Whole-Body Perfusion in Neonates and Infants Undergoing Aortic Arch Surgery—Working Towards the New Standard
Abstract
:1. Introduction
2. Methods
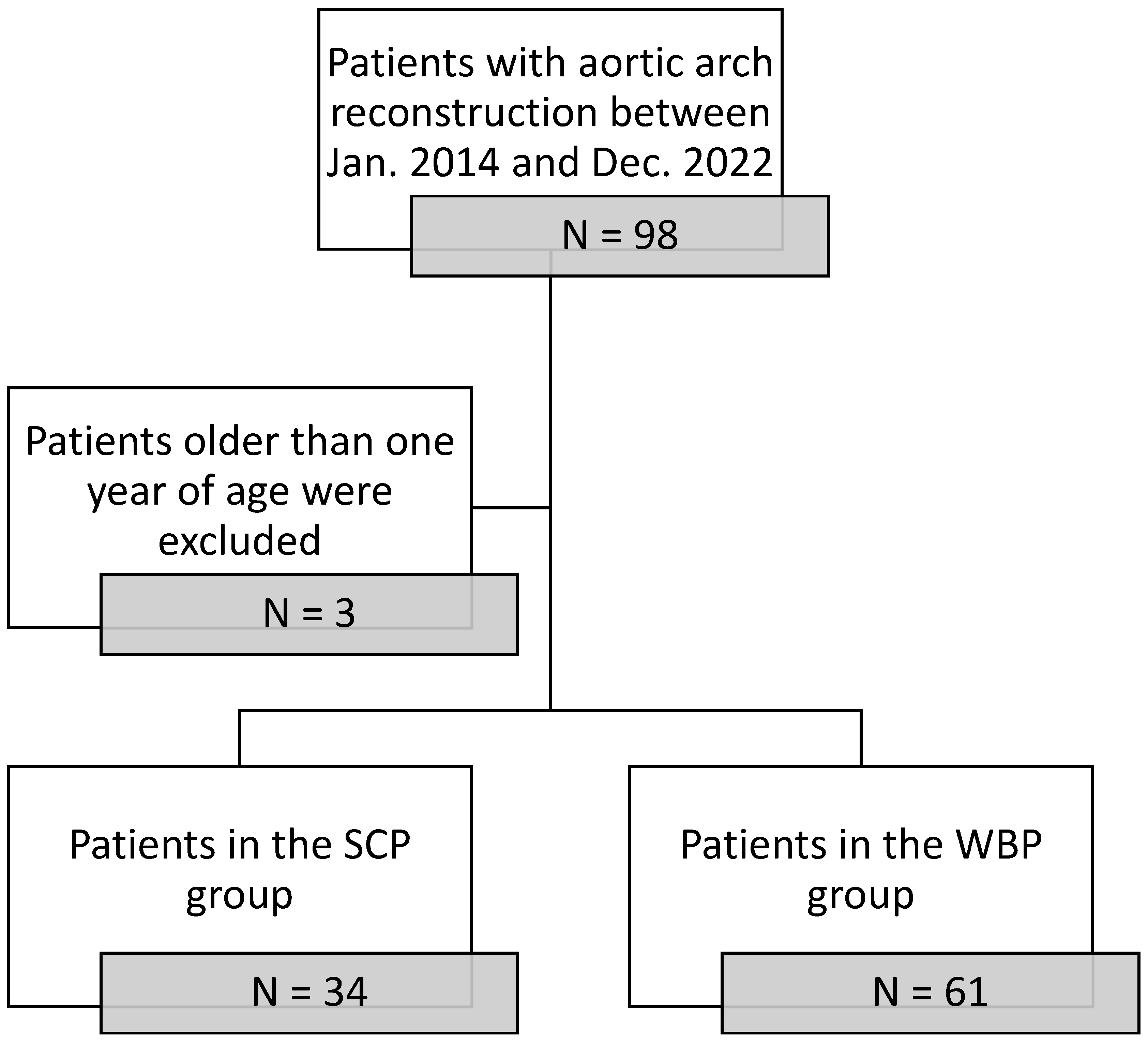
2.1. Patient Selection and Grouping
2.2. Perfusion Strategies
2.3. Parameter Analysis
2.4. Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Newburger, J.W.; Jonas, R.A.; Wernovsky, G.; Wypij, D.; Hickey, P.R.; Kuban, K.C.; Farrell, D.M.; Holmes, G.L.; Helmers, S.L.; Constantinou, J.; et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N. Engl. J. Med. 1993, 329, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Wypij, D.; Newburger, J.W.; Rappaport, L.A.; duPlessis, A.J.; Jonas, R.A.; Wernovsky, G.; Lin, M.; Bellinger, D.C. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: The Boston Circulatory Arrest Trial. J. Thorac. Cardiovasc. Surg. 2003, 126, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Eggli, K.D.; Contant, C.; Baylen, B.G.; Myers, J.L. Postoperative neurologic complications after open heart surgery on young infants. Arch. Pediatr. Adolesc. Med. 1995, 149, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Levy, E.M.; Hammermeister, K.E.; Grover, F.; Daley, J. Independent association between acute renal failure and mortality following cardiac surgery. Am. J. Med. 1998, 104, 343–348. [Google Scholar] [CrossRef]
- Clancy, R.R.; McGaurn, S.A.; Wernovsky, G.; Gaynor, J.W.; Spray, T.L.; Norwood, W.I.; Jacobs, M.L.; Goin, J.E. Risk of seizures in survivors of newborn heart surgery using deep hypothermic circulatory arrest. Pediatrics 2003, 111, 592–601. [Google Scholar] [CrossRef]
- Rajagopal, S.K.; Emani, S.M.; Roy, N.; Westgate, L.; Bacha, E.A. Acute kidney injury and regional abdominal perfusion during neonatal aortic arch reconstruction. J. Thorac. Cardiovasc. Surg. 2010, 140, 453–458. [Google Scholar] [CrossRef]
- Boburg, R.S.; Rosenberger, P.; Kling, S.; Jost, W.; Schlensak, C.; Magunia, H. Selective lower body perfusion during aortic arch surgery in neonates and small children. Perfusion 2020, 35, 621–625. [Google Scholar] [CrossRef]
- Raees, M.A.; Morgan, C.D.; Pinto, V.L.; Westrick, A.C.; Shannon, C.N.; Christian, K.G.; Mettler, B.A.; Bichell, D.P. Neonatal Aortic Arch Reconstruction with Direct Splanchnic Perfusion Avoids Deep Hypothermia. Ann. Thorac. Surg. 2017, 104, 2054–2063. [Google Scholar] [CrossRef]
