A Nation-Wide Evaluation of Suboptimal Lipid-Lowering Treatment Patterns Among Patients Undergoing Intervention for Acute Coronary Syndrome in Hungary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Assessment of Endpoints
2.3. Statistical Analysis
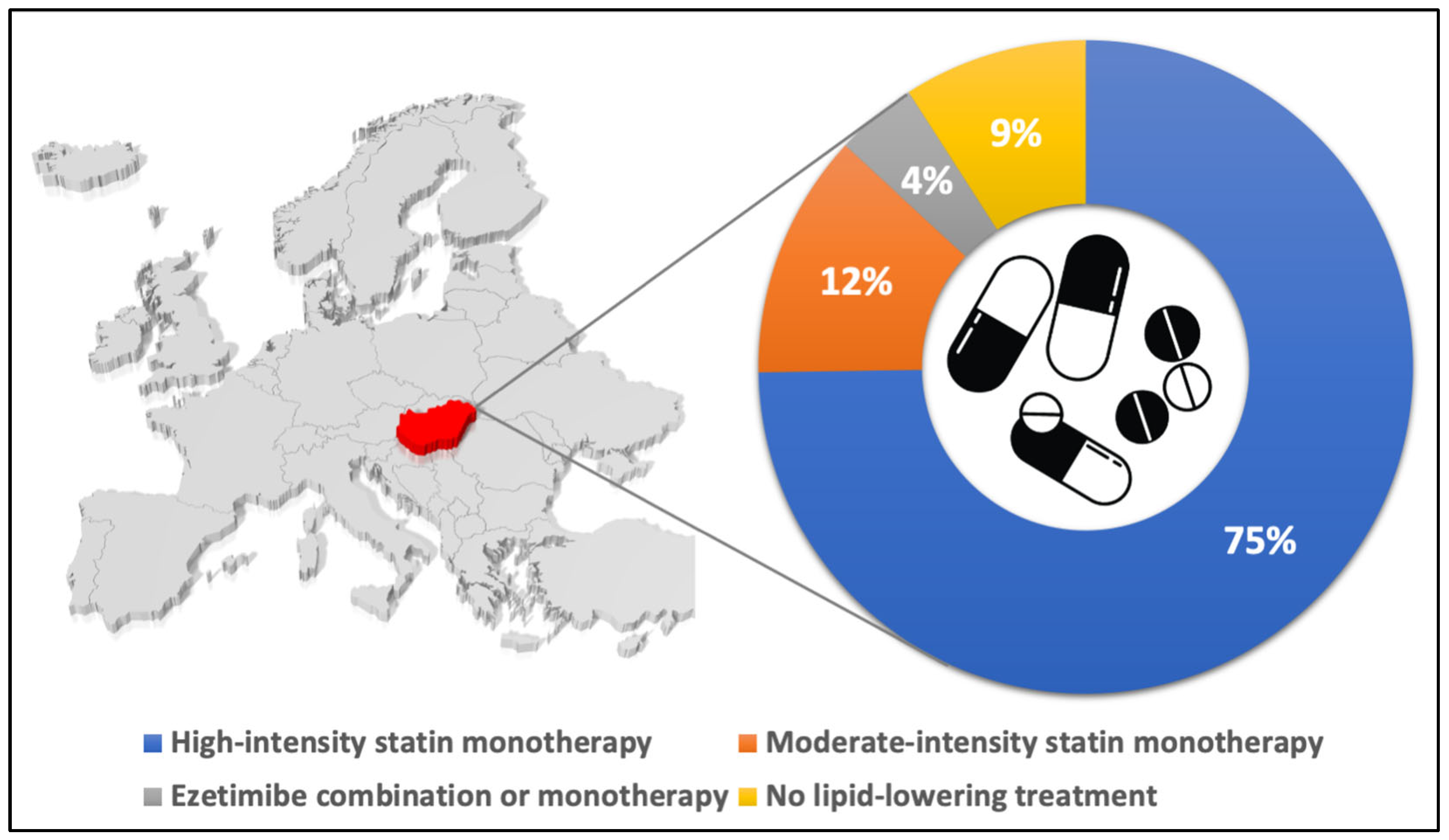
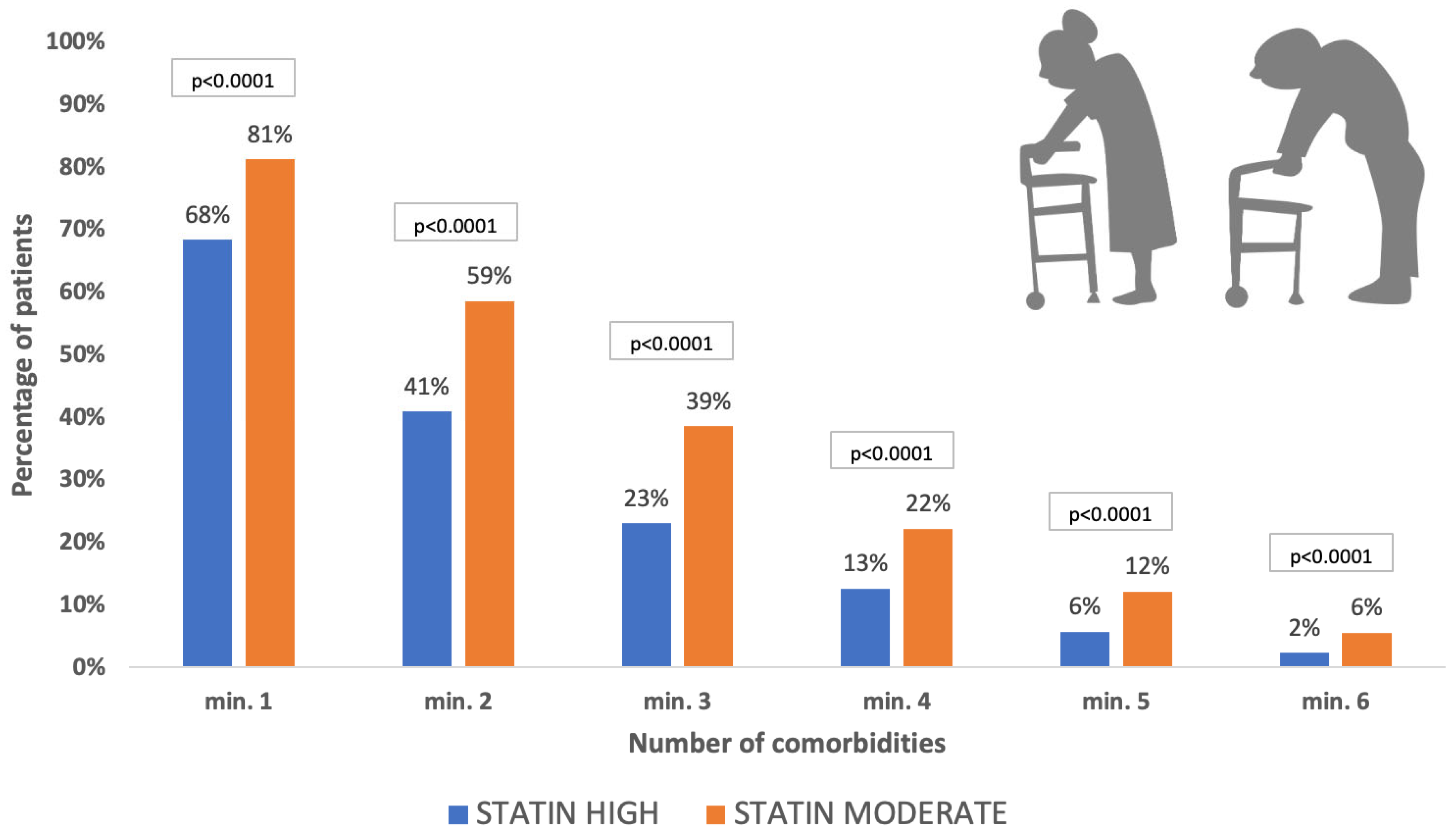
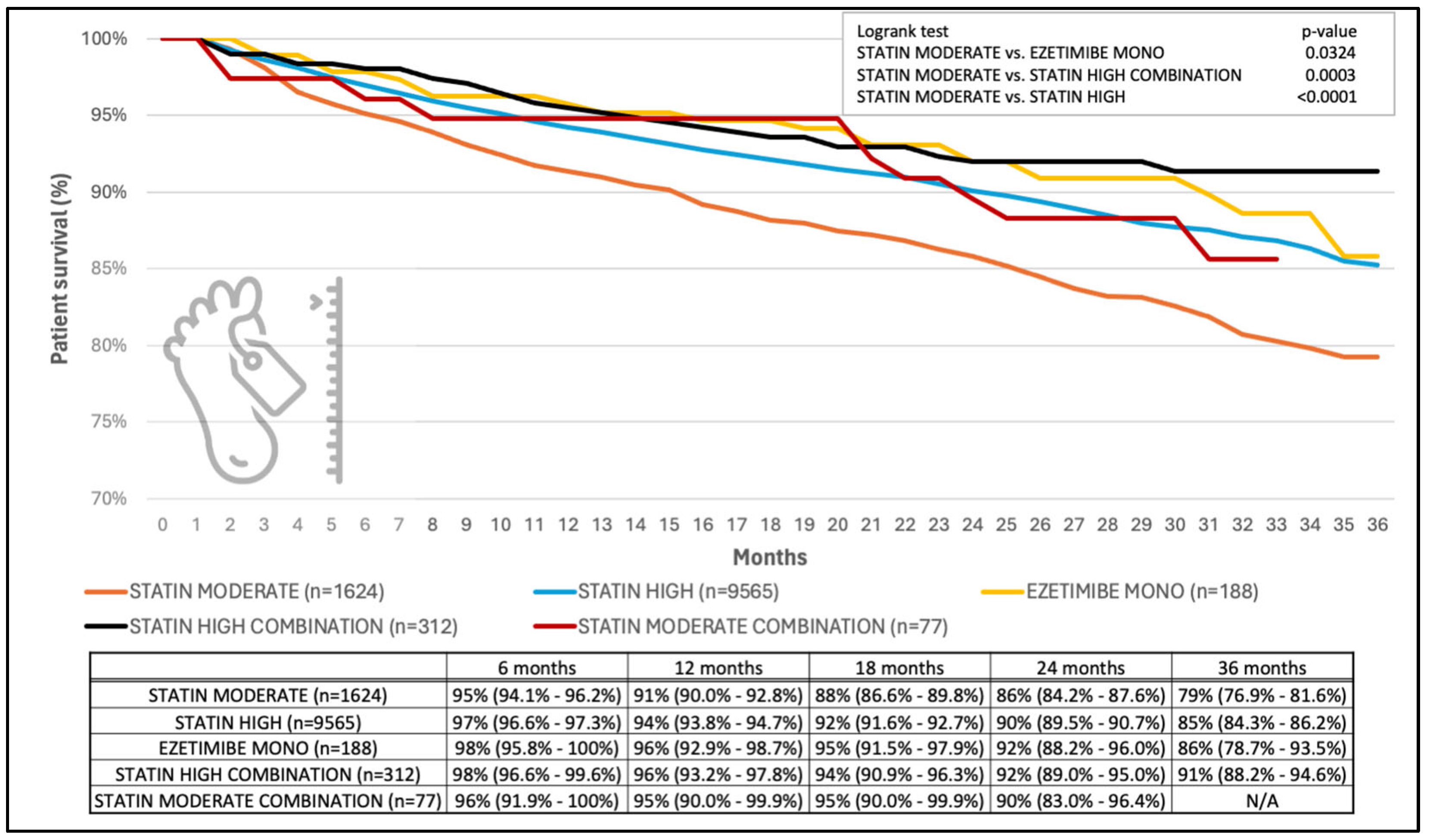
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- The Cholesterol Treatment Trialists’ Collaboration; Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [CrossRef]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; De Mets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef] [PubMed]
- Krychtiuk, K.A.; Ahrens, I.; Drexel, H.; Halvorsen, S.; Hassager, C.; Huber, K.; Kurpas, D.; Niessner, A.; Schiele, F.; Semb, A.G.; et al. Acute LDL-C reduction post ACS: Strike early and strike strong: From evidence to clinical practice. A clinical consensus statement of the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Association of Preventive Cardiology (EAPC) and the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 939–949. [Google Scholar] [CrossRef]
- Writing Committee; Lloyd-Jones, D.M.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Covington, A.M.; De Palma, S.M.; Minissian, M.B.; Orringer, C.E.; Smith, S.C., Jr.; et al. 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 80, 1366–1418. [Google Scholar] [CrossRef]
- Banach, M.; Penson, P.E.; Farnier, M.; Fras, Z.; Latkovskis, G.; Laufs, U.; Paneni, F.; Parini, P.; Pirro, M.; Reiner, Z.; et al. Bempedoic acid in the management of lipid disorders and cardiovascular risk. 2023 position paper of the International Lipid Expert Panel (ILEP). Prog. Cardiovasc. Dis. 2023, 79, 2–11. [Google Scholar] [CrossRef]
- Pogran, E.; Burger, A.L.; Zweiker, D.; Kaufmann, C.C.; Muthspiel, M.; Rega-Kaun, G.; Wenkstetten-Holub, A.; Wojta, J.; Drexel, H.; Huber, K. Lipid-Lowering Therapy after Acute Coronary Syndrome. J. Clin. Med. 2024, 13, 2043. [Google Scholar] [CrossRef]
- Nelson, A.J.; Haynes, K.; Shambhu, S.; Eapen, Z.; Cziraky, M.J.; Nanna, M.G.; Calvert, S.B.; Gallagher, K.; Pagidipati, N.J.; Granger, C.B. High-Intensity Statin Use Among Patients With Atherosclerosis in the U.S. J. Am. Coll. Cardiol. 2022, 79, 1802–1813. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Molemans, B.; Schoonen, W.M.; Giovas, P.; Bray, S.; Kiru, G.; Murphy, J.; Banach, M.; De Servi, S.; Gaita, D.; et al. EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: The DA VINCI study. Eur. J. Prev. Cardiol. 2021, 28, 1279–1289. [Google Scholar] [CrossRef]
