Contemporary Single-Center Experience of Complete Aortic Arch Replacement Employing the Frozen Elephant Trunk Technique in Patients with Extensive Aortic Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Indications
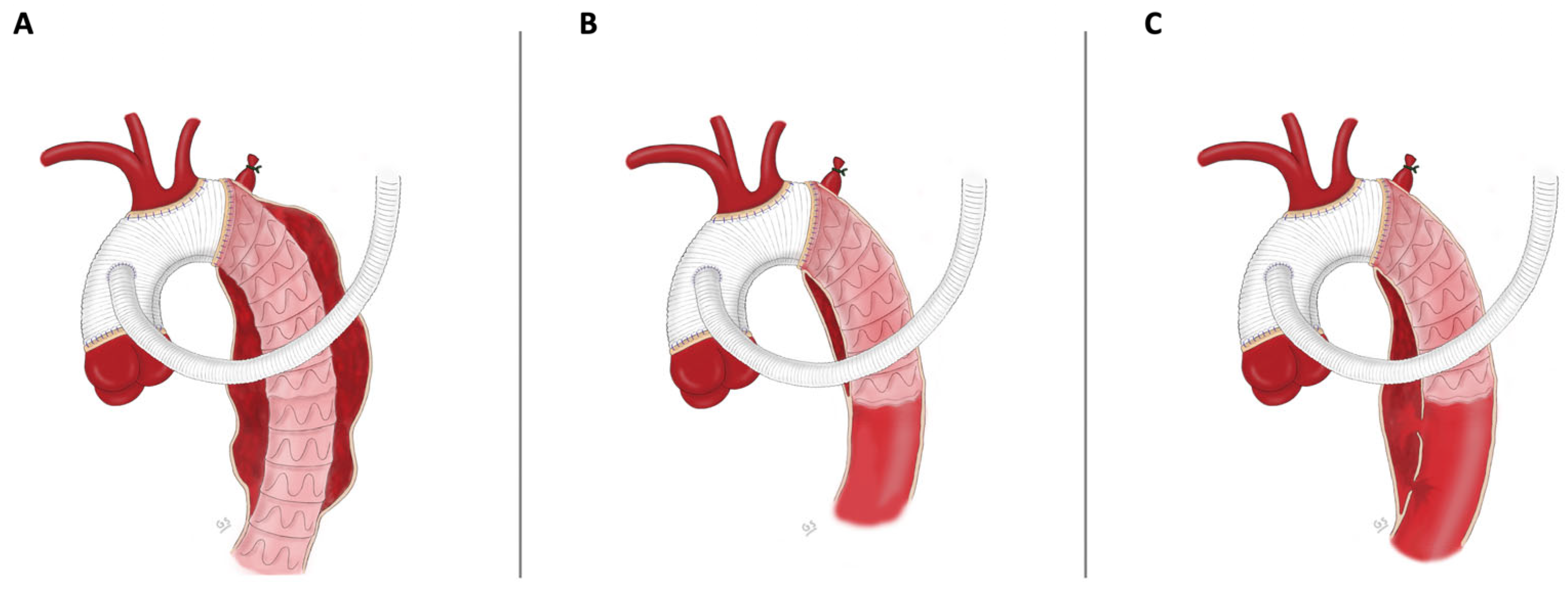
2.3. Surgical Technique
3. Results
3.1. Baseline Characteristics
3.2. Intraoperative Data
3.3. Early Outcome
3.4. Follow-Up
3.5. Secondary Aortic Interventions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borst, H.; Walterbusch, G.; Schaps, D. Extensive Aortic Replacement using “Elephant Trunk” Prosthesis. Thorac Cardiovasc. Surg. 1983, 31, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Bachet, J.; Bavaria, J.; Carrel, T.P.; De Paulis, R.; Di Bartolomeo, R.; Etz, C.D.; Grabenwöger, M.; Grimm, M.; Haverich, A.; et al. Current status and recommendations for use of the frozen elephant trunk technique: A position paper by the Vascular Domain of EACTS. Eur. J. Cardiothorac. Surg. 2015, 47, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, R.; Di Marco, L.; Armaro, A.; Marsilli, D.; Leone, A.; Pilato, E.; Pacini, D. Treatment of complex disease of the thoracic aorta: The frozen elephant trunk technique with the E-vita open prosthesis☆. Eur. J. Cardio-Thorac. Surg. 2009, 35, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Gkremoutis, A.; Zierer, A.; Schmitz-Rixen, T.; Ahmad, A.E.-S.; Kaiser, E.; Keese, M.; Schmandra, T. Staged treatment of mega aortic syndrome using the frozen elephant trunk and hybrid thoracoabdominal repair. J. Thorac. Cardiovasc. Surg. 2017, 154, 1842–1849. [Google Scholar] [CrossRef]
- Czerny, M.; Berger, T.; Della Corte, A.; Kreibich, M.; Lescan, M.; A Mestres, C.; Quintana, E.; Rylski, B.; Schoenhoff, F.; EACTS/STS Scientific Document Group; et al. Clinical cases referring to the 2023 EACTS/STS guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur. J. Cardio-Thoracic Surg. 2024, 65, ezad426. [Google Scholar] [CrossRef]
- Leone, A.; Beckmann, E.; Aandreas, M.; Di Marco, L.; Pantaleo, A.; Reggiani, L.B.; Haverich, A.; Di Bartolomeo, R.; Pacini, D.; Sherestha, M. Total aortic arch replacement with frozen elephant trunk technique: Results from two European institutes. J. Thorac. Cardiovasc. Surg. 2020, 159, 1201–1211. [Google Scholar] [CrossRef]
- Shrestha, M.; Martens, A.; Kaufeld, T.; Beckmann, E.; Bertele, S.; Krueger, H.; Neuser, J.; Fleissner, F.; Ius, F.; Alhadi, F.A.; et al. Single-centre experience with the frozen elephant trunk technique in 251 patients over 15 years†. Eur. J. Cardio-Thoracic Surg. 2017, 52, 858–866. [Google Scholar] [CrossRef]