- Hammel, J.M.; Deptula, J.J.; Karamlou, T.; Wedemeyer, E.; Abdullah, I.; Duncan, K.F. Newborn aortic arch reconstruction with descending aortic cannulation improves postoperative renal function. Ann. Thorac. Surg. 2013, 96, 1721–1726. [Google Scholar] [CrossRef]
- Fernandez-Doblas, J.; Ortega-Loubon, C.; Perez-Andreu, J.; Lines, M.; Fernandez-Molina, M.; Abella, R.F. Selective visceral perfusion improves renal flow and hepatic function in neonatal aortic arch repair. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 395–401. [Google Scholar] [CrossRef]
- Sandoval Boburg, R.; Berger, R.; Mustafi, M.; Faust, C.; Magunia, H.; Neunhoeffer, F.; Hofbeck, M.; Rosenberger, P.; Schlensak, C. Whole-body perfusion improves intraoperative transfusions in neonatal aortic arch surgery. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad065. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, R.; Faust, C.; Kruger, T.; Mustafi, M.; Magunia, H.; Neunhoeffer, F.; Hofbeck, M.; Schlensak, C. Impact of Whole-Body Perfusion in Postoperative Outcomes After Aortic Arch Reconstruction Surgery in Neonates and Infants. Heart Surg. Forum 2022, 25, E222–E228. [Google Scholar] [CrossRef] [PubMed]
- Pigula, F.A.; Nemoto, E.M.; Griffith, B.P.; Siewers, R.D. Regional low-flow perfusion provides cerebral circulatory support during neonatal aortic arch reconstruction. J. Thorac. Cardiovasc. Surg. 2000, 119, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Pigula, F.A.; Gandhi, S.K.; Siewers, R.D.; Davis, P.J.; Webber, S.A.; Nemoto, E.M. Regional low-flow perfusion provides somatic circulatory support during neonatal aortic arch surgery. Ann. Thorac. Surg. 2001, 72, 401–406; discussion 406–407. [Google Scholar] [CrossRef]
- Algra, S.O.; Kornmann, V.N.; van der Tweel, I.; Schouten, A.N.; Jansen, N.J.; Haas, F. Increasing duration of circulatory arrest, but not antegrade cerebral perfusion, prolongs postoperative recovery after neonatal cardiac surgery. J. Thorac. Cardiovasc. Surg. 2012, 143, 375–382. [Google Scholar] [CrossRef]
- Algra, S.O.; Schouten, A.N.; van Oeveren, W.; van der Tweel, I.; Schoof, P.H.; Jansen, N.J.; Haas, F. Low-flow antegrade cerebral perfusion attenuates early renal and intestinal injury during neonatal aortic arch reconstruction. J. Thorac. Cardiovasc. Surg. 2012, 144, 1323–1328.e2. [Google Scholar] [CrossRef]
