Exploring Metabolic Mechanisms in Calcific Tendinopathy and Shoulder Arthrofibrosis: Insights and Therapeutic Implications
Abstract
1. Introduction
2. Background and Etiology
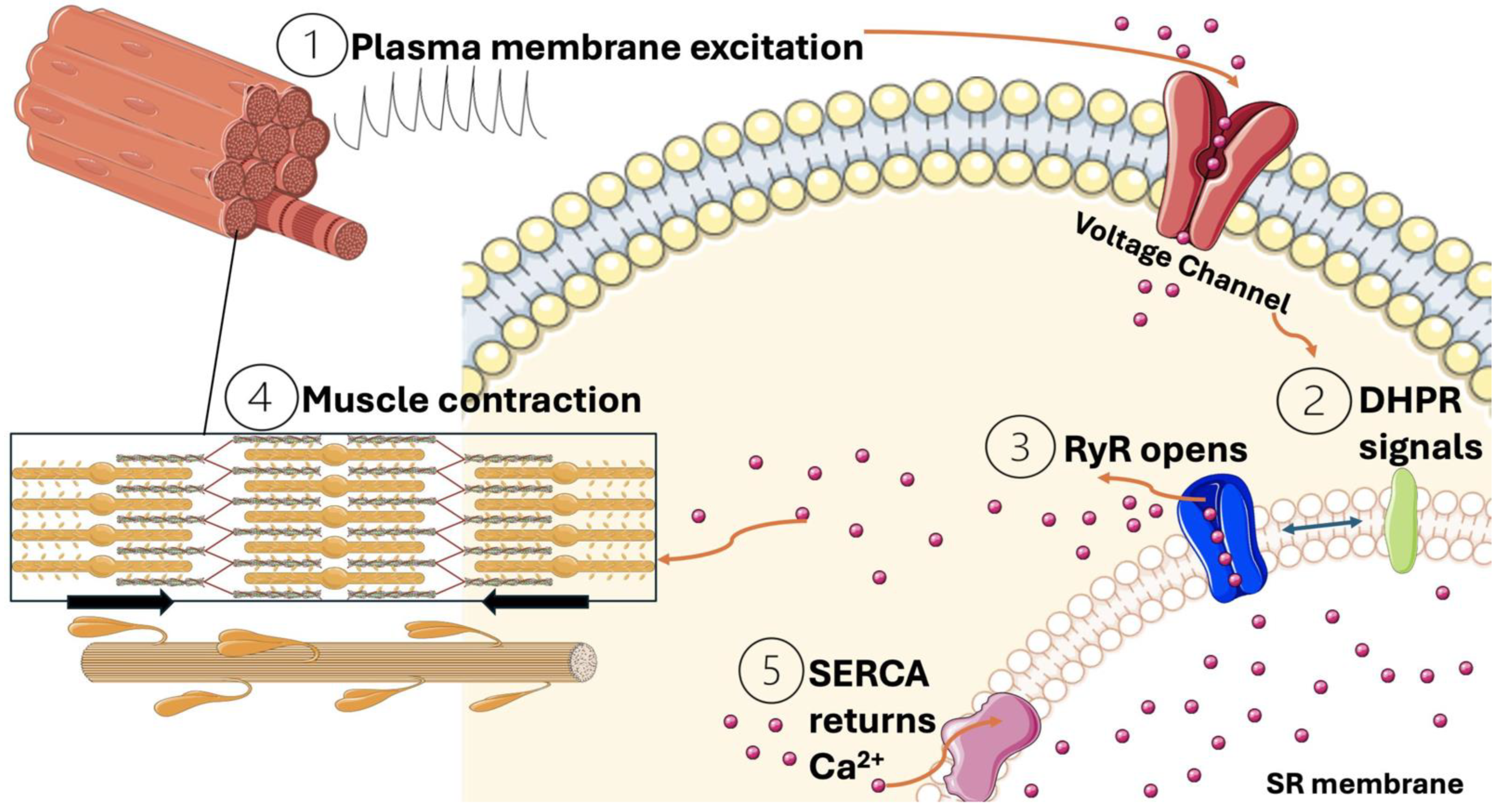
3. Calcium as a Vital Signaling Molecule During Muscle Contraction
4. Calcium’s Role in Tendon Stem Cell Modulation and Proliferation
5. Modulation of Calcium Channels
6. Calcium Homeostasis in Skeletal Muscle Fibro-Adipogenic Progenitor Cells
7. Calcium as an Activator of Signaling Cascade
8. Calcium Homeostasis Under Hypoxic Conditions
9. Concluding Remarks and Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | Adhesive capsulitis |
| BMI | Body mass index |
| BMP | Bone morphogenetic protein |
| COL1A1 | Alpha1 chain of type I collagen |
| CRYab | Crystallin alpha B |
| DHPR | Dihydropyridine receptors |
| ECC | Excitation coupling contraction |
| ECM | Extracellular matrix |
| ERK | Extracellular signal regulated kinase |
| ER | Estrogen receptor |
| FAPs | Fibro-adipogenic progenitors |
| GSK3β | Glycogen synthase kinase 3 |
| HIF | Hypoxia inducible factors |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| IP3 | Inositol 1,4,5 trisphosphate |
| MAPK | Mitogen activated protein kinases |
| MMP | Matrix metalloproteinase |
| MSCs | Mesenchymal stem cells |
| MuSC | Muscle stem cell |
| NFAT | Nuclear factor of activated T cells |
| OSX | Osterix |
| PDGFRα | Platelet-derived growth factor receptor alpha isoform |
| PHDs | Prolyl hydroxylase enzymes |
| PI3K | Phosphatidylinositol 3 kinase |
| PTGES | Prostaglandin E Synthase |
| RCCT | Rotator cuff calcific tendinopathy |
| RCT | Rotator cuff tears |
| ROM | Range of motion |
| ROS | Reactive oxygen species |
| RUNX2 | Runt-related transcriptional factor 2 |
| RyR | Ryanodine receptors |
| SCA | Stem cell antigen |
| SCN4A | Sodium channels alpha subunit |
| SCX | Scleraxis |
| SERCA | Sarcoplasmic reticulum calcium ATPase pump |
| SMAD | Suppressor of mothers against decapentaplegic |
| TGFβ | Transforming growth factor β |
| TNMD | Tenomodulin |
| TSCs | Tendon stem cells |
| VEGF | Vascular endothelial growth factor |
References
- Chianca, V.; Albano, D.; Messina, C.; Midiri, F.; Mauri, G.; Aliprandi, A.; Catapano, M.; Pescatori, L.C.; Monaco, C.G.; Gitto, S.; et al. Rotator Cuff Calcific Tendinopathy: From Diagnosis to Treatment. Acta Bio Medica Atenei Parm. 2018, 89, 186–196. [Google Scholar]
- Akbar, M.; Crowe, L.A.N.; McLean, M.; Garcia-Melchor, E.; MacDonald, L.; Carter, K.; Fazzi, U.G.; Martin, D.; Arthur, A.; Reilly, J.H.; et al. Translational Targeting of Inflammation and Fibrosis in Frozen Shoulder: Molecular Dissection of the T Cell/IL-17A Axis. Proc. Natl. Acad. Sci. USA 2021, 118, e2102715118. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.; Alyami, A.H.; Althaqafi, R.M.M.; Alzeyadi, A.; Alrubaei, F.S.; Alyami, A.A.; Singer, M.S.; Saati, A.A.; Alotaibi, W.T.; Alsharif, M.O. Cytokines’ Role in the Pathogenesis and Their Targeting for the Prevention of Frozen Shoulder: A Narrative Review. Cureus 2023, 15, e36070. [Google Scholar] [CrossRef] [PubMed]
- Brindisino, F.; Girardi, G.; Crestani, M.; Fiore, A.; Giovannico, G.; Garzonio, F.; Venturin, D.; Struyf, F. Effectiveness of Electrophysical Agents in Subjects with Frozen Shoulder: A Systematic Review and Meta-Analysis. Disabil. Rehabil. 2023, 46, 3513–3534. [Google Scholar] [CrossRef]
- Cao, W.; Chen, J.; Pu, J.; Fan, Y.; Cao, Y. Risk Factors for the Onset of Frozen Shoulder in Middle-Aged and Elderly Subjects Within 1 Year of Discharge From a Hospitalization That Involved Intravenous Infusion: A Prospective Cohort Study. Front. Med. 2022, 9, 911532. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, J.; Zhu, T.; Cui, J.; Deng, Z.; Chen, K.; Chang, C.; Geng, Y.; Chen, F.; Ouyang, K.; et al. Pathological Changes of Frozen Shoulder in Rat Model and the Therapeutic Effect of PPAR-γ Agonist. J. Orthop. Res. 2021, 39, 891–901. [Google Scholar] [CrossRef]
- Cher, J.Z.B.; Akbar, M.; Kitson, S.; Crowe, L.A.N.; Garcia-Melchor, E.; Hannah, S.C.; McLean, M.; Fazzi, U.G.; Kerr, S.C.; Murrell, G.A.C.; et al. Alarmins in Frozen Shoulder: A Molecular Association Between Inflammation and Pain. Am. J. Sports Med. 2018, 46, 671–678. [Google Scholar] [CrossRef]
- Cho, C.-H.; Song, K.-S.; Kim, B.-S.; Kim, D.H.; Lho, Y.-M. Biological Aspect of Pathophysiology for Frozen Shoulder. BioMed Res. Int. 2018, 2018, 7274517. [Google Scholar] [CrossRef]
- De La Serna, D.; Navarro-Ledesma, S.; Alayón, F.; López, E.; Pruimboom, L. A Comprehensive View of Frozen Shoulder: A Mystery Syndrome. Front. Med. 2021, 8, 663703. [Google Scholar] [CrossRef]
- Erickson, B.J.; Shishani, Y.; Bishop, M.E.; Romeo, A.A.; Gobezie, R. Adhesive Capsulitis: Demographics and Predictive Factors for Success Following Steroid Injections and Surgical Intervention. Arthrosc. Sports Med. Rehabil. 2019, 1, e35–e40. [Google Scholar] [CrossRef]