- Vrablik, M.; Seifert, B.; Parkhomenko, A.; Banach, M.; Jozwiak, J.J.; Kiss, R.G.; Gaita, D.; Raslova, K.; Zachlederova, M.; Bray, S.; et al. Lipid-lowering therapy use in primary and secondary care in Central and Eastern Europe: DA VINCI observational study. Atherosclerosis 2021, 334, 66–75. [Google Scholar] [CrossRef]
- Mark, L.; Dani, G.; Ozsvath, L.; Szabo-Gyorke, I.; Katona, A.; Jambrik, Z. Akut koronáriaszindróma miatt intervención átesett betegeink lipidcsökkentő kezelése és ajánlás a beavatkozás utáni ellenőrzésekre [The lipid lowering therapy of patients after intervention due to acute coronary syndrome and a recommendation for the further controls]. Cardiol. Hung. 2020, 50, 29–34. [Google Scholar] [CrossRef]
- Reiber, I.; Márk, L.; Együd, F.; Mező, I.; Császár, A. Nagy intenzitású rosuvastatin vagy rosuvastatin/ezetimib kombinációs lipidcsökkentő terápia hatékonysága hypercholesterinaemiás igen nagy kardiovaszkuláris rizikójú magyar betegekben—a 3T-FIGHT vizsgálat első eredményei [Efficacy of high-intensity rosuvastatin or rosuvastatin/ezetimibe combined lipid-lowering therapy in Hungarian patients with hypercholesterolemia at very-high cardiovascular risk—the first results of the 3T-FIGHT STUDY]. Cardiol. Hung. 2023, 53, 633–639. [Google Scholar] [CrossRef]
- Chou, R.; Cantor, A.; Dana, T.; Wagner, J.; Ahmed, A.; Fu, R.; Ferencik, M. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: A Systematic Review for the U.S. Preventive Services Task Force; U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews: Rockville, MD, USA, 2022; Volume 328, pp. 754–771. [Google Scholar]
- Anderson, T.S.; Jing, B.; Wray, C.M.; Ngo, S.; Xu, E.; Fung, K.; Steinman, M.A. Comparison of Pharmacy Database Methods for Determining Prevalent Chronic Medication Use. Med. Care 2019, 57, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Gencer, B.; Marston, N.A.; Im, K.; Cannon, C.P.; Sever, P.; Keech, A.; Braunwald, E.; Giugliano, R.P.; Sabatine, M.S. Efficacy and safety of lowering LDL cholesterol in older patients: A systematic review and meta-analysis of randomised controlled trials. Lancet 2020, 396, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Berteotti, M.; Profili, F.; Nreu, B.; Casolo, G.; Zuppiroli, A.; Mannucci, E.; Marcucci, R.; Francesconi, P. LDL-cholesterol target levels achievement in high-risk patients: An (un)expected gender bias. