstract
1. Introduction
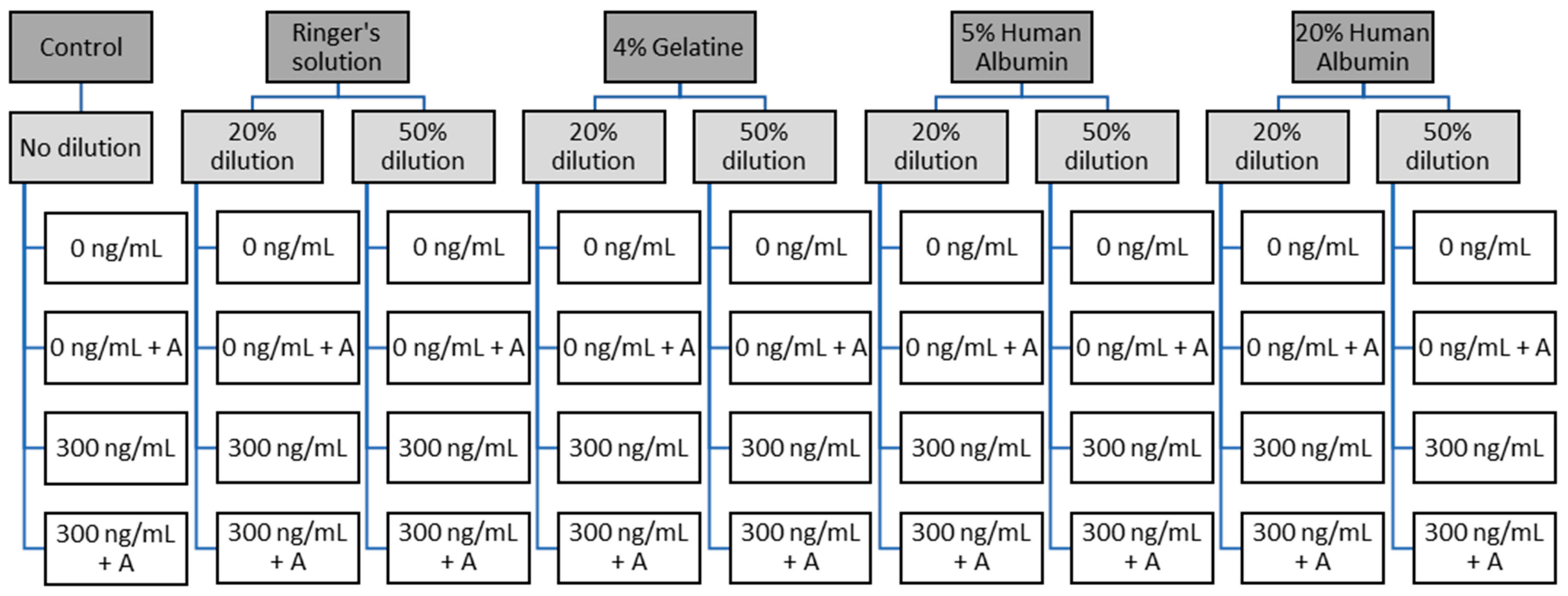
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Participants and Blood Drawing
2.3. Sample Preparation and Analytical Methods
2.3.1. Viscoelastometry
2.3.2. Plasmatic Coagulation Parameters and Thrombin Generation Assays
2.3.3. Chromogenic Anti-FXa Assay
2.4. Statistical Analysis