- Kingston, K.; Curry, E.J.; Galvin, J.W.; Li, X. Shoulder Adhesive Capsulitis: Epidemiology and Predictors of Surgery. J. Shoulder Elb. Surg. 2018, 27, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Kraal, T.; Lübbers, J.; Van Den Bekerom, M.P.J.; Alessie, J.; Van Kooyk, Y.; Eygendaal, D.; Koorevaar, R.C.T. The Puzzling Pathophysiology of Frozen Shoulders—A Scoping Review. J. Exp. Orthop. 2020, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.T.; MacFarlane, R.J.; Khan, Y.; Waseem, M. The Frozen Shoulder: Myths and Realities. Open Orthop. J. 2013, 7, 352–355. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, G.; Li, Q.; Yin, Y.; Fu, W.; He, K.; Pen, X. Risk Factors and Predictive Models for Frozen Shoulder. Sci. Rep. 2024, 14, 15261. [Google Scholar] [CrossRef]
- Merolla, G.; Bhat, M.G.; Paladini, P.; Porcellini, G. Complications of Calcific Tendinitis of the Shoulder: A Concise Review. J. Orthop. Traumatol. Off. J. Ital. Soc. Orthop. Traumatol. 2015, 16, 175–183. [Google Scholar] [CrossRef]
- Phansopkar, P.; Qureshi, M.I. A Review on Current Notion in Frozen Shoulder: A Mystery Shoulder. Cureus 2022, 14, e29362. [Google Scholar] [CrossRef]
- Millar, N.L.; Meakins, A.; Struyf, F.; Willmore, E.; Campbell, A.L.; Kirwan, P.D.; Akbar, M.; Moore, L.; Ronquillo, J.C.; Murrell, G.A.C.; et al. Frozen Shoulder. Nat. Rev. Dis. Primer 2022, 8, 59. [Google Scholar] [CrossRef]
- Kelley, M.J.; Shaffer, M.A.; Kuhn, J.E.; Michener, L.A.; Seitz, A.L.; Uhl, T.L.; Godges, J.J.; McClure, P. Shoulder Pain and Mobility Deficits: Adhesive Capsulitis: Clinical Practice Guidelines Linked to the International Classification of Functioning, Disability, and Health From the Orthopaedic Section of the American Physical Therapy Association. J. Orthop. Sports Phys. Ther. 2013, 43, A1–A31. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Ando, A.; Onoda, Y.; Takemura, T.; Minowa, T.; Hanagata, N.; Tsuchiya, M.; Watanabe, T.; Chimoto, E.; Suda, H.; et al. Coexistence of Fibrotic and Chondrogenic Process in the Capsule of Idiopathic Frozen Shoulders. Osteoarthr. Cartil. 2012, 20, 241–249. [Google Scholar] [CrossRef]
- Le, H.V.; Lee, S.J.; Nazarian, A.; Rodriguez, E.K. Adhesive Capsulitis of the Shoulder: Review of Pathophysiology and Current Clinical Treatments. Shoulder Elb. 2017, 9, 75–84. [Google Scholar] [CrossRef]
- Zappia, M.; Reginelli, A.; Russo, A.; D’Agosto, G.F.; Di Pietto, F.; Genovese, E.A.; Coppolino, F.; Brunese, L. Long Head of the Biceps Tendon and Rotator Interval. Musculoskelet. Surg. 2013, 97, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Draghi, F.; Scudeller, L.; Draghi, A.G.; Bortolotto, C. Prevalence of Subacromial-Subdeltoid Bursitis in Shoulder Pain: An Ultrasonographic Study. J. Ultrasound 2015, 18, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Picasso, R.; Pistoia, F.; Zaottini, F.; Marcenaro, G.; Miguel-Pérez, M.; Tagliafico, A.S.; Martinoli, C. Adhesive Capsulitis of the Shoulder: Current Concepts on the Diagnostic Work-Up and Evidence-Based Protocol for Radiological Evaluation. Diagnostics 2023, 13, 3410. [Google Scholar] [CrossRef] [PubMed]
- Hand, C.; Clipsham, K.; Rees, J.L.; Carr, A.J. Long-Term Outcome of Frozen Shoulder. J. Shoulder Elb. Surg. 2008, 17, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Sansone, V.; Maiorano, E.; Galluzzo, A.; Pascale, V. Calcific Tendinopathy of the Shoulder: Clinical Perspectives into the Mechanisms, Pathogenesis, and Treatment. Orthop. Res. Rev. 2018, 10, 63–72. [Google Scholar] [CrossRef]
- Della Valle, V.; Bassi, E.M.; Calliada, F. Migration of Calcium Deposits into Subacromial–Subdeltoid Bursa and into Humeral Head as a Rare Complication of Calcifying Tendinitis: Sonography and Imaging. J. Ultrasound 2015, 18, 259–263. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, I.-W.; Lee, S.; Shin, S.-J. Diagnosis and Treatment of Calcific Tendinitis of the Shoulder. Clin. Shoulder Elb. 2020, 23, 210–216. [Google Scholar] [CrossRef]
- Becciolini, M.; Bonacchi, G.; Galletti, S. Intramuscular Migration of Calcific Tendinopathy in the Rotator Cuff: Ultrasound Appearance and a Review of the Literature. J. Ultrasound 2016, 19, 175–181. [Google Scholar] [CrossRef]
- Loew, M.; Schnetzke, M.; Lichtenberg, S. Current Treatment Concepts of Calcifying Tendinitis of the Shoulder: A Systematic Review. Obere Extrem. 2021, 16, 85–93. [Google Scholar] [CrossRef]
- Spinnato, P.; Masuzzo, O.; Tuè, G.; Tucci, F.; Bevere, A.; Vita, F.; Cavallo, M.; Marinelli, A.; Miceli, M. A Novel Ultrasound-Guided Interventional Procedure for the Combined Treatment of Rotator Cuff Calcific Tendinopathy Complicated with Adhesive Capsulitis: The “Rizzoli” Technique. Acad. Radiol. 2023, 30, 2437–2438. [Google Scholar] [CrossRef]
- Furuhata, R.; Matsumura, N.; Yoshiyama, A.; Kamata, Y.; Takahashi, M.; Morioka, H. Seasonal Variation in the Onset of Acute Calcific Tendinitis of Rotator Cuff. BMC Musculoskelet. Disord. 2020, 21, 741. [Google Scholar] [CrossRef] [PubMed]