- Tsagakis, K.; Pacini, D.; Grabenwöger, M.; Borger, M.A.; Goebel, N.; Hemmer, W.; Santos, A.L.; Sioris, T.; Widenka, K.; Risteski, P.; et al. Results of frozen elephant trunk from the international E-vita Open registry. Ann. Cardiothorac. Surg. 2020, 9, 178–188. [Google Scholar] [CrossRef]
- Baraki, H.; Hagl, C.; Khaladj, N.; Kallenbach, K.; Weidemann, J.; Haverich, A.; Karck, M. The Frozen Elephant Trunk Technique for Treatment of Thoracic Aortic Aneurysms. Ann. Thorac. Surg. 2007, 83, S819–S823. [Google Scholar] [CrossRef]
- Hellgren, T.; Wanhainen, A.; Astudillo, R.; Vikholm, P.; Hellgren, L.; Mani, K. Outcomes of aortic arch repair using the frozen elephant trunk technique: Analysis of a Scandinavian center’s results over 14 years. J. Cardiovasc. Surg. 2023, 64, 215–223. Available online: https://www.minervamedica.it/index2.php?show=R37Y2023N02A0215 (accessed on 1 July 2024). [CrossRef]
- Arnold, Z.; Geisler, D.; Aschacher, T.; Winkler, B.; Lenz, V.; Crailsheim, I.; Folkmann, S.; Harrer, M.; Moidl, R.; Grabenwöger, M.; et al. Long-Term Results with 187 Frozen Elephant Trunk Procedures. J. Clin. Med. 2023, 12, 4143. [Google Scholar] [CrossRef] [PubMed]
- Coselli, J.S.; Roselli, E.E.; Preventza, O.; Malaisrie, S.C.; Stewart, A.; Stelzer, P.; Takayama, H.; Chen, E.P.; Estrera, A.L.; Gleason, T.G.; et al. Total aortic arch replacement using a frozen elephant trunk device: Results of a 1-year US multicenter trial. J. Thorac. Cardiovasc. Surg. 2024, 167, 1680–1692.e2. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, T.-H.; Lee, H.; Kim, M.S.; Heo, W.; Yoo, K.-J.; Cho, B.-K.; Song, S.-W. One-year outcomes of total arch replacement and frozen elephant trunk using the E-vita Open NEO. Eur. J. Cardio-Thoracic Surg. 2024, 65, ezae017. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.E.-S.; Silaschi, M.; Borger, M.; Seidiramool, V.; Hamiko, M.; Leontyev, S.; Zierer, A.; Doss, M.; Etz, C.D.; Benedikt, P.; et al. The Frozen Elephant Technique Using a Novel Hybrid Prosthesis for Extensive Aortic Arch Disease: A Multicentre Study. Adv. Ther. 2023, 40, 1104–1113. [Google Scholar] [CrossRef]
- Katayama, A.; Uchida, N.; Katayama, K.; Arakawa, M.; Sueda, T. The frozen elephant trunk technique for acute type A aortic dissection: Results from 15 years of experience†. Eur. J. Cardio-Thoracic Surg. 2015, 47, 355–3600. [Google Scholar] [CrossRef]
- Berger, T.; Kreibich, M.; Mueller, F.; Rylski, B.; Kondov, S.; Schröfel, H.; Pingpoh, C.; Beyersdorf, F.; Siepe, M.; Czerny, M. The frozen elephant trunk technique for aortic dissection is safe after previous aortic repair. Eur. J. Cardio-Thoracic Surg. 2021, 59, 130–136. [Google Scholar] [CrossRef]