3. Results
3.1. Blood Count
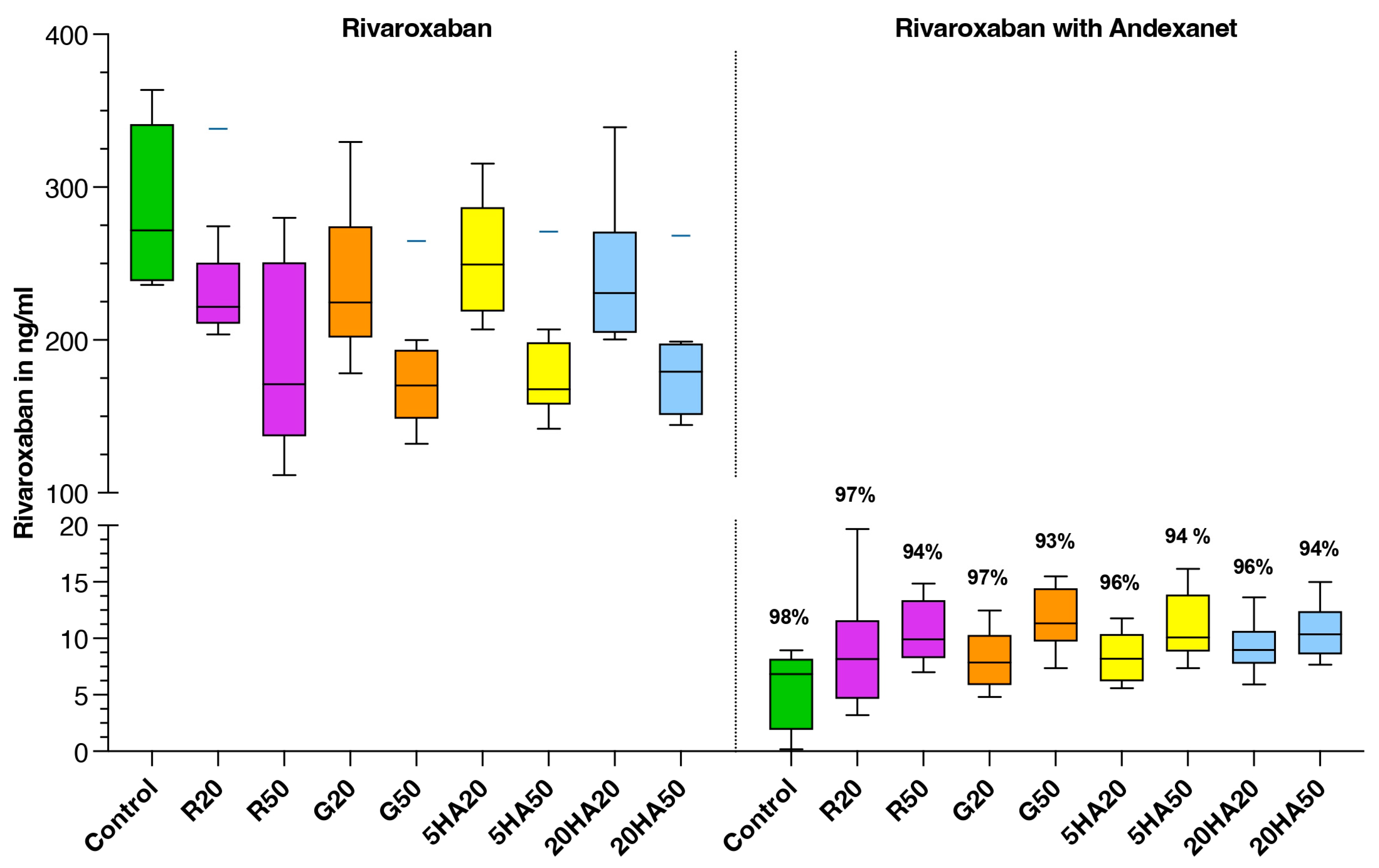
3.2. Plasma Rivaroxaban Levels
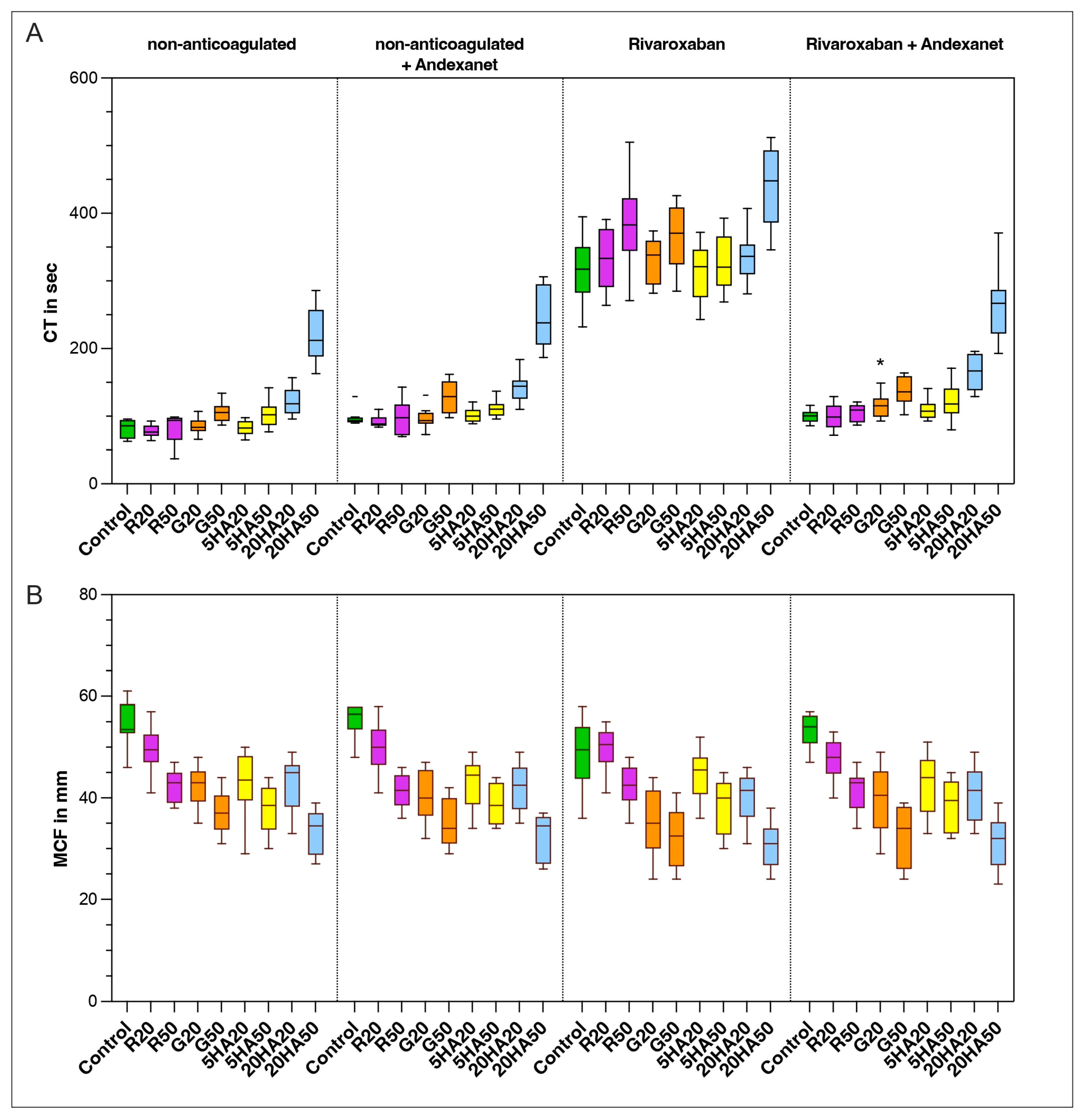
3.3. Thromboelastic Coagulation Parameters
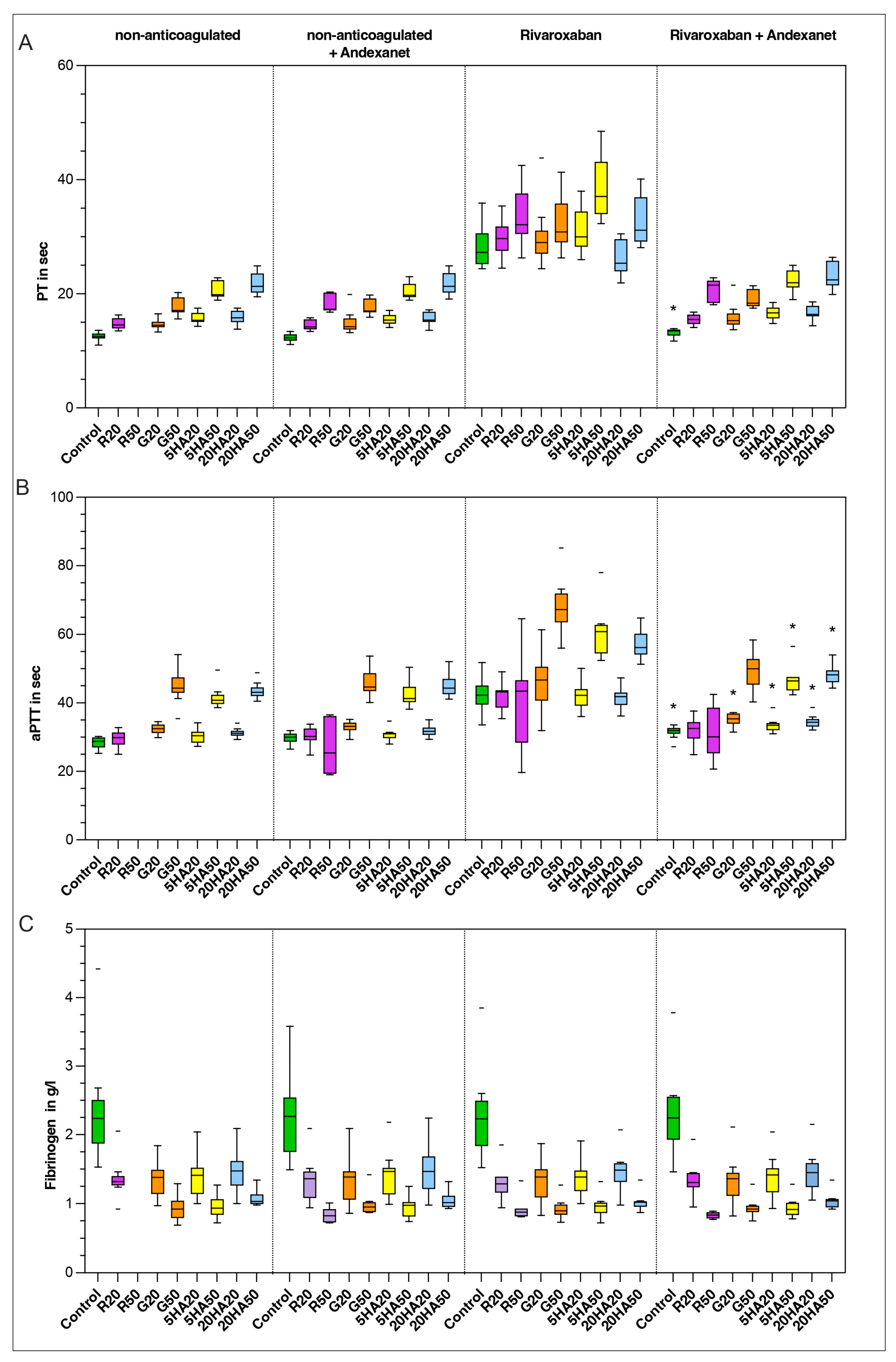
3.4. Plasma-Based Coagulation Parameters
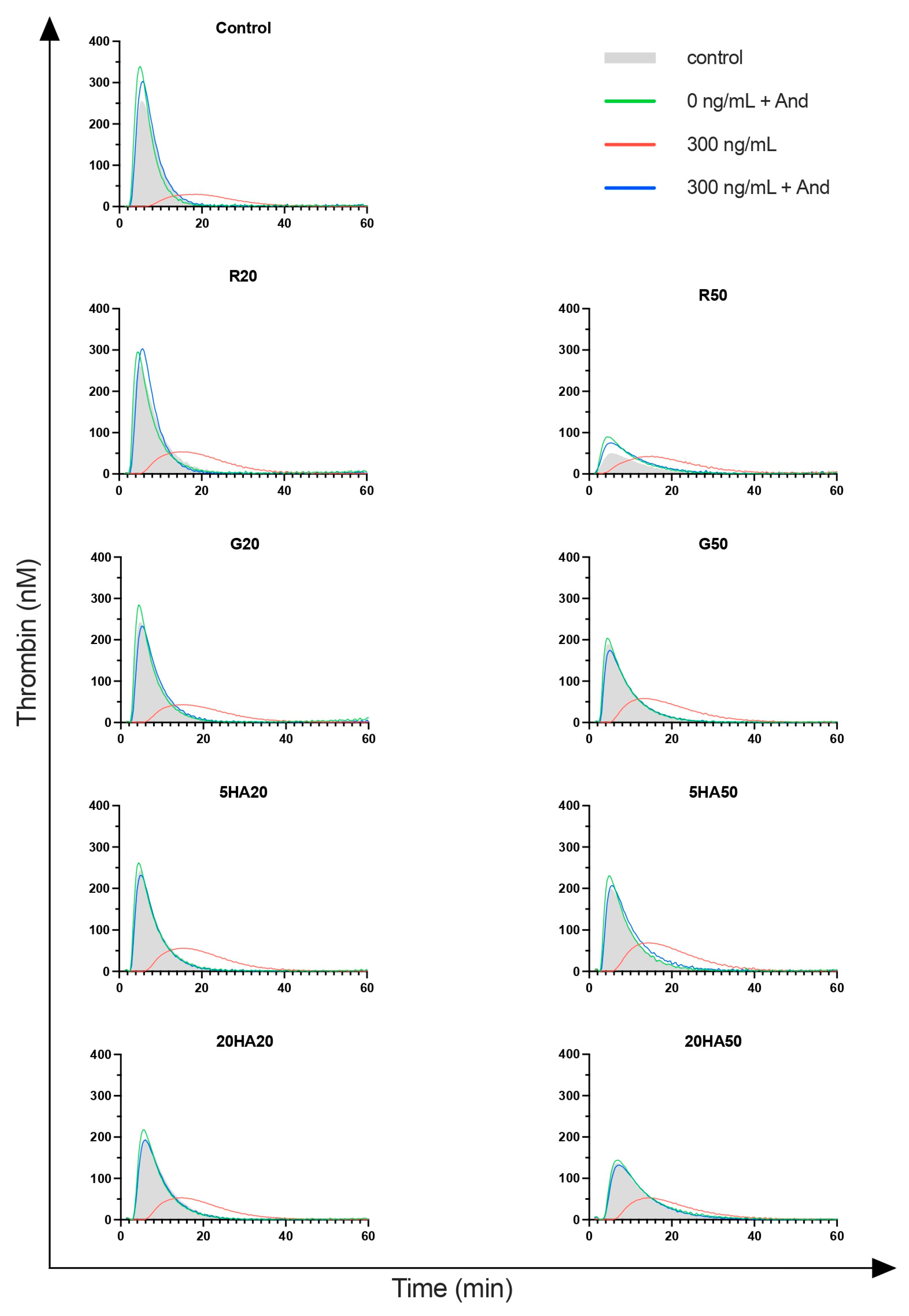
3.5. Thrombin Generation
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perreault, S.; de Denus, S.; White-Guay, B.; Côté, R.; Schnitzer, M.E.; Dubé, M.; Dorais, M.; Tardif, J. Oral Anticoagulant Prescription Trends, Profile Use, and Determinants of Adherence in Patients with Atrial Fibrillation. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; A Morrow, D.; A Murphy, S.; Kuder, J.F.; Deenadayalu, N.; Jarolim, P.; Betcher, J.; Shi, M.; et al. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: An analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 2015, 385, 2288–2295. [Google Scholar] [CrossRef]
- Sarode, R.; Welsby, I.J.; Hoffman, M. Clinical Relevance of Preclinical and Clinical Studies of Four-Factor Prothrombin Complex Concentrate for Treatment of Bleeding Related to Direct Oral Anticoagulants. Ann. Emerg. Med. 2023, 82, 341–361. [Google Scholar] [CrossRef]
- Agency, E.M. Ondexxya, Andexanet Alfa 2019. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ondexxya#authorisation-details-section (accessed on 28 October 2024).