- Ricci, V.; Mezian, K.; Chang, K.-V.; Özçakar, L. Clinical/Sonographic Assessment and Management of Calcific Tendinopathy of the Shoulder: A Narrative Review. Diagnostics 2022, 12, 3097. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-H.; Bae, K.-C.; Kim, D.-H. Treatment Strategy for Frozen Shoulder. Clin. Orthop. Surg. 2019, 11, 249. [Google Scholar] [CrossRef]
- Hyatt, H.W.; Powers, S.K. Disturbances in Calcium Homeostasis Promotes Skeletal Muscle Atrophy: Lessons From Ventilator-Induced Diaphragm Wasting. Front. Physiol. 2020, 11, 615351. [Google Scholar] [CrossRef]
- Sun, X.; Wang, W.; Dong, Y.; Wang, Y.; Zhang, M.; Wang, Z.; Yu, X.; Huang, J.; Cai, H. Relationship between Calcium Circulation-Related Factors and Muscle Strength in Rat Sciatic Nerve Injury Model. Iran. J. Basic Med. Sci. 2020, 23, 654–662. [Google Scholar]
- Jayasinghe, I.D.; Munro, M.; Baddeley, D.; Launikonis, B.S.; Soeller, C. Observation of the Molecular Organization of Calcium Release Sites in Fast- and Slow-Twitch Skeletal Muscle with Nanoscale Imaging. J. R. Soc. Interface 2014, 11, 20140570. [Google Scholar] [CrossRef]
- Mukund, K.; Subramaniam, S. Skeletal Muscle: A Review of Molecular Structure and Function, in Health and Disease. WIREs Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium Signalling: Dynamics, Homeostasis and Remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Saran, S.; Babhulkar, J.A.; Gupta, H.; Chari, B. Imaging of Calcific Tendinopathy: Natural History, Migration Patterns, Pitfalls, and Management: A Review. Br. J. Radiol. 2024, 97, 1099–1111. [Google Scholar] [CrossRef]
- Lee, K.J.; Clegg, P.D.; Comerford, E.J.; Canty-Laird, E.G. A Comparison of the Stem Cell Characteristics of Murine Tenocytes and Tendon-Derived Stem Cells. BMC Musculoskelet. Disord. 2018, 19, 116. [Google Scholar] [CrossRef]
- Tuè, G.; Masuzzo, O.; Tucci, F.; Cavallo, M.; Parmeggiani, A.; Vita, F.; Patti, A.; Donati, D.; Marinelli, A.; Miceli, M.; et al. Can Secondary Adhesive Capsulitis Complicate Calcific Tendinitis of the Rotator Cuff? An Ultrasound Imaging Analysis. Clin. Pract. 2024, 14, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Meißner, J.D.; Kubis, H.; Scheibe, R.J.; Gros, G. Reversible Ca2+-induced Fast-to-slow Transition in Primary Skeletal Muscle Culture Cells at the mRNA Level. J. Physiol. 2000, 523, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, J.; Zhou, Y.; Thampatty, B.P.; Wang, J.H.-C. Tendon Stem/Progenitor Cells and Their Interactions with Extracellular Matrix and Mechanical Loading. Stem Cells Int. 2019, 2019, 3674647. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.-M.; Zhang, L.; et al. Identification of Tendon Stem/Progenitor Cells and the Role of the Extracellular Matrix in Their Niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Millar, N.L.; Reilly, J.H.; Kerr, S.C.; Campbell, A.L.; Little, K.J.; Leach, W.J.; Rooney, B.P.; Murrell, G.A.C.; McInnes, I.B. Hypoxia: A Critical Regulator of Early Human Tendinopathy. Ann. Rheum. Dis. 2012, 71, 302. [Google Scholar] [CrossRef]
- Steinmann, S.; Pfeifer, C.G.; Brochhausen, C.; Docheva, D. Spectrum of Tendon Pathologies: Triggers, Trails and End-State. Int. J. Mol. Sci. 2020, 21, 844. [Google Scholar] [CrossRef]
- Rui, Y.; Chan, L.; Chan, K.; Fu, S.; Gang, L. Does Erroneous Differentiation of Tendon-Derived Stem Cells Contribute to the Pathogenesis of Calcifying Tendinopathy? Chin. Med. J. 2011, 124, 606–610. [Google Scholar]
- Robinson, D.M.; Schowalter, S.; McInnis, K.C. Update on Evaluation and Management of Calcific Tendinopathy. Curr. Phys. Med. Rehabil. Rep. 2021, 9, 57–69. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, C.; Yin, L.; Liu, Y.; He, Y.; Li, S.; Shen, H. A Review of the Role of Tendon Stem Cells in Tendon-Bone Regeneration. Med. Sci. Monit. 2023, 29, e940805. [Google Scholar] [CrossRef]
- Passini, F.S.; Jaeger, P.K.; Saab, A.S.; Hanlon, S.; Chittim, N.A.; Arlt, M.J.; Ferrari, K.D.; Haenni, D.; Caprara, S.; Bollhalder, M.; et al. Shear-Stress Sensing by PIEZO1 Regulates Tendon Stiffness in Rodents and Influences Jumping Performance in Humans. Nat. Biomed. Eng. 2021, 5, 1457–1471. [Google Scholar] [CrossRef]
- Wunderli, S.L.; Widmer, J.; Amrein, N.; Foolen, J.; Silvan, U.; Leupin, O.; Snedeker, J.G. Minimal Mechanical Load and Tissue Culture Conditions Preserve Native Cell Phenotype and Morphology in Tendon—A Novel Ex Vivo Mouse Explant Model. J. Orthop. Res. 2018, 36, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Tohidnezhad, M.; Zander, J.; Slowik, A.; Kubo, Y.; Dursun, G.; Willenberg, W.; Zendedel, A.; Kweider, N.; Stoffel, M.; Pufe, T. Impact of Uniaxial Stretching on Both Gliding and Traction Areas of Tendon Explants in a Novel Bioreactor. Int. J. Mol. Sci. 2020, 21, 2925. [Google Scholar] [CrossRef] [PubMed]
- Maeda, E.; Shelton, J.C.; Bader, D.L.; Lee, D.A. Time Dependence of Cyclic Tensile Strain on Collagen Production in Tendon Fascicles. Biochem. Biophys. Res. Commun. 2007, 362, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Maeda, E.; Shelton, J.C.; Bader, D.L.; Lee, D.A. Differential Regulation of Gene Expression in Isolated Tendon Fascicles Exposed to Cyclic Tensile Strain in Vitro. J. Appl. Physiol. 2009, 106, 506–512. [Google Scholar] [CrossRef]