- Demal, T.J.; Bax, L.; Brickwedel, J.; Kölbel, T.; Vettorazzi, E.; Sitzmann, F.; Reichenspurner, H.; Detter, C. Outcome of the frozen elephant trunk procedure as a redo operation. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 85–92. [Google Scholar] [CrossRef]
- Preventza, O.; Liao, J.L.; Olive, J.K.; Simpson, K.; Critsinelis, A.C.; Price, M.D.; Galati, M.; Cornwell, L.D.; Orozco-Sevilla, V.; Omer, S.; et al. Neurologic complications after the frozen elephant trunk procedure: A meta-analysis of more than 3000 patients. J. Thorac. Cardiovasc. Surg. 2020, 160, 20–33.e4. [Google Scholar] [CrossRef]
- Choudhury, R.Y.; Basharat, K.; Zahra, S.A.; Tran, T.; Rimmer, L.; Harky, A.; Idhrees, M.; Bashir, M. “Proximalization is Advancement”—Zone 3 Frozen Elephant Trunk vs Zone 2 Frozen Elephant Trunk: A Literature Review. Vasc. Endovascular. Surg. 2021, 55, 612–618. [Google Scholar] [CrossRef]
- Mousavizadeh, M.; Bashir, M.; Jubouri, M.; Tan, S.Z.; Borzeshi, E.Z.; Ilkhani, S.; Banar, S.; Nakhaei, P.; Rezaei, Y.; Idhrees, M.; et al. Zone proximalization in frozen elephant trunk: What is the optimal zone for open intervention? A systematic review and meta-analysis. J. Cardiovasc. Surg. 2022, 63, 265–274. Available online: https://www.minervamedica.it/index2.php?show=R37Y2022N03A0265 (accessed on 31 December 2023). [CrossRef]
- Leone, A.; Di Marco, L.; Coppola, G.; Amodio, C.; Berardi, M.; Mariani, C.; Votano, D.; Reggiani, M.L.B.; Di Bartolomeo, R.; Pacini, D. Open distal anastomosis in the frozen elephant trunk technique: Initial experiences and preliminary results of arch zone 2 versus arch zone 3. Eur. J. Cardio-Thoracic Surg. 2019, 56, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Zierer, A.; Ahmad, A.E.-S.; Papadopoulos, N.; Moritz, A.; Diegeler, A.; Urbanski, P.P. Selective antegrade cerebral perfusion and mild (28 °C–30 °C) systemic hypothermic circulatory arrest for aortic arch replacement: Results from 1002 patients. J. Thorac. Cardiovasc. Surg. 2012, 144, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.E.-S.; Risteski, P.; Ay, M.; Papadopoulos, N.; Moritz, A.; Zierer, A. Moderate Hypothermic Circulatory Arrest (≥28 °C) with Selective Antegrade Cerebral Perfusion for Total Arch Replacement with Frozen Elephant Trunk Technique. Thorac. Cardiovasc. Surg. 2019, 67, 345–350. [Google Scholar]