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Di Mario, S.; Philip, K. Gender Disparities in Health Resource Utilization in Patients with Atherosclerotic Cardiovascular Disease: A Retrospective Cross-Sectional Study. Adv. Ther. 2019, 36, 3424–3434. [Google Scholar] [CrossRef]
- Rodriguez, F.; Olufade, T.O.; Ramey, D.R.; Friedman, H.S.; Navaratnam, P.; Heithoff, K.; Foody, J.M. Gender Disparities in Lipid-Lowering Therapy in Cardiovascular Disease: Insights from a Managed Care Population. J. Womens Health 2016, 25, 697–706. [Google Scholar] [CrossRef]
- Ciliberti, G.; Guerra, F.; Pizzi, C.; Merlo, M.; Zilio, F.; Bianco, F.; Mancone, M.; Zaffalon, D.; Gioscia, R.; Bergamaschi, L.; et al. Characteristics of patients with recurrent acute myocardial infarction after MINOCA. Prog. Cardiovasc. Dis. 2023, 81, 42–47. [Google Scholar] [CrossRef]
- Rajtar-Salwa, R.; Bobrowska, B.; Batko, J.; Bartus, S.; Petkow-Dimitrow, P.; Krawczyk-Ozog, A. Lipid-Lowering Therapy after Acute Coronary Syndrome in Outpatient Practice-How to Achieve Goal. J. Clin. Med. 2023, 12, 6579. [Google Scholar] [CrossRef]
- Wongvibulsin, S.; Habeos, E.E.; Huynh, P.P.; Xun, H.; Shan, R.; Porosnicu Rodriguez, K.A.; Wang, J.; Gandapur, Y.K.; Osuji, N.; Shah, L.M.; et al. Digital Health Interventions for Cardiac Rehabilitation: Systematic Literature Review. J. Med. Internet Res. 2021, 23, e18773. [Google Scholar] [CrossRef]
- Ofori-Asenso, R.; Jakhu, A.; Zomer, E.; Curtis, A.J.; Korhonen, M.J.; Nelson, M.; Gambhir, M.; Tonkin, A.; Liew, D.; Zoungas, S. Adherence and Persistence Among Statin Users Aged 65 Years and Over: A Systematic Review and Meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Danese, M.D.; Kutikova, L.; Catterick, D.; Sorio-Vilela, F.; Gleeson, M.; Kondapally Seshasai, S.R.; Brownrigg, J.; Ray, K.K. Association of a Combined Measure of Adherence and Treatment Intensity With Cardiovascular Outcomes in Patients With Atherosclerosis or Other Cardiovascular Risk Factors Treated With Statins and/or Ezetimibe. JAMA Netw. Open 2018, 1, e185554. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Haq, I.; Bilitou, A.; Manu, M.C.; Burden, A.; Aguiar, C.; Arca, M.; Connolly, D.L.; Eriksson, M.; Ferrieres, J.; et al. Treatment gaps in the implementation of LDL cholesterol control among high- and very high-risk patients in Europe between 2020 and 2021: The multinational observational SANTORINI study. Lancet Reg. Health Eur. 2023, 29, 100624. [Google Scholar] [CrossRef] [PubMed]