- Siegal, D.M.; Curnutte, J.T.; Connolly, S.J.; Lu, G.; Conley, P.B.; Wiens, B.L.; Mathur, V.S.; Castillo, J.; Bronson, M.D.; Leeds, J.M.; et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N. Engl. J. Med. 2015, 373, 2413–2424. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Crowther, M.; Eikelboom, J.W.; Gibson, C.M.; Curnutte, J.T.; Lawrence, J.H.; Yue, P.; Bronson, M.D.; Lu, G.; Conley, P.B.; et al. Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. N. Engl. J. Med. 2019, 380, 1326–1335. [Google Scholar] [CrossRef]
- Connolly, S.J.; Sharma, M.; Cohen, A.T.; Demchuk, A.M.; Członkowska, A.; Lindgren, A.G.; Molina, C.A.; Bereczki, D.; Toni, D.; Seiffge, D.J.; et al. Andexanet for Factor Xa Inhibitor–Associated Acute Intracerebral Hemorrhage. N. Engl. J. Med. 2024, 390, 1745–1755. [Google Scholar] [CrossRef]
- Rossaint, R.; Afshari, A.; Bouillon, B.; Cerny, V.; Cimpoesu, D.; Curry, N.; Duranteau, J.; Filipescu, D.; Grottke, O.; Grønlykke, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Sixth edition. Crit. Care 2023, 27, 1–45. [Google Scholar] [CrossRef]
- Wang, C.H.; Hsieh, W.H.; Chou, H.C.; Huang, Y.S.; Shen, J.H.; Yeo, Y.H.; Chang, H.E.; Chen, S.C.; Lee, C.C. Liberal versus restricted fluid resuscitation strategies in trauma patients: A systematic review and meta-analysis of randomized controlled trials and observational studies. Crit. Care Med. 2014, 42, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Jonge, E. Clinical relevance of the effects of plasma expanders on coagulation. Semin. Thromb. Hemost. 2007, 33, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Portola Netherlands BV, in Agreement with the European Medicines Agency and the Medicines & Healthcare products Regulatory Agency, MHRA. Commercial Anti-FXa Activity Assays Are Unsuitable for Measuring Anti-FXa Activity Following Administration of Andexanet Alfa 2020. Available online: https://assets.publishing.service.gov.uk/media/5f1701b9d3bf7f5964e33867/Ondexxya-DHCP.pdf (accessed on 28 October 2024).
- Angelillo-Scherrer, A.; Casini, A.; Studt, J.-D.; Gerber, B.; Alberio, L.A.; Fontana, P. Recommendations for the use of andexanet alfa in the management of bleeding in patients on oral factor Xa inhibitors in Switzerland: Guideline from the Working Party Hemostasis of the Swiss Society of Hematology. Swiss Med. Wkly. 2023, 153, 40113. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef]
- Lu, G.; Lin, J.; Bui, K.; Curnutte, J.T.; Conley, P.B. Andexanet versus prothrombin complex concentrates: Differences in reversal of factor Xa inhibitors in in vitro thrombin generation. Res. Pract. Thromb. Haemost. 2020, 4, 1282–1294. [Google Scholar] [CrossRef]
- Yoshii, R.; Sawa, T.; Kawajiri, H.; Amaya, F.; Tanaka, K.A.; Ogawa, S. A comparison of the ClotPro system with rotational thromboelastometry in cardiac surgery: A prospective observational study. Sci. Rep. 2022, 12, 17269. [Google Scholar] [CrossRef]