- Berger, T.; Weiss, G.; Voetsch, A.; Arnold, Z.; Kreibich, M.; Rylski, B.; Krombholz-Reindl, P.; Winkler, A.; Mach, M.; Geisler, D.; et al. Multicentre experience with two frozen elephant trunk prostheses in the treatment of acute aortic dissection†. Eur. J. Cardio-Thoracic Surg. 2019, 56, 572–578. [Google Scholar] [CrossRef]
- Easo, J.; Weigang, E.; Hölzl, P.P.; Horst, M.; Hoffmann, I.; Blettner, M.; Dapunt, O.E. Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection—analysis of the German Registry for Acute Aortic Dissection type A (GERAADA). Ann. Cardiothorac. Surg. 2013, 2, 175–180. [Google Scholar] [CrossRef]
- Conzelmann, L.O.; Weigang, E.; Mehlhorn, U.; Abugameh, A.; Hoffmann, I.; Blettner, M.; Etz, C.D.; Czerny, M.; Vahl, C.F. Mortality in patients with acute aortic dissection type A: Analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur. J. Cardio-Thoracic Surg. 2016, 49, e44–e52. [Google Scholar] [CrossRef]
- De Rango, P.; Ferrer, C.; Coscarella, C.; Musumeci, F.; Verzini, F.; Pogany, G.; Montalto, A.; Cao, P. Contemporary comparison of aortic arch repair by endovascular and open surgical reconstructions. J. Vasc. Surg. 2015, 61, 339–346. [Google Scholar] [CrossRef]
- Riambau, V.; Böckler, D.; Brunkwall, J.; Cao, P.; Chiesa, R.; Coppi, G.; Czerny, M.; Fraedrich, G.; Haulon, S.; Jacobs, M.; et al. Editor’s Choice—Management of Descending Thoracic Aorta Diseases. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef]
- McWilliams, R.G.; Murphy, M.; Hartley, D.; Lawrence-Brown, M.M.D.; Harris, P.L. In Situ Stent-Graft Fenestration to Preserve the Left Subclavian Artery. J. Endovasc. Ther. 2004, 11, 170–174. [Google Scholar] [CrossRef]
- Rizza, A.; Trimarchi, G.; Di Sibio, S.; Bastiani, L.; Murzi, M.; Palmieri, C.; Foffa, I.; Berti, S. Preliminary Outcomes of Zone 2 Thoracic Endovascular Aortic Repair Using Castor Single-Branched Stent Grafts: A Single-Center Experience. J. Clin. Med. 2023, 12, 7593. [Google Scholar] [CrossRef]
- Folkmann, S.; Weiss, G.; Pisarik, H.; Czerny, M.; Grabenwoger, M. Thoracoabdominal aortic aneurysm repair after frozen elephant trunk procedure. Eur. J. Cardio-Thorac. Surg. 2015, 47, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Kreibich, M.; Berger, T.; Walter, T.; Potratz, P.; Discher, P.; Kondov, S.; Beyersdorf, F.; Siepe, M.; Gottardi, R.; Czerny, M.; et al. Downstream thoracic endovascular aortic repair following the frozen elephant trunk procedure. Cardiovasc. Diagn. Ther. 2022, 12, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Haensig, M.; Schmidt, A.; Staab, H.; Steiner, S.; Scheinert, D.; Branzan, D. Endovascular Repair of the