- Lubieniecki, P.; Wolyniec, M.; Poltyn-Zaradna, K.; Zatonska, K.; Szuba, A. Lipid-Lowering Therapy in PURE Poland Cohort Study. J. Clin. Med. 2023, 13, 60. [Google Scholar] [CrossRef]
- Laufs, U.; Catapano, A.L.; De Caterina, R.; Schiele, F.; Sionis, A.; Zaman, A.; Jukema, J.W. The effect of the 2019 ESC/EAS dyslipidaemia guidelines on low-density lipoprotein cholesterol goal achievement in patients with acute coronary syndromes: The ACS EuroPath IV project. Vasc. Pharmacol. 2023, 148, 107141. [Google Scholar] [CrossRef]
- Vasko, P.A.J.; Bäck, M.; Dahlbom, L.; Engblom, H.; Erlinge, D.; Friberg, O.; Hagström, E.; Johansson, P.; Settergren, M.; Svensson, S. SWEDEHEART Annual Report 2022. Upps. Clin. Res. Cent. 2023, 1–276. [Google Scholar]
- Banach, M.; Penson, P.E.; Vrablik, M.; Bunc, M.; Dyrbus, K.; Fedacko, J.; Gaita, D.; Gierlotka, M.; Jarai, Z.; Magda, S.L.; et al. Optimal use of lipid-lowering therapy after acute coronary syndromes: A Position Paper endorsed by the International Lipid Expert Panel (ILEP). Pharmacol. Res. 2021, 166, 105499. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Reeskamp, L.F.; Laufs, U.; Banach, M.; Mach, F.; Tokgozoglu, L.S.; Connolly, D.L.; Gerrits, A.J.; Stroes, E.S.G.; Masana, L.; et al. Combination lipid-lowering therapy as first-line strategy in very high-risk patients. Eur. Heart J. 2022, 43, 830–833. [Google Scholar] [CrossRef]
- Banach, M.; Reiner, Z.; Cicero, A.F.G.; Sabouret, P.; Viigimaa, M.; Sahebkar, A.; Postadzhiyan, A.; Gaita, D.; Pella, D.; Penson, P.E. 2022: The year in cardiovascular disease—The year of upfront lipid lowering combination therapy. Arch. Med. Sci. 2022, 18, 1429–1434. [Google Scholar] [CrossRef]
- Banach, M.; Surma, S.; Toth, P.P.; The International Lipid Expert Panel. 2023: The year in cardiovascular disease—The year of new and prospective lipid lowering therapies. Can we render dyslipidemia a rare disease by 2024? Arch. Med. Sci. 2023, 19, 1602–1615. [Google Scholar] [CrossRef]