- Devkota, A.C.; Tsuzaki, M.; Almekinders, L.C.; Banes, A.J.; Weinhold, P.S. Distributing a Fixed Amount of Cyclic Loading to Tendon Explants over Longer Periods Induces Greater Cellular and Mechanical Responses. J. Orthop. Res. 2007, 25, 1078–1086. [Google Scholar] [CrossRef]
- Wang, T.; Lin, Z.; Ni, M.; Thien, C.; Day, R.E.; Gardiner, B.; Rubenson, J.; Kirk, T.B.; Smith, D.W.; Wang, A. Cyclic Mechanical Stimulation Rescues Achilles Tendon from Degeneration in a Bioreactor System. J. Orthop. Res. 2015, 33, 1888–1896. [Google Scholar] [CrossRef]
- Screen, H.R.; Shelton, J.C.; Bader, D.L.; Lee, D.A. Cyclic Tensile Strain Upregulates Collagen Synthesis in Isolated Tendon Fascicles. Biochem. Biophys. Res. Commun. 2005, 336, 424–429. [Google Scholar] [CrossRef]
- Legerlotz, K.; Jones, G.; Screen, H.; Riley, G. Cyclic Loading of Tendon Fascicles Using a Novel Fatigue Loading System Increases Interleukin-6 Expression by Tenocytes. Scand. J. Med. Sci. Sports 2013, 23, 31–37. [Google Scholar] [CrossRef]
- Szczesny, S.E.; Aeppli, C.; David, A.; Mauck, R.L. Fatigue Loading of Tendon Results in Collagen Kinking and Denaturation but Does Not Change Local Tissue Mechanics. J. Biomech. 2018, 71, 251–256. [Google Scholar] [CrossRef]
- Cho, C.-H.; Lho, Y.-M.; Hwang, I.; Kim, D.H. Role of Matrix Metalloproteinases 2 and 9 in the Development of Frozen Shoulder: Human Data and Experimental Analysis in a Rat Contracture Model. J. Shoulder Elb. Surg. 2019, 28, 1265–1272. [Google Scholar] [CrossRef]
- Wu, S.Y.; Kim, W.; Kremen, T.J. In Vitro Cellular Strain Models of Tendon Biology and Tenogenic Differentiation. Front. Bioeng. Biotechnol. 2022, 10, 826748. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zeng, R.; He, E.; Zhang, I.; Ding, C.; Zhang, A. Piezo-Type Mechanosensitive Ion Channel Component 1 (Piezo1): A Promising Therapeutic Target and Its Modulators: Miniperspective. J. Med. Chem. 2022, 65, 6441–6453. [Google Scholar] [CrossRef] [PubMed]
- Szabó, L.; Balogh, N.; Tóth, A.; Angyal, Á.; Gönczi, M.; Csiki, D.M.; Tóth, C.; Balatoni, I.; Jeney, V.; Csernoch, L. The Mechanosensitive Piezo1 Channels Contribute to the Arterial Medial Calcification. Front. Physiol. 2022, 13, 1037230. [Google Scholar] [CrossRef] [PubMed]
- Thien, N.D.; Hai-Nam, N.; Anh, D.T.; Baecker, D. Piezo1 and Its Inhibitors: Overview and Perspectives. Eur. J. Med. Chem. 2024, 273, 116502. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, M.; Meng, K.; Zhou, C.; Jia, X.; Li, X.; Cui, D.; Yu, M.; Tang, Y.; Li, M.; et al. Salvianolic Acid B Attenuates Inflammation and Prevent Pathologic Fibrosis by Inhibiting CD36-Mediated Activation of the PI3K-Akt Signaling Pathway in Frozen Shoulder. Front. Pharmacol. 2023, 14, 1230174. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Chen, C.; Cui, D.; Wang, Z.; Li, M.; Long, Y.; Zhang, J.; Li, C.; Wang, Z.; et al. A Mussel-Inspired, Antibacterial, Antioxidant, Injectable Composite Hydrogel for the Sustain Delivery of Salvianolic Acid B for the Treatment of Frozen Shoulder. Bioact. Mater. 2024, 40, 396–416. [Google Scholar] [CrossRef]
- Cao, C.; Ren, Y.; Barnett, A.S.; Mirando, A.J.; Rouse, D.; Mun, S.H.; Park-Min, K.-H.; McNulty, A.L.; Guilak, F.; Karner, C.M. Increased Ca2+ Signaling through CaV1. 2 Promotes Bone Formation and Prevents Estrogen Deficiency–Induced Bone Loss. JCI Insight 2017, 2, e95512. [Google Scholar] [CrossRef]
- Cao, C.; Oswald, A.B.; Fabella, B.A.; Ren, Y.; Rodriguiz, R.; Trainor, G.; Greenblatt, M.B.; Hilton, M.J.; Pitt, G.S. The CaV1. 2 L-Type Calcium Channel Regulates Bone Homeostasis in the Middle and Inner Ear. Bone 2019, 125, 160–168. [Google Scholar] [CrossRef]
- Ramachandran, K.V.; Hennessey, J.A.; Barnett, A.S.; Yin, X.; Stadt, H.A.; Foster, E.; Shah, R.A.; Yazawa, M.; Dolmetsch, R.E.; Kirby, M.L. Calcium Influx through L-Type CaV1.2 Ca2+ Channels Regulates Mandibular Development. J. Clin. Investig. 2013, 123, 1638–1646. [Google Scholar] [CrossRef]
- Li, H.; Korcari, A.; Ciufo, D.; Mendias, C.L.; Rodeo, S.A.; Buckley, M.R.; Loiselle, A.E.; Pitt, G.S.; Cao, C. Increased Ca2+ Signaling through CaV1.2 Induces Tendon Hypertrophy with Increased Collagen Fibrillogenesis and Biomechanical Properties. FASEB J. 2023, 37, e23007. [Google Scholar] [CrossRef]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of Adipogenesis by Wnt Signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.A.; Van Velthoven, C.T.; Fukumoto, K.D.; Cheung, T.H.; Rando, T.A. Intronic Polyadenylation of PDGFRα in Resident Stem Cells Attenuates Muscle Fibrosis. Nature 2016, 540, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, G.; Rosina, M.; Reggio, A. Signaling Pathways Regulating the Fate of Fibro/Adipogenic Progenitors (FAPs) in Skeletal Muscle Regeneration and Disease. FEBS J. 2022, 289, 6484–6517. [Google Scholar] [CrossRef] [PubMed]