Thoracic or Thoracoabdominal Aorta Following the Frozen Elephant Trunk Procedure. Ann. Thorac. Surg. 2020, 109, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Huber, F.; Schachner, B.; Hagleitner, G.; Binder, R.K.; Pichler, P.; Zierer, A. Extensive thoracoabdominal aortic electro septotomy: A case report of a novel approach in the treatment of chronic aortic dissections. SAGE Open Med. Case Rep. 2024, 12, 2050313X231225867. [Google Scholar] [CrossRef]

| Preoperative Characteristics | TAA (n = 32) | AAD (n = 32) | CAD (n = 68) | Total (n = 132) | p |
|---|---|---|---|---|---|
| Age, y | 68 (62–73) | 62 (51–69) | 60 (53–67) | 63 (55–69) | 0.005 *† |
| Male | 18 (56.3%) | 22 (68.8%) | 47 (69.1%) | 87 (65.9%) | 0.416 |
| Height | 170 (162–178) | 175 (169–182) | 175 (168–180) | 174 (168–180) | 0.053 |
| Weight | 71 (61–178) | 83 (72–98) | 81 (73–96) | 80 (70–95) | 0.030 * |
| Heart failure | 4 (12.5%) | 1 (3.1%) | 1 (1.5%) | 6 (4.5%) | 0.047 |
| COPD | 6 (18.8%) | 1 (3.1%) | 5 (7.4%) | 12 (9.1%) | 0.115 |
| Hypertension | 20 (62.5%) | 19 (59.4%) | 48 (70.6%) | 87 (65.9%) | 0.647 |
| Chronic kidney disease | 9 (28.1%) | 3 (9.4%) | 11 (16.2%) | 23 (17.4%) | 0.156 |
| Preoperative dialysis | 1 (3.1%) | 0 | 2 (2.9%) | 3 (2.3%) | 0.999 |
| Peripheral arterial disease | 4 (12.5%) | 0 | 1 (1.5%) | 5 (3.8%) | 0.037 |
| Cerebrovascular disease | 2 (6.3%) | 1 (3,1%) | 1 (1.5%) | 4 (3.0%) | 0.434 |
| Diabetes | 2 (6.3%) | 2 (6.3%) | 5 (7.4%) | 9 (6.8%) | 0.999 |
| Redo-Procedure | 7 (21.9%) | 3 (9.4%) | 38 (55.9%) | 48 (36.4%) | <0.001 †‡ |
| Intraoperative Data | TAA (n = 32) | AAD (n = 32) | CAD (n = 68) | Total (n = 132) | p |
|---|---|---|---|---|---|
| Procedure time, min | 337 (299–355) | 344 (313–404) | 332 (282–376) | 336 (300–373) | 0.145 |
| Cardiopulmonary bypass time, min | 188 (144–204) | 190 (179–223) | 175 (155–200) | 180 (161–205) | 0.022 ‡ |
| Cardiac ischemia time, min | 83 (71–117) | 109 (90–123) | 78 (66–102) | 89 (70–113) | 0.001 *‡ |
| ASCP time, min | 42 (35–54) | 50 (41–56) | 43 (38–54) | 45 (38–54) | 0.099 |
| ASCP number | |||||
| Unilateral | 10 (31.3%) | 19 (59.4%) | 30 (44.1%) | 59 (44.7%) | |
| Bilateral | 22 (68.8%) | 13 (40.6%) | 38 (55.9%) | 73 (55.3%) | |
| Distal Anastomosis | |||||
| Zone 2 | 15 (46.9%) | 19 (59.4%) | 38 (55.9%) | 72 (54.5%) | |
| Zone 3 | 17 (53.1%) | 13 (40.6%) | 13 (40.6%) | 60 (45.5%) | |