- Mark, L.; Paragh, G.; Karadi, I.; Reiber, I.; Pados, G.; Kiss, Z. How can we further improve the LDL-cholesterol target level achievement rate based on the Hungarian MULTI GAP 2011 study results and considering the new European dyslipidemia guidelines? Arch. Med. Sci. 2012, 8, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-K.; Hong, S.-J.; Lee, Y.-J.; Hong, S.J.; Yun, K.H.; Hong, B.-K.; Heo, J.H.; Rha, S.-W.; Cho, Y.-H.; Lee, S.-J.; et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): A randomised, open-label, non-inferiority trial. Lancet 2022, 400, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Lewek, J.; Niedziela, J.; Desperak, P.; Dyrbus, K.; Osadnik, T.; Jankowski, P.; Witkowski, A.; Bielecka-Dabrowa, A.; Dudek, D.; Gierlotka, M.; et al. Intensive Statin Therapy Versus Upfront Combination Therapy of Statin and Ezetimibe in Patients With Acute Coronary Syndrome: A Propensity Score Matching Analysis Based on the PL-ACS Data. J. Am. Heart Assoc. 2023, 12, e030414. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Joo, J.H.; Park, S.; Kim, C.; Choi, D.-W.; Hong, S.-J.; Ahn, C.-M.; Kim, J.-S.; Kim, B.-K.; Ko, Y.-G.; et al. Combination Lipid-Lowering Therapy in Patients Undergoing Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2023, 82, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.K.; Tilberry, S.; Gregor, C.; Yaeger, L.H.; Hu, Y.; Sturm, A.C.; Seaton, T.L.; Waltz, T.J.; Rahm, A.K.; Goldberg, A.; et al. Implementation strategies to improve statin utilization in individuals with hypercholesterolemia: A systematic review and meta-analysis. Implement. Sci. 2021, 16, 40. [Google Scholar] [CrossRef]
- Karlsson, A.K.C.; Orre, M.; Nilsson, J. International Price Comparison 2022: An Analysis of Swedish Pharmaceutical Prices in Relation to 19 Other European Countries. 2022, pp. 1–64. Available online: https://www.tlv.se/download/18.12c69789187230f29b822802/1680069871440/report_international_price_comparison_2022_130-2023.pdf (accessed on 31 October 2024).
- Repasy, B.; Gazso, T.; Elmer, D.; Ponusz-Kovacs, D.; Kajos, F.L.; Csakvari, T.; Kovacs, B.; Boncz, I. The long-term effect of generic price competition on the Hungarian statin market. BMC Health Serv. Res. 2023, 23, 447. [Google Scholar] [CrossRef]
- Boruzs, K.; Juhasz, A.; Nagy, C.; Adany, R.; Biro, K. Relationship between Statin Utilization and Socioeconomic Deprivation in Hungary. Front. Pharmacol. 2016, 7, 66. [Google Scholar] [CrossRef]
- Ohm, J.; Kuja-Halkola, R.; Warnqvist, A.; Habel, H.; Skoglund, P.H.; Sundstrom, J.; Hambraeus, K.; Jernberg, T.; Svensson, P. Socioeconomic Disparities and Mediators for Recurrent Atherosclerotic Cardiovascular Disease Events After a First Myocardial Infarction. Circulation 2023, 148, 256–267. [Google Scholar] [CrossRef]
