Facial Surface Electromyography: A Novel Approach to Facial Nerve Functional Evaluation after Vestibular Schwannoma Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
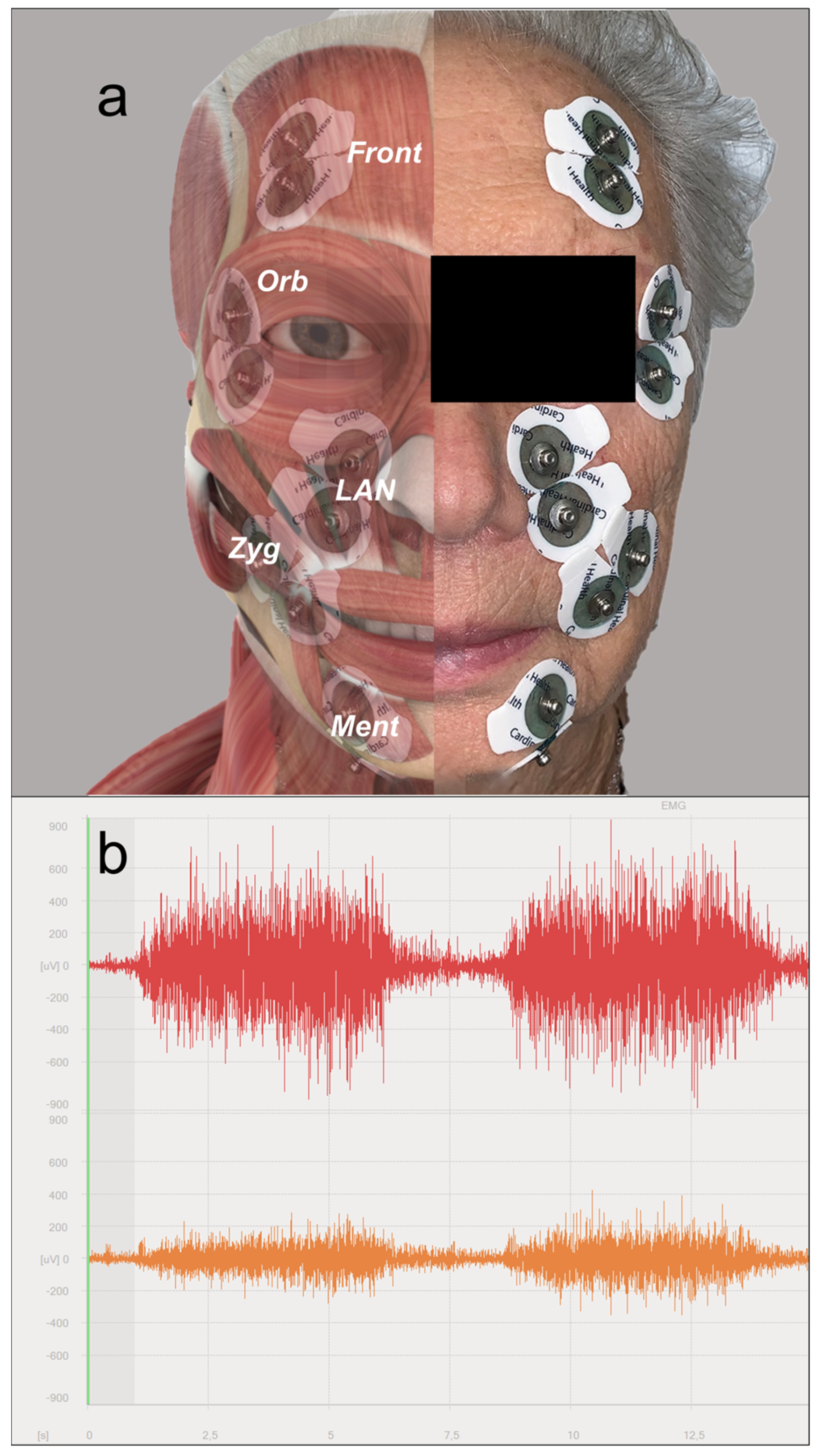
2.2. Surface Electromyography (sEMG)
- Mentalis (lip pucker);
- Levator labii alaeque nasi (snarl);
- Zygomatic/risorius (open-mouth smile);
- Orbicularis oculi (eye closure);
- Frontalis (forehead wrinkle).