| Concomitant Procedure Aortic valve replacement Bentall procedure David procedure CABG TEVAR: Myectomy Septotomy | 12 (37.5%) 3 (9.4%) 1 (3.1%) 0 7 (21.9%) 1 (3.1%) 0 0 | 7 (21.9%) 0 3 (9.4%) 0 1 (3.1%) 1 (3.1%) 1 (3.1%) 1 (3.1%) | 17 (25%) 3 (4.4%) 1 (1.5%) 1 (1.5%) 2 (2.9%) 7 (10.3%) 0 3 (4.4%) | 36 (27.3%) 6 (4.5%) 5 (3.8%) 1 (0.8%) 10 (7.6) 9 (6.8%) 1 (0.8%) 4 (3.0%) | 0.195 |
| Postoperative Data | TAA (n = 32) | AAD (n = 32) | CAD (n = 68) | Total (n = 132) | p |
|---|---|---|---|---|---|
| Stroke | 1 (3.1%) | 4 (12.5%) | 4 (5.9%) | 9 (6.8%) | 0.389 |
| Spinal cord injury | |||||
| Transient | 3 (9.4%) | 2 (6.3%) | 4 (5.9%) | 9 (6.8%) | 0.898 |
| Permanent | 1 (3.1%) | 1 (3.1%) | 1 (1.5%) | 3 (2.3%) | 0.611 |
| Hemodialysis | |||||
| Transient | 7 (21.9%) | 10 (31.3%) | 6 (8.8%) | 23 (17.4%) | 0.016 ‡ |
| Permanent | 1 (3.1%) | 3 (9.4%) | 1 (1.5%) | 5 (3.8%) | 0.106 |
| Prolonged ventilation (>72 h) | 3 (9.4%) | 10 (31.3%) | 2 (2.9%) | 15 (11.4%) | <0.001 ‡ |
| Rethoracotomy | 0 | 6 (18.8%) | 6 (8.8%) | 12 (9.1%) | 0.034 * |
| ICU median stay, d | 4 (2–6) | 6 (3–10) | 4 (2–6) | 4 (2–7) | 0.042 ‡ |
| Hospital median stay, d | 21 (12–35) | 16 (10–33) | 21 (15–27) | 21 (13–30) | 0.732 |
| Endovascular Reintervention | 15 (46.9%) | 9 (28.1%) | 30 (44.1%) | 54 (40.9%) | 0.232 |
| In-hospital mortality (30 d) | 1 (3.1%) | 7 (21.9%) | 2 (2.9%) | 10 (7.6%) | 0.004 ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoeberl, A.-K.; Huber, F.; Schachner, B.; Preinfalk, V.; Zierer, A. Contemporary Single-Center Experience of Complete Aortic Arch Replacement Employing the Frozen Elephant Trunk Technique in Patients with Extensive Aortic Disease. J. Clin. Med. 2024, 13, 6640. https://doi.org/10.3390/jcm13226640
Schoeberl A-K, Huber F, Schachner B, Preinfalk V, Zierer A. Contemporary Single-Center Experience of Complete Aortic Arch Replacement Employing the Frozen Elephant Trunk Technique in Patients with Extensive Aortic Disease. Journal of Clinical Medicine. 2024; 13(22):6640. https://doi.org/10.3390/jcm13226640
Chicago/Turabian StyleSchoeberl, Armin-Kai, Florian Huber, Bruno Schachner, Valentina Preinfalk, and Andreas Zierer. 2024. "Contemporary Single-Center Experience of Complete Aortic Arch Replacement Employing the Frozen Elephant Trunk Technique in Patients with Extensive Aortic Disease" Journal of Clinical Medicine 13, no. 22: 6640. https://doi.org/10.3390/jcm13226640
APA StyleSchoeberl, A.-K., Huber, F., Schachner, B., Preinfalk, V., & Zierer, A. (2024). Contemporary Single-Center Experience of Complete Aortic Arch Replacement Employing the Frozen Elephant Trunk Technique in Patients with Extensive Aortic Disease. Journal of Clinical Medicine, 13(22), 6640. https://doi.org/10.3390/jcm13226640