- Scicali, R.; Piro, S.; Ferrara, V.; Di Mauro, S.; Filippello, A.; Scamporrino, A.; Romano, M.; Purrello, F.; Di Pino, A. Direct and Indirect Effects of SARS-CoV-2 Pandemic in Subjects with Familial Hypercholesterolemia: A Single Lipid-Center Real-World Evaluation. J. Clin. Med. 2021, 10, 4363. [Google Scholar] [CrossRef]
- Mark, L.; Fulop, P.; Lorincz, H.; Dani, G.; Tajtine, K.F.; Thury, A.; Paragh, G. The Evaluation of Lipid-Lowering Treatment in Patients with Acute Coronary Syndrome in a Hungarian Invasive Centre in 2015, 2017, and during the COVID-19 Pandemic—The Comparison of the Achieved LDL-Cholesterol Values Calculated with Friedewald and Martin-Hopkins Methods. J. Clin. Med. 2024, 13, 3398. [Google Scholar] [CrossRef]
- Bytyci, I.; Penson, P.E.; Mikhailidis, D.P.; Wong, N.D.; Hernandez, A.V.; Sahebkar, A.; Thompson, P.D.; Mazidi, M.; Rysz, J.; Pella, D.; et al. Prevalence of statin intolerance: A meta-analysis. Eur. Heart. J. 2022, 43, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Vrablik, M.; Sarkanova, I.; Brecikova, K.; Sedova, P.; Satny, M.; Tichopad, A. Low LDL-C goal attainment in patients at very high cardiovascular risk due to lacking observance of the guidelines on dyslipidaemias. PLoS ONE 2023, 18, e0272883. [Google Scholar] [CrossRef] [PubMed]

| LLT | Low Intensity | Moderate Intensity | High Intensity |
|---|---|---|---|
| Atorvastatin | N/A | 10–30 mg | 40–80 mg |
| Ezetimibe | 10 mg | N/A | N/A |
| Fluvastatin | 20–40 mg | 80 mg | N/A |
| Pravastatin | 10–20 mg | 40–80 mg | N/A |
| Rosuvastatin | N/A | 5–15 mg | 20–40 mg |
| Simvastatin | 10 mg | 20–40 mg | N/A |
| Baseline Characteristics and Comorbidities | Statin High Combination | Statin Moderate Combination | Statin Low Combination | Statin High | Statin Moderate | Ezetimibe Monotherapy |
|---|---|---|---|---|---|---|
| No. | 312 | 77 | 193 | 9565 | 1624 | 188 |
| Mean follow-up time (days, ±SD) | 861 (±185.49) | 852.8 (±194.77) | 891.9 (±189,97) | 870.6 (±210.3) | 848.8 (±243.71) | 891.9 (±189.97) |
| Age (y, mean ± SD) | 63.9 (±10.75) | 66.1 (±8.81) | 67.0 (±10) | 64.5 (±11.88) | 68.6 (±11.68) | 66.9 (±9.81) |
| Male, n (%) | 192 (61.54) | 46 (59.74) | 112 (58.03) | 6155 (64.35) | 946 (58.25) | 108 (57.45) |
| Heart failure, n (%) | 68 (21.79) | 22 (28.57) | 54 (27.98) | 1902 (19.88) | 523 (32.2) | 52 (27.66) |
| Peripheral arterial disease, n (%) | 89 (28.53) | 22 (28.57) | 49 (25.39) | 1758 (18.38) | 431 (26.54) | 48 (25.53) |