- Algra, S.O.; Jansen, N.J.; van der Tweel, I.; Schouten, A.N.; Groenendaal, F.; Toet, M.; van Oeveren, W.; van Haastert, I.C.; Schoof, P.H.; de Vries, L.S.; et al. Neurological injury after neonatal cardiac surgery: A randomized, controlled trial of 2 perfusion techniques. Circulation 2014, 129, 224–233. [Google Scholar] [CrossRef]
- Miyaji, K.; Miyamoto, T.; Kohira, S.; Itatani, K.; Tomoyasu, T.; Inoue, N.; Ohara, K. Regional high-flow cerebral perfusion improves both cerebral and somatic tissue oxygenation in aortic arch repair. Ann. Thorac. Surg. 2010, 90, 593–599. [Google Scholar] [CrossRef]
- Tlaskal, T.; Vojtovic, P.; Reich, O.; Hucin, B.; Gebauer, R.; Kucera, V. Improved results after the primary repair of interrupted aortic arch: Impact of a new management protocol with isolated cerebral perfusion. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2010, 38, 52–58. [Google Scholar] [CrossRef]
- Fernandes, P.; Cleland, A.; Adams, C.; Chu, M.W. Clinical and biochemical outcomes for additive mesenteric and lower body perfusion during hypothermic circulatory arrest for complex total aortic arch replacement surgery. Perfusion 2012, 27, 493–501. [Google Scholar] [CrossRef]
- Kulyabin, Y.Y.; Gorbatykh, Y.N.; Soynov, I.A.; Zubritskiy, A.V.; Voitov, A.V.; Bogachev-Prokophiev, A.V. Selective Antegrade Cerebral Perfusion with or without Additional Lower Body Perfusion during Aortic Arch Reconstruction in Infants. World J. Pediatr. Congenit. Heart Surg. 2020, 11, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kulyabin, Y.Y.; Bogachev-Prokophiev, A.V.; Soynov, I.A.; Omelchenko, A.Y.; Zubritskiy, A.V.; Gorbatykh, Y.N. Clinical Assessment of Perfusion Techniques During Surgical Repair of Coarctation of Aorta with Aortic Arch Hypoplasia in Neonates: A Pilot Prospective Randomized Study. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Misra, A.; Forbes, T.J.; Kobayashi, D. Femoral Artery Thrombosis after Pediatric Cardiac Catheterization. Pediatr. Cardiol. 2021, 42, 753–761. [Google Scholar] [CrossRef] [PubMed]

| Total Cohort N = 95 | WBP N = 61 | SCP N = 34 | p-Value | |
|---|---|---|---|---|
| Age (d) | 12.5 (8–33.5) | 12 (8–45) | 13.5 (8–28) | 0.7 |
| Sex (m) | 63 (66%) | 40 (66%) | 23 (68%) | 0.83 |