- Kharraz, Y.; Guerra, J.; Pessina, P.; Serrano, A.L.; Muñoz-Cánoves, P. Understanding the Process of Fibrosis in Duchenne Muscular Dystrophy. BioMed Res. Int. 2014, 2014, 965631. [Google Scholar] [CrossRef]
- Joe, A.W.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M. Muscle Injury Activates Resident Fibro/Adipogenic Progenitors That Facilitate Myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal Progenitors Distinct from Satellite Cells Contribute to Ectopic Fat Cell Formation in Skeletal Muscle. Nat. Cell Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef]
- Pessina, P.; Cabrera, D.; Morales, M.G.; Riquelme, C.A.; Gutiérrez, J.; Serrano, A.L.; Brandan, E.; Muñoz-Cánoves, P. Novel and Optimized Strategies for Inducing Fibrosis in Vivo: Focus on Duchenne Muscular Dystrophy. Skelet. Muscle 2014, 4, 7. [Google Scholar] [CrossRef]
- Martin, A.B.; Cardenas, M.A.; Andersen, R.K.; Bowman, A.I.; Hillier, E.A.; Bensmaia, S.; Fuglevand, A.J.; Gothard, K.M. A Context-Dependent Switch from Sensing to Feeling in the Primate Amygdala. Cell Rep. 2023, 42, 112056. [Google Scholar] [CrossRef]
- Yang, R.; Tang, Y.; Hou, J.; Yu, M.; Long, Y.; Yamuhanmode, A.; Li, Q.; Li, F.; Zhang, Y.; Warsame, M.; et al. Fibrosis in Frozen Shoulder: Activation of IL-6 through PI3K-Akt Signaling Pathway in Synovial Fibroblast. Mol. Immunol. 2022, 150, 29–38. [Google Scholar] [CrossRef]
- Bonnieu, A.; Carnac, G.; Vernus, B. Myostatin in the Pathophysiology of Skeletal Muscle. Curr. Genom. 2007, 8, 415–422. [Google Scholar] [CrossRef]
- Yang, M.; Liu, C.; Jiang, N.; Liu, Y.; Luo, S.; Li, C.; Zhao, H.; Han, Y.; Chen, W.; Li, L.; et al. Myostatin: A Potential Therapeutic Target for Metabolic Syndrome. Front. Endocrinol. 2023, 14, 1181913. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Dong, Y.; Chen, Z.; Mitch, W.E.; Zhang, L. The Pathway to Muscle Fibrosis Depends on Myostatin Stimulating the Differentiation of Fibro/Adipogenic Progenitor Cells in Chronic Kidney Disease. Kidney Int. 2017, 91, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Bhasin, S.; Klickstein, L.; Krishnan, V.; Rooks, D. Challenges and Future Prospects of Targeting Myostatin/Activin A Signaling to Treat Diseases of Muscle Loss and Metabolic Dysfunction. J. Gerontol. Ser. A 2023, 78, 32–37. [Google Scholar] [CrossRef]
- McGowan, T.A.; Madesh, M.; Zhu, Y.; Wang, L.; Russo, M.; Deelman, L.; Henning, R.; Joseph, S.; Hajnoczky, G.; Sharma, K. TGF-β-Induced Ca2+ Influx Involves the Type III IP3 Receptor and Regulates Actin Cytoskeleton. Am. J. Physiol. Ren. Physiol. 2002, 282, F910–F920. [Google Scholar] [CrossRef]
- Wicks, S.J.; Lui, S.; Abdel-Wahab, N.; Mason, R.M.; Chantry, A. Inactivation of Smad-Transforming Growth Factor β Signaling by Ca2+-Calmodulin-Dependent Protein Kinase II. Mol. Cell. Biol. 2000, 20, 8103–8111. [Google Scholar] [CrossRef]
- Pacher, P.; Sharma, K.; Csordás, G.; Zhu, Y.; Hajnóczky, G. Uncoupling of ER-Mitochondrial Calcium Communication by Transforming Growth Factor-β. Am. J. Physiol. Ren. Physiol. 2008, 295, F1303–F1312. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β Signaling in Health, Disease and Therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar]
- Song, Z.; Wang, Y.; Zhang, F.; Yao, F.; Sun, C. Calcium Signaling Pathways: Key Pathways in the Regulation of Obesity. Int. J. Mol. Sci. 2019, 20, 2768. [Google Scholar] [CrossRef]
- Coultrap, S.J.; Buard, I.; Kulbe, J.R.; Dell’Acqua, M.L.; Bayer, K.U. CaMKII Autonomy Is Substrate-Dependent and Further Stimulated by Ca2+/Calmodulin. J. Biol. Chem. 2010, 285, 17930–17937. [Google Scholar] [CrossRef]
- Stratton, M.M.; Chao, L.H.; Schulman, H.; Kuriyan, J. Structural Studies on the Regulation of Ca2+/Calmodulin Dependent Protein Kinase II. Curr. Opin. Struct. Biol. 2013, 23, 292–301. [Google Scholar] [CrossRef]
- Chao, L.H.; Stratton, M.M.; Lee, I.-H.; Rosenberg, O.S.; Levitz, J.; Mandell, D.J.; Kortemme, T.; Groves, J.T.; Schulman, H.; Kuriyan, J. A Mechanism for Tunable Autoinhibition in the Structure of a Human Ca2+/Calmodulin- Dependent Kinase II Holoenzyme. Cell 2011, 146, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, M.; Xu, Y.; Wu, S.; Saeed, M.; Sun, C. ColXV Promotes Adipocyte Differentiation via Inhibiting DNA Methylation and cAMP/PKA Pathway in Mice. Oncotarget 2017, 8, 60135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wada, N.; Hashinaga, T.; Otabe, S.; Yuan, X.; Kurita, Y.; Kakino, S.; Ohoki, T.; Nakayama, H.; Fukutani, T.; Tajiri, Y. Selective Modulation of Wnt Ligands and Their Receptors in Adipose Tissue by Chronic Hyperadiponectinemia. PLoS ONE 2013, 8, e67712. [Google Scholar] [CrossRef] [PubMed]
- Benchoula, K.; Mediani, A.; Hwa, W.E. The Functions of Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) in Diabetes Progression. J. Cell Commun. Signal. 2023, 17, 25–34. [Google Scholar] [CrossRef]