| Cerebrovascular disease, n (%) | 88 (28.21) | 30 (38.96) | 55 (28.5) | 1676 (17.52) | 437 (26.91) | 53 (28.19) |
| Diabetes, n (%) | 136 (43.59) | 43 (55.84) | 84 (43.52) | 2997 (31.33) | 625 (38.49) | 82 (43.62) |
| Chronic pulmonary disease, n (%) | 74 (23.72) | 16 (20.78) | 44 (22.8) | 1585 (16.57) | 372 (22.91) | 43 (22.87) |
| Hepatic disease, n (%) | 20 (6.41) | 7 (9.09) | 12 (6.22) | 304 (3.18) | 92 (5.67) | 12 (6.38) |
| Renal disease, n (%) | 39 (12.5) | 12 (15.58) | 30 (15.54) | 835 (8.73) | 293 (18.04) | 29 (15.43) |
| Gastrointestinal ulceration, n (%) | 2 (0.64) | - | - | 150 (1.57) | 36 (2.22) | - |
| Tumor, n (%) | 54 (17.31) | 18 (23.38) | 33 (17.1) | 1548 (16.18) | 344 (21.18) | 32 (17.02) |
| Metastatic tumor, n (%) | 2 (0.64) | - | - | 82 (0.86) | 13 (0.8) | - |
| Leukemia/lymphoma, n (%) | 1 (0.32) | 1 (1.3) | 5 (2.59) | 42 (0.44) | 15 (0.92) | 5 (2.66) |
| Lymphoma, n (%) | 1 (0.32) | - | - | 40 (0.42) | 9 (0.55) | - |
| Connective tissue disease, n (%) | 5 (1.6) | 1 (1.3) | 11 (5.7) | 125 (1.31) | 26 (1.6) | 11 (5.85) |
| Alcohol use disorder, n (%) | - | 2 (2.6) | 1 (0.52) | 203 (2.12) | 47 (2.89) | 1 (0.53) |
| Dementia/mental condition, n (%) | - | - | 1 (0.52) | 117 (1.22) | 31 (1.91) | 1 (0.53) |
| Mental condition, n (%) | 45 (14.42) | 15 (19.48) | 27 (13.99) | 1187 (12.41) | 235 (14.47) | 27 (14.36) |
| Baseline Characteristics and Comorbidities | Statin High Combination (SHC) | Statin High (SH) | Statin Moderate (SM) | SHC vs. SH (p-Values) | SHC vs. SM (p-Values) | SH vs. SM (p-Values) |
|---|---|---|---|---|---|---|
| No. | 312 | 9565 | 1624 | - | - | - |
| Age (y, mean) | 63.9 | 64.5 | 68.6 | 1.0000 | 0.0010 | 0.0010 |
| Male (%) | 61.54 | 64.35 | 58.25 | 1.0000 | 1.0000 | <0.0001 |
| Heart failure (%) | 21.79 | 19.88 | 32.2 | 1.0000 | 0.0026 | <0.0001 |
| Peripheral arterial disease (%) | 28.53 | 18.38 | 26.54 | <0.0001 | 1.0000 | <0.0001 |
| Cerebrovascular disease (%) | 28.21 | 17.52 | 26.91 | <0.0001 | 1.0000 | <0.0001 |
| Diabetes (%) | 43.59 | 31.33 | 38.49 | <0.0001 | 0.9089 | <0.0001 |
| Chronic pulmonary disease (%) | 23.72 | 16.57 | 22.91 | 0.0089 | 1.0000 | <0.0001 |
| Hepatic disease (%) | 6.41 | 3.18 | 5.67 | 0.0161 | 1.0000 | <0.0001 |
| Renal disease (%) | 12.5 | 8.73 | 18.04 | 0.2102 | 0.1738 | <0.0001 |
| Gastrointestinal ulceration (%) | 0.64 | 1.57 | 2.22 | 1.0000 | 0.5950 | 0.5289 |
| Tumor (%) | 17.31 | 16.18 | 21.18 | 1.0000 | 1.0000 | <0.0001 |