| Length (cm) | 50 (49–53) | 51 (49–54) | 50 (49–52) | 0.19 |
| Weight (kg) | 3.45 (3.1–3.8) | 3.5 (3.1–3.9) | 3.4 (3.1–3.7) | 0.71 |
| Preoperative Parameters | ||||
| Creatinine (mg/dL) | 0.5 (0.35–0.7) | 0.4 (0.3–0.6) | 0.55 (0.3–0.7) | 0.16 |
| ASAT (mg/dL) | 34 (27–48) | 34 (27–51) | 34 (27–47.5) | 0.82 |
| ALAT (mg/dL) | 17 (10–30.5) | 17 (9.5–29) | 17 (11–35) | 0.54 |
| LDH (mg/dL) | 346.5 (281–452) | 349 (284–461) | 343.5 (261–442) | 0.7 |
| INR | 1.2 (1.1–1.2) | 1.2(1.1–1.25) | 1.1(1–1.2) | <0.01 |
| WBP N = 61 | SCP N = 34 | p-Value | |
|---|---|---|---|
| Lowest temperature (°C) | 30.86 ± 2.56 | 26.94 ± 2.13 | <0.01 |
| CPB time (min) | 133.61 ± 65.97 | 174.82 ± 64.95 | <0.01 |
| Aortic cross-clamp (min) | 72.67 ± 44.03 | 85.72 ± 47.94 | 0.09 |
| LBP time (min) | 54 ± 38.98 | N/A | |
| Max. lactate value during reperfusion (mg/dL) | 1.9 (1.6–2.8) | 4.3 (3.25–5.55) | <0.01 |
| Intraoperative Transfusions | |||
| RBC (mL) | 320 (182.5–560) | 600 (300–775) | <0.01 |
| FFP (mL) | 280 (150–300) | 600 (342.5–600) | <0.01 |
| TP (mL) | 60 (25–190) | 300 (250–300) | <0.01 |
| WBP N = 61 | SCP N = 34 | p-Value | |
|---|---|---|---|
| Preoperative Parameters | |||
| ALAT (mg/dL) | 72 (48.5–104) | 84 (62–135) | 0.1 |
| ASAT (mg/dL) | 13 (9–19.5) | 20.5 (16–28) | <0.01 |
| LDH (mg/dL) | 408 (346.5–553.5) | 445.5 (349–543) | 0.4 |
| INR | 1.1 (1–1.2) | 1.1 (1–1.1) | 0.1 |
| Postoperative Parameters after 24 h | |||
| Creatinine (mg/dL) | 0.52 ± 0.21 | 0.64 ± 0.25 | 0.02 |
| ALAT (mg/dL) | 51 (37–100.5) | 49 (37–152) | 0.78 |
| ASAT (mg/dL) | 10 (8–16.5) | 12.5 (8–25) | 0.23 |
| LDH (mg/dL) | 361 (287.5–530) | 374.5 (315–669) | 0.3 |
| INR | 1.2 (1.1–1.3) | 1.1 (1.1–1.2) | 0.02 |
| Urine output (mL/h) | 6.54 (4.63–10.17) | 3.46 (2.88–4.71) | <0.01 |
| Postoperative Parameters after 72 h | |||
| Creatinine (mg/dL) | 0.5 (0.4–0.6) | 0.6 (0.5–0.9) | 0.02 |
| ALAT (md/dL) | 30.5 (20–46.5) | 24 (17–45) | 0.5 |
| ASAT (md/dL) | 8.5 (5–15) | 7.5 (4–14) | 0.33 |
| LDH (md/dL) | 326 (261–539) | 326.5 (256–485) | 0.98 |
| INR | 1.1 (1.05–1.2) | 1.1 (1–1.2) | 0.41 |
| Urine output (mL/h) | 23.25 (19.25–27.17) | 20.77 (14.38–26.50) | 0.06 |
| Duration of mechanical ventilation (d) | 6 (3–11) | 8 (6–14) | 0.02 |
| Length of stay at the PICU (d) | 13 (8–26) | 15 (9–28) | 0.38 |