- Deng, G.; Wei, Y. The Causal Relationship between Hypothyroidism and Frozen Shoulder: A Two-Sample Mendelian Randomization. Medicine 2023, 102, e35650. [Google Scholar] [CrossRef]
- Carter, P.H.; Schipani, E. The Roles of Parathyroid Hormone and Calcitonin in Bone Remodeling: Prospects for Novel Therapeutics. Endocr. Metab. Immune Disord. Drug Targets Former. Curr. Drug Targets Immune Endocr. Metab. Disord. 2006, 6, 59–76. [Google Scholar] [CrossRef]
- Babić Leko, M.; Pleić, N.; Gunjača, I.; Zemunik, T. Environmental Factors That Affect Parathyroid Hormone and Calcitonin Levels. Int. J. Mol. Sci. 2021, 23, 44. [Google Scholar] [CrossRef]
- Rouhani, A.; Mardani-Kivi, M.; Bazavar, M.; Barzgar, M.; Tabrizi, A.; Hashemi-Motlagh, K.; Saheb-Ekhtiari, K. Calcitonin Effects on Shoulder Adhesive Capsulitis. Eur. J. Orthop. Surg. Traumatol. 2016, 26, 575–580. [Google Scholar] [CrossRef]
- Yang, R.; Deng, H.; Hou, J.; Li, W.; Zhang, C.; Yu, M.; Tang, Y.; Li, Q.; Li, F.; Song, B.; et al. Investigation of Salmon Calcitonin in Regulating Fibrosis-related Molecule Production and Cell-substrate Adhesion in Frozen Shoulder Synovial/Capsular Fibroblasts. J. Orthop. Res. 2020, 38, 1375–1385. [Google Scholar] [CrossRef]
- Longo, U.G.; Mazzola, A.; Carotti, S.; Francesconi, M.; Catapano, S.; Magrì, F.; Perrone, G.; Morini, S.; De Salvatore, S.; Denaro, V. The Role of Estrogen and Progesterone Receptors in the Rotator Cuff Disease: A Retrospective Cohort Stud y. BMC Musculoskelet. Disord. 2021, 22, 891. [Google Scholar] [CrossRef]
- Cogan, C.J.; Cevallos, N.; Freshman, R.D.; Lansdown, D.; Feeley, B.T.; Zhang, A.L. Evaluating Utilization Trends in Adhesive Capsulitis of the Shoulder: A Retrospective Cohort Analysis of a Large Database. Orthop. J. Sports Med. 2022, 10, 23259671211069577. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, E.; Kennedy, J.; Ford, A.; Reinke, E.; Green, C.; Poehlein, E.; Wittstein, J. Poster 188: Is Hormone Replacing Therapy Associated with Reduced Risk of Adhesive Capsulitis in Menopausal Women? A Single Center Analysis. Orthop. J. Sports Med. 2023, 11, 2325967123S00174. [Google Scholar] [CrossRef]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Ström, A.; Treuter, E.; Warner, M.; et al. Estrogen Receptors: How Do They Signal and What Are Their Targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef] [PubMed]
- Granéli, C.; Thorfve, A.; Ruetschi, U.; Brisby, H.; Thomsen, P.; Lindahl, A.; Karlsson, C. Novel Markers of Osteogenic and Adipogenic Differentiation of Human Bone Marrow Stromal Cells Identified Using a Quantitative Proteomics Approach. Stem Cell Res. 2014, 12, 153–165. [Google Scholar] [CrossRef]
- Viti, F.; Landini, M.; Mezzelani, A.; Petecchia, L.; Milanesi, L.; Scaglione, S. Osteogenic Differentiation of MSC through Calcium Signaling Activation: Transcriptomics and Functional Analysis. PLoS ONE 2016, 11, e0148173. [Google Scholar] [CrossRef]
- Barradas, A.M.; Fernandes, H.A.; Groen, N.; Chai, Y.C.; Schrooten, J.; van de Peppel, J.; Van Leeuwen, J.P.; Van Blitterswijk, C.A.; de Boer, J. A Calcium-Induced Signaling Cascade Leading to Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stromal Cells. Biomaterials 2012, 33, 3205–3215. [Google Scholar] [CrossRef]
- Regan, J.N.; Waning, D.L.; Guise, T.A. Skeletal Muscle Ca2+ Mishandling: Another Effect of Bone-to-Muscle Signaling. Semin. Cell Dev. Biol. 2016, 49, 24–29. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, L.; Song, B.; Cui, Z.; Chen, G.; Yu, Z.; Song, B. Insulin-like Growth Factor-2 (IGF-2) in Fibrosis. Biomolecules 2022, 12, 1557. [Google Scholar] [CrossRef]
- Monaco, S.; Illario, M.; Rusciano, M.R.; Gragnaniello, G.; Di Spigna, G.; Leggiero, E.; Pastore, L.; Fenzi, G.; Rossi, G.; Vitale, M. Insulin Stimulates Fibroblast Proliferation through Calcium-Calmodulin-Dependent Kinase II. Cell Cycle 2009, 8, 2024–2030. [Google Scholar] [CrossRef]
- Aquino-Martínez, R.; Artigas, N.; Gámez, B.; Rosa, J.L.; Ventura, F. Extracellular Calcium Promotes Bone Formation from Bone Marrow Mesenchymal Stem Cells by Amplifying the Effects of BMP-2 on SMAD Signalling. PLoS ONE 2017, 12, e0178158. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad Pathways in TGF-β Signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carballo, E.; Ulsamer, A.; Susperregui, A.R.; Manzanares-Céspedes, C.; Sánchez-García, E.; Bartrons, R.; Rosa, J.L.; Ventura, F. Conserved Regulatory Motifs in Osteogenic Gene Promoters Integrate Cooperative Effects of Canonical Wnt and BMP Pathways. J. Bone Miner. Res. 2011, 26, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Gámez, B.; Rodríguez-Carballo, E.; Graupera, M.; Rosa, J.L.; Ventura, F. Class I PI-3-kinase Signaling Is Critical for Bone Formation through Regulation of SMAD1 Activity in Osteoblasts. J. Bone Miner. Res. 2016, 31, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Mandal, C.C.; Das, F.; Ganapathy, S.; Harris, S.E.; Choudhury, G.G.; Ghosh-Choudhury, N. Bone Morphogenetic Protein-2 (BMP-2) Activates NFATc1 Transcription Factor via an Autoregulatory Loop Involving Smad/Akt/Ca2+ Signaling. J. Biol. Chem. 2016, 291, 1148–1161. [Google Scholar] [CrossRef]