| Metastatic tumor (%) | 0.64 | 0.86 | 0.8 | 1.0000 | 1.0000 | 1.0000 |
| Leukemia/lymphoma (%) | 0.32 | 0.44 | 0.92 | 1.0000 | 1.0000 | 0.1121 |
| Lymphoma (%) | 0.32 | 0.42 | 0.55 | 1.0000 | 1.0000 | 1.0000 |
| Connective tissue disease (%) | 1.6 | 1.31 | 1.6 | 1.0000 | 1.0000 | 1.0000 |
| Alcohol use disorder (%) | - | 2.12 | 2.89 | 0.0932 | 0.0235 | 0.5170 |
| Dementia/mental condition (%) | - | 1.22 | 1.91 | 0.4445 | 0.1250 | 0.2281 |
| Mental condition (%) | 14.42 | 12.41 | 14.47 | 1.0000 | 1.0000 | 0.2116 |
| Lipid-Lowering Therapy | Adherence | Persistence 1-Year | Persistence 2-Year |
|---|---|---|---|
| Moderate-dose statin | 56% | 52% | 38% |
| Ezetimibe mono and low-dose statin | 61% | 57% | 48% |
| High-dose statin | 63% | 59% | 45% |
| Combination, moderate-dose statin | 66% | 66% | 45% |
| Combination, high-dose statin | 69% | 67% | 54% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, G.G.; Mark, L.; Gerencser, A.; Reiber, I.; Kiss, N.; Rokszin, G.; Fabian, I.; Csanadi, Z.; Karadi, I.; Aradi, D.; et al. A Nation-Wide Evaluation of Suboptimal Lipid-Lowering Treatment Patterns Among Patients Undergoing Intervention for Acute Coronary Syndrome in Hungary. J. Clin. Med. 2024, 13, 6562. https://doi.org/10.3390/jcm13216562
Nagy GG, Mark L, Gerencser A, Reiber I, Kiss N, Rokszin G, Fabian I, Csanadi Z, Karadi I, Aradi D, et al. A Nation-Wide Evaluation of Suboptimal Lipid-Lowering Treatment Patterns Among Patients Undergoing Intervention for Acute Coronary Syndrome in Hungary. Journal of Clinical Medicine. 2024; 13(21):6562. https://doi.org/10.3390/jcm13216562
Chicago/Turabian StyleNagy, Gergely Gyorgy, Laszlo Mark, Andrea Gerencser, Istvan Reiber, Norbert Kiss, Gyorgy Rokszin, Ibolya Fabian, Zoltan Csanadi, Istvan Karadi, Daniel Aradi, and et al. 2024. "A Nation-Wide Evaluation of Suboptimal Lipid-Lowering Treatment Patterns Among Patients Undergoing Intervention for Acute Coronary Syndrome in Hungary" Journal of Clinical Medicine 13, no. 21: 6562. https://doi.org/10.3390/jcm13216562
APA StyleNagy, G. G., Mark, L., Gerencser, A., Reiber, I., Kiss, N., Rokszin, G., Fabian, I., Csanadi, Z., Karadi, I., Aradi, D., Bajnok, L., & Paragh, G. (2024). A Nation-Wide Evaluation of Suboptimal Lipid-Lowering Treatment Patterns Among Patients Undergoing Intervention for Acute Coronary Syndrome in Hungary. Journal of Clinical Medicine, 13(21), 6562. https://doi.org/10.3390/jcm13216562