- Lu, G.; Conley, P.B.; Leeds, J.M.; Karbarz, M.J.; Levy, G.G.; Mathur, V.S.; Castillo, J.; Crowther, M.; Curnutte, J.T. A phase 2 PK/PD study of andexanet alfa for reversal of rivaroxaban and edoxaban anticoagulation in healthy volunteers. Blood Adv. 2020, 4, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Lindhoff-Last, E.; Birschmann, I.; Kuhn, J.; Lindau, S.; Konstantinides, S.; Grottke, O.; Nowak-Göttl, U.; Lucks, J.; Zydek, B.; von Heymann, C.; et al. Pharmacokinetics of Direct Oral Anticoagulants in Emergency Situations: Results of the Prospective Observational RADOA-Registry. Thromb. Haemost. 2021, 122, 552–559. [Google Scholar] [CrossRef]
- Milling, T.J.; Middeldorp, S.; Xu, L.; Koch, B.; Demchuk, A.; Eikelboom, J.W.; Verhamme, P.; Cohen, A.T.; Beyer-Westendorf, J.; Gibson, C.M.; et al. Final Study Report of Andexanet Alfa for Major Bleeding with Factor Xa Inhibitors. Circulation 2023, 147, 1026–1038. [Google Scholar] [CrossRef]
- Viuff, D.; Lauritzen, B.; Pusateri, A.E.; Andersen, S.; Rojkjaer, R.; Johansson, P.I. Effect of haemodilution, acidosis, and hypothermia on the activity of recombinant factor VIIa (NovoSeven). Br. J. Anaesth. 2008, 101, 324–331. [Google Scholar] [CrossRef]
- Chambers, L.A.; Chow, S.J.; Shaffer, L.E. Frequency and Characteristics of Coagulopathy in Trauma Patients Treated With a Low- or High-Plasma-Content Massive Transfusion Protocol. Am. J. Clin. Pathol. 2011, 136, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Ruttmann, T.G.; James, M.F.; Viljoen, J.F. Haemodilution induces a hypercoagulable state. Br. J. Anaesth. 1996, 76, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Sinauridze, E.I.; Gorbatenko, A.S.; Seregina, E.A.; Lipets, E.N.; Ataullakhanov, F.I. Moderate plasma dilution using artificial plasma expanders shifts the haemostatic balance to hypercoagulation. Sci. Rep. 2017, 7, 843. [Google Scholar] [CrossRef] [PubMed]
- E Fletcher, J.; Heard, C.M. Possible mechanism to explain increased coagulability of blood after haemodilution. Br. J. Anaesth. 1997, 78, 478. [Google Scholar] [CrossRef]
- Oberladstätter, D.; Schlimp, C.J.; Zipperle, J.; Osuchowski, M.F.; Voelckel, W.; Grottke, O.; Schöchl, H. Impact of Idarucizumab and Andexanet Alfa on DOAC Plasma Concentration and ClotPro® Clotting Time: An Ex Vivo Spiking Study in A Cohort of Trauma Patients. J. Clin. Med. 2021, 10, 3476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wienhold, J.; Rossaint, R.; Vandeput, E.; Grottke, O. The Efficacy of Andexanet Alfa for the Reversal of Factor Xa Inhibitors Is Not Influenced by Hemodilution with Different Volume Expanders. J. Clin. Med. 2024, 13, 6706. https://doi.org/10.3390/jcm13226706
Wienhold J, Rossaint R, Vandeput E, Grottke O. The Efficacy of Andexanet Alfa for the Reversal of Factor Xa Inhibitors Is Not Influenced by Hemodilution with Different Volume Expanders. Journal of Clinical Medicine. 2024; 13(22):6706. https://doi.org/10.3390/jcm13226706
Chicago/Turabian StyleWienhold, Jan, Rolf Rossaint, Eline Vandeput, and Oliver Grottke. 2024. "The Efficacy of Andexanet Alfa for the Reversal of Factor Xa Inhibitors Is Not Influenced by Hemodilution with Different Volume Expanders" Journal of Clinical Medicine 13, no. 22: 6706. https://doi.org/10.3390/jcm13226706
APA StyleWienhold, J., Rossaint, R., Vandeput, E., & Grottke, O. (2024). The Efficacy of Andexanet Alfa for the Reversal of Factor Xa Inhibitors Is Not Influenced by Hemodilution with Different Volume Expanders. Journal of Clinical Medicine, 13(22), 6706. https://doi.org/10.3390/jcm13226706