- Koga, T.; Matsui, Y.; Asagiri, M.; Kodama, T.; de Crombrugghe, B.; Nakashima, K.; Takayanagi, H. NFAT and Osterix Cooperatively Regulate Bone Formation. Nat. Med. 2005, 11, 880–885. [Google Scholar] [CrossRef]
- Fromigué, O.; Haÿ, E.; Barbara, A.; Marie, P.J. Essential Role of Nuclear Factor of Activated T Cells (NFAT)-Mediated Wnt Signaling in Osteoblast Differentiation Induced by Strontium Ranelate. J. Biol. Chem. 2010, 285, 25251–25258. [Google Scholar] [CrossRef]
- Fuentealba, L.C.; Eivers, E.; Ikeda, A.; Hurtado, C.; Kuroda, H.; Pera, E.M.; De Robertis, E.M. Integrating Patterning Signals: Wnt/GSK3 Regulates the Duration of the BMP/Smad1 Signal. Cell 2007, 131, 980–993. [Google Scholar] [CrossRef]
- Majumdar, M.K.; Thiede, M.A.; Haynesworth, S.E.; Bruder, S.P.; Gerson, S.L. Human Marrow-Derived Mesenchymal Stem Cells (MSCs) Express Hematopoietic Cytokines and Support Long-Term Hematopoiesis When Differentiated toward Stromal and Osteogenic Lineages. J. Hematother. Stem Cell Res. 2000, 9, 841–848. [Google Scholar] [CrossRef]
- Rezaee, F.; Rellick, S.L.; Piedimonte, G.; Akers, S.M.; O’Leary, H.A.; Martin, K.; Craig, M.D.; Gibson, L.F. Neurotrophins Regulate Bone Marrow Stromal Cell IL-6 Expression through the MAPK Pathway. PLoS ONE 2010, 5, e9690. [Google Scholar] [CrossRef]
- Lee, M.W.; Kim, D.S.; Ryu, S.; Jang, I.K.; Kim, H.J.; Yang, J.M.; Lee, D.-H.; Lee, S.H.; Son, M.H.; Cheuh, H.W. Effect of Ex Vivo Culture Conditions on Immunosuppression by Human Mesenchymal Stem Cells. BioMed Res. Int. 2013, 2013, 154919. [Google Scholar]
- Ren, J.; Jin, P.; Sabatino, M.; Balakumaran, A.; Feng, J.; Kuznetsov, S.A.; Klein, H.G.; Robey, P.G.; Stroncek, D.F. Global Transcriptome Analysis of Human Bone Marrow Stromal Cells (BMSC) Reveals Proliferative, Mobile and Interactive Cells That Produce Abundant Extracellular Matrix Proteins, Some of Which May Affect BMSC Potency. Cytotherapy 2011, 13, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; James, A.W.; Blough, J.; Donneys, A.; Wang, S.C.; Cederna, P.S.; Buchman, S.R.; Levi, B. Reconciling the Effects of Inflammatory Cytokines on Mesenchymal Cell Osteogenic Differentiation. J. Surg. Res. 2013, 185, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, C.; Yang, W.; Jiang, T. Effect of Dexamethasone on TGF-Β1/Smad3 Signalling Pathway in Airway Remodelling Model of Asthmatic Rats. J. Coll. Physicians Surg. Pak. 2019, 29, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Ecker Cohen, O.; Neuman, S.; Natan, Y.; Levy, A.; Blum, Y.D.; Amselem, S.; Bavli, D.; Ben, Y. Amorphous Calcium Carbonate Enhances Osteogenic Differentiation and Myotube Formation of Human Bone Marrow Derived Mesenchymal Stem Cells and Primary Skeletal Muscle Cells under Microgravity Conditions. Life Sci. Space Res. 2024, 41, 146–157. [Google Scholar] [CrossRef]
- Huston, P. A Sedentary and Unhealthy Lifestyle Fuels Chronic Disease Progression by Changing Interstitial Cell Behaviour: A Network Analysis. Front. Physiol. 2022, 13, 904107. [Google Scholar] [CrossRef]
- Taylor, C.T.; Scholz, C.C. The Effect of HIF on Metabolism and Immunity. Nat. Rev. Nephrol. 2022, 18, 573–587. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, B.; Li, Y.; Liu, X.; Guo, S.; Wang, C.; Li, S.; Wang, D. The Role of Vascular Endothelial Growth Factor in Tendon Healing. Front. Physiol. 2021, 12, 766080. [Google Scholar] [CrossRef]
- Wilgus, T.A. Vascular Endothelial Growth Factor and Cutaneous Scarring. Adv. Wound Care 2019, 8, 671–678. [Google Scholar] [CrossRef]
- Willson, J.A.; Arienti, S.; Sadiku, P.; Reyes, L.; Coelho, P.; Morrison, T.; Rinaldi, G.; Dockrell, D.H.; Whyte, M.K.B.; Walmsley, S.R. Neutrophil HIF-1α Stabilization Is Augmented by Mitochondrial ROS Produced via the Glycerol 3-Phosphate Shuttle. Blood 2022, 139, 281–286. [Google Scholar] [CrossRef]
- Lee, A.; Derricks, K.; Minns, M.; Ji, S.; Chi, C.; Nugent, M.A.; Trinkaus-Randall, V. Hypoxia-Induced Changes in Ca2+ Mobilization and Protein Phosphorylation Implicated in Impaired Wound Healing. Am. J. Physiol. Cell Physiol. 2014, 306, C972–C985. [Google Scholar] [CrossRef]
- Rossi, A.; Pizzo, P.; Filadi, R. Calcium, Mitochondria and Cell Metabolism: A Functional Triangle in Bioenergetics. Biochim. Biophys. Acta BBA Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef]
- Seta, K.A.; Yuan, Y.; Spicer, Z.; Lu, G.; Bedard, J.; Ferguson, T.K.; Pathrose, P.; Cole-Strauss, A.; Kaufhold, A.; Millhorn, D.E. The Role of Calcium in Hypoxia-Induced Signal Transduction and Gene Expression. Cell Calcium 2004, 36, 331–340. [Google Scholar] [CrossRef]

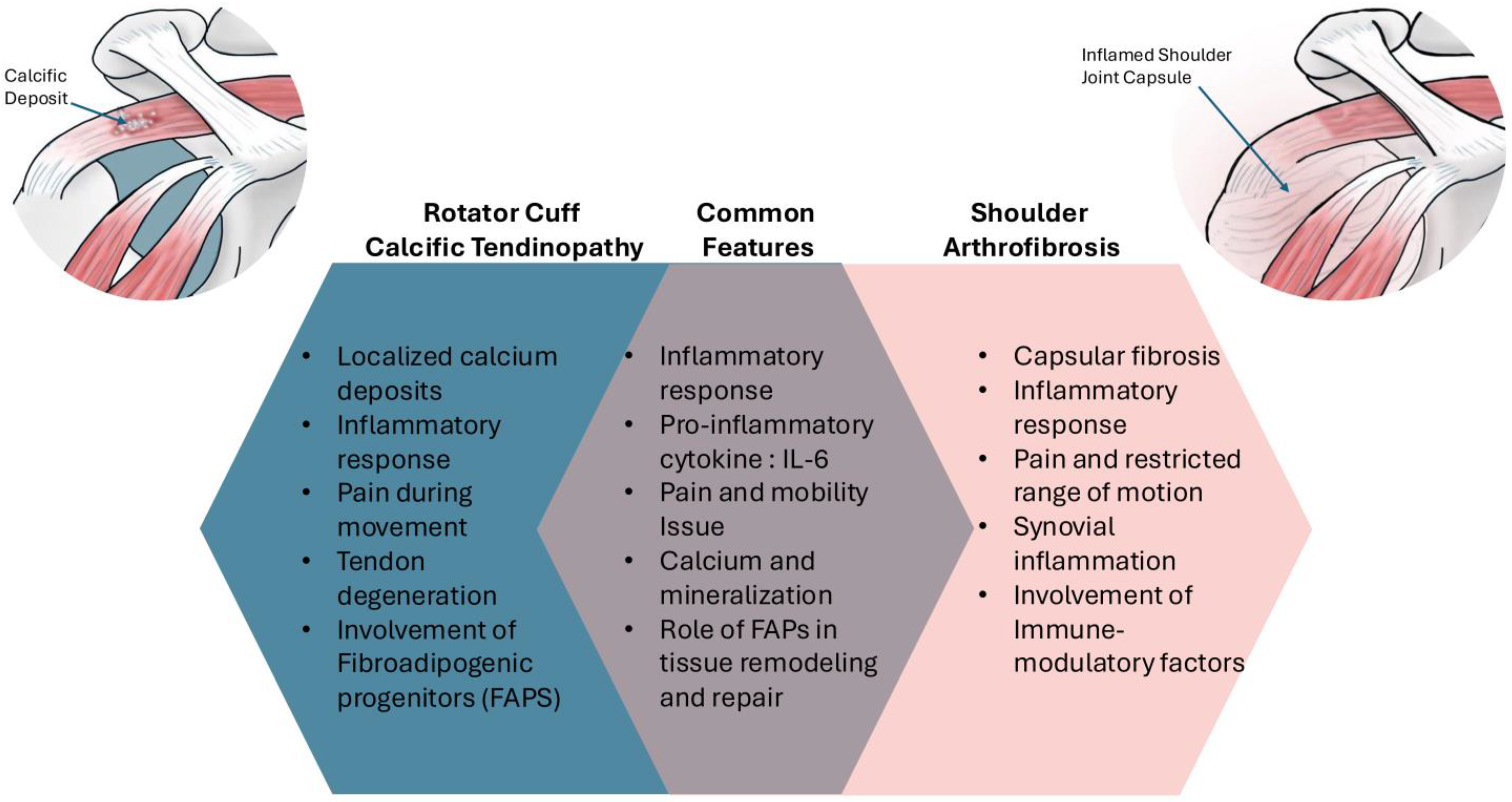

| Pathophysiological Link | Calcific Tendinopathy | Adhesive Capsulitis | Mechanisms/Implications |
|---|---|---|---|