| WBP N = 61 | SCP N = 34 | p-Value | |
|---|---|---|---|
| RRT | 4 (6.6%) | 5 (14.7%) | 0.19 |
| Neurological deficits | 2 (3.3%) | 6 (17.6%) | 0.01 |
| Open thorax | 34 (55.7%) | 22 (64.7%) | 0.39 |
| NEC | 8 (13.1%) | 4 (11.8%) | 0.85 |
| Postoperative bleeding complications | 8 (13.1%) | 5 (14.7%) | 0.82 |
| Intraoperative ECLS | 14 (23.0%) | 8 (23.5%) | 0.95 |
| Postoperative ECLS | 7 (11.5%) | 1 (2.9%) | 0.15 |
| Punction-related complications | 4 (6.6%) | - | |
| Multiorgan failure | 1 (1.6%) | 2 (5.9%) | 0.25 |
| 30-day mortality | 3 (4.9%) | 2 (5.9%) | 0.84 |
| Outcome | Regression Coefficient | Odds Ratio | CI (95%) | p-Value |
|---|---|---|---|---|
| Dialysis | −0.011 | 0.989 | 0.983–0.995 | <0.01 |
| Neurological deficits | −0.006 | 0.994 | 0.988–1 | 0.06 |
| 30-day mortality | −3.346 | 0.035 | 0.003–0.391 | <0.01 |
| NEC | 0.018 | 1.018 | 1.001–1.035 | 0.04 |
| Multiorgan failure | −6.003 | 0.002 | 0–0.275 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval Boburg, R.; Doll, I.; Rustenbach, C.J.; Berger, R.; Jost, W.; Magunia, H.; Nordmeyer, J.; Michel, J.; Schlensak, C. Whole-Body Perfusion in Neonates and Infants Undergoing Aortic Arch Surgery—Working Towards the New Standard. J. Clin. Med. 2024, 13, 6170. https://doi.org/10.3390/jcm13206170
Sandoval Boburg R, Doll I, Rustenbach CJ, Berger R, Jost W, Magunia H, Nordmeyer J, Michel J, Schlensak C. Whole-Body Perfusion in Neonates and Infants Undergoing Aortic Arch Surgery—Working Towards the New Standard. Journal of Clinical Medicine. 2024; 13(20):6170. https://doi.org/10.3390/jcm13206170
Chicago/Turabian StyleSandoval Boburg, Rodrigo, Isabelle Doll, Christian Jörg Rustenbach, Rafal Berger, Walter Jost, Harry Magunia, Johannes Nordmeyer, Jörg Michel, and Christian Schlensak. 2024. "Whole-Body Perfusion in Neonates and Infants Undergoing Aortic Arch Surgery—Working Towards the New Standard" Journal of Clinical Medicine 13, no. 20: 6170. https://doi.org/10.3390/jcm13206170
APA StyleSandoval Boburg, R., Doll, I., Rustenbach, C. J., Berger, R., Jost, W., Magunia, H., Nordmeyer, J., Michel, J., & Schlensak, C. (2024). Whole-Body Perfusion in Neonates and Infants Undergoing Aortic Arch Surgery—Working Towards the New Standard. Journal of Clinical Medicine, 13(20), 6170. https://doi.org/10.3390/jcm13206170