| Chronic Micro-Leakage of Calcium | Calcium moves from tendon fibers into the joint’s synovial recesses, leading to inflammation | Inflammatory response in the shoulder capsule | This chronic leakage can initiate synovial inflammation, linking both conditions |
| Chemical Synovitis | Calcium deposits trigger a chemical inflammatory response | Results in pain and stiffness in the shoulder | Chemical irritation from calcium can exacerbate capsular inflammation |
| Intra-Articular Calcium Debris | Calcium debris from the rotator cuff tends to diffuse into the joint space during procedures | Associated with higher postoperative adhesive capsulitis risk | Arthroscopic interventions can inadvertently introduce calcium debris, promoting inflammation |
| Prolonged Pain-Induced Hypomobility | Chronic pain leads to decreased shoulder mobility | Results in fibrosis and further stiffness of the shoulder capsule | Limited movement due to pain can worsen both conditions |
| Inflammatory Response | Activation of immune pathways due to calcium accumulation | Inflammatory processes in the shoulder capsule | Shared inflammatory mechanisms can link the two conditions |
| Synovial/Capsular Inflammation | Initiated by the presence of calcium in the joint | Characterized by thickening and loss of mobility | Inflammation in the synovial tissue may lead to adhesive capsulitis |
| Pathway/Component | Role/Function | Mechanisms/Interactions | Implications |
|---|---|---|---|
| Fibro-Adipogenic Progenitors (FAPs) | Muscle-resident interstitial cells with mesenchymal stem cell properties; essential for muscle homeostasis and regeneration | Differentiate into activated fibroblasts, adipocytes, and osteogenic cells, providing signals for muscle stem cell (MuSC) expansion and myogenesis | Dysregulation can lead to fibrosis and impaired muscle regeneration |
| Calcium Signaling | Critical for various cellular processes, including muscle contraction and cell growth | Calmodulin binds calcium, modulating activity of kinases and phosphatases; influences TGF-β signaling pathways | Calcium overload can lead to fibrosis and adipogenesis |
| TGF-β Signaling | Plays a key role in regulating muscle regeneration and fibrosis | Activates SMAD2, SMAD3 (canonical pathways) and PI3K-AKT, p38 MAPK (non-canonical pathways) | Dysregulation can lead to excessive fibrosis and impaired muscle repair |
| Calmodulin | Calcium-binding protein that mediates calcium signaling | Binds Ca2+, interacts with SMAD proteins, influences transcriptional activity of TGF-β | Overexpression can inhibit TGF-β effects, impacting fibrosis |
| IP3 Pathway | Mediates calcium release from intracellular stores | Activation of phospholipase C leads to IP3 generation, triggering calcium release and influx | Critical for TGF-β-induced calcium signaling |
| Myostatin (GDF8) | Regulates muscle growth and differentiation | Influences FAP activation via p38 MAPK and AKT pathways | Involved in various muscular disorders and metabolic conditions |
| Bone Morphogenetic Proteins (BMPs) | Induce osteogenic differentiation in mesenchymal stem cells (MSCs) | Bind to serine–threonine kinase receptors, activating SMAD and non-SMAD pathways | Promote bone formation and interact with calcium signaling |
| Inflammatory Cytokines (IL-6, IL-1) | Mediate inflammatory responses, linked to fibrosis and musculoskeletal disorders | Secreted by FAPs and osteoblasts, influencing MAPK signaling for skeletogenesis | Associated with the progression of rotator cuff diseases |
| Calcium Homeostasis | Maintained by parathyroid hormone (PTH) and calcitonin | PTH elevates plasma calcium levels by stimulating osteoclast activity; calcitonin decreases calcium levels | Imbalances can affect bone and muscle health |
| Calcium Dynamics in MSCs | Essential for osteogenic differentiation | Calcium and phosphate ions activate BMP/SMAD and RAS signaling pathways | Dysregulation can affect bone remodeling and contribute to disorder |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, S.; Sargeant, M.S.; Patel, R.; Jayaram, P. Exploring Metabolic Mechanisms in Calcific Tendinopathy and Shoulder Arthrofibrosis: Insights and Therapeutic Implications. J. Clin. Med. 2024, 13, 6641. https://doi.org/10.3390/jcm13226641
Alam S, Sargeant MS, Patel R, Jayaram P. Exploring Metabolic Mechanisms in Calcific Tendinopathy and Shoulder Arthrofibrosis: Insights and Therapeutic Implications. Journal of Clinical Medicine. 2024; 13(22):6641. https://doi.org/10.3390/jcm13226641
Chicago/Turabian StyleAlam, Shahenvaz, Marisa Shauna Sargeant, Ronak Patel, and Prathap Jayaram. 2024. "Exploring Metabolic Mechanisms in Calcific Tendinopathy and Shoulder Arthrofibrosis: Insights and Therapeutic Implications" Journal of Clinical Medicine 13, no. 22: 6641. https://doi.org/10.3390/jcm13226641
APA StyleAlam, S., Sargeant, M. S., Patel, R., & Jayaram, P. (2024). Exploring Metabolic Mechanisms in Calcific Tendinopathy and Shoulder Arthrofibrosis: Insights and Therapeutic Implications. Journal of Clinical Medicine, 13(22), 6641. https://doi.org/10.3390/jcm13226641