2.3. Analysis of the EMG Signal
2.4. Statistical Analysis
3. Results
3.1. Clinical Features and Outcomes
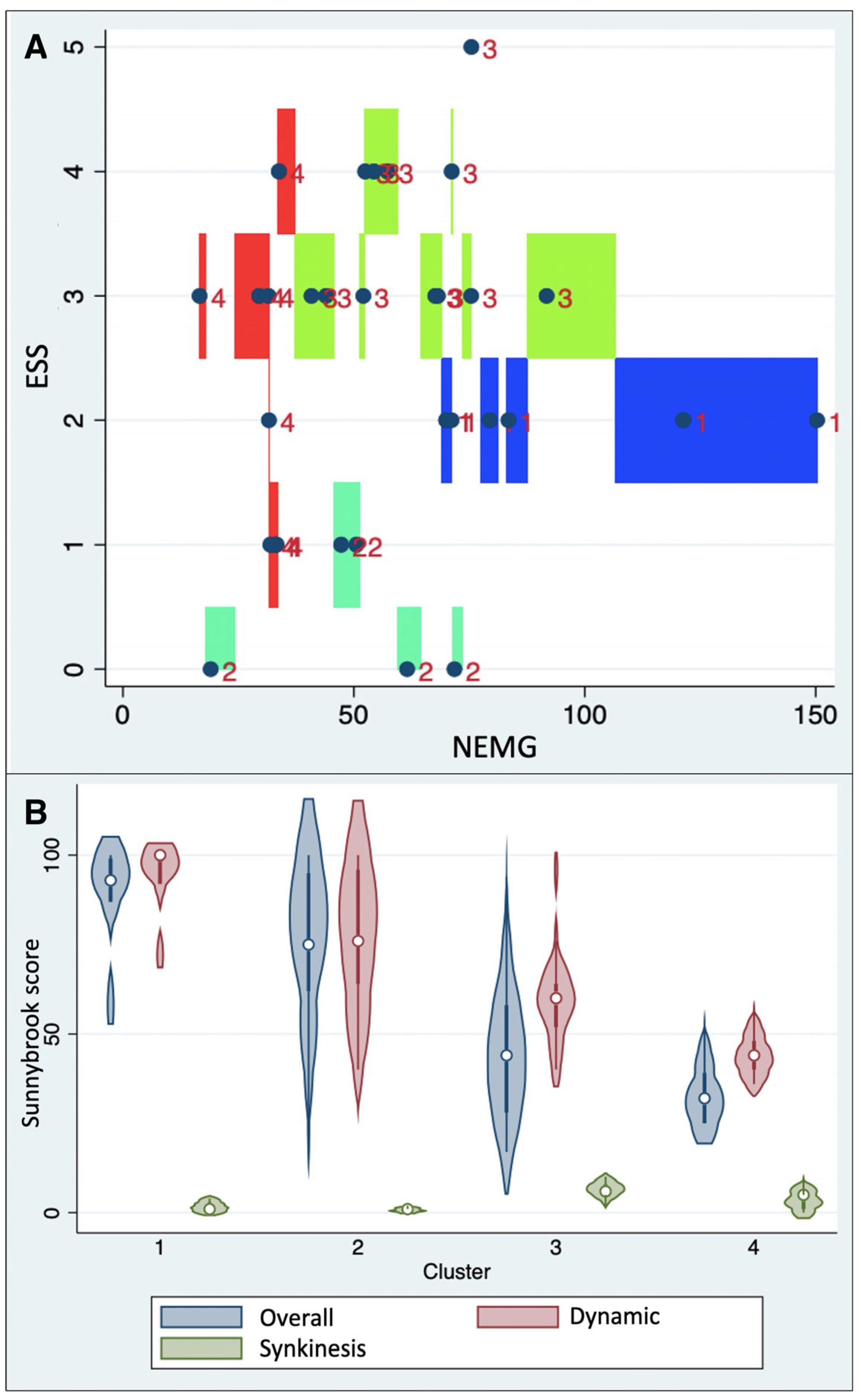
3.2. sEMG Patterns on the Affected Hemiface and on the Healthy Side
- Cluster 1 (median NEMG: 79.4% IQR 70.0–121.2%; median ESS: 2.0, IQR: 2.0–2.0);
- Cluster 2 (median NEMG: 50.5% IQR 47.3–61.5%; median ESS: 0.0, IQR: 0.0–1.0);
- Cluster 3 (median NEMG: 62.5% IQR 52.3–73.3%; median ESS: 3.0, IQR: 3.0–4.0);
- Cluster 4 (median NEMG: 37.8% IQR 30.5–33.1%; median ESS: 2.5, IQR: 1.0–3.0).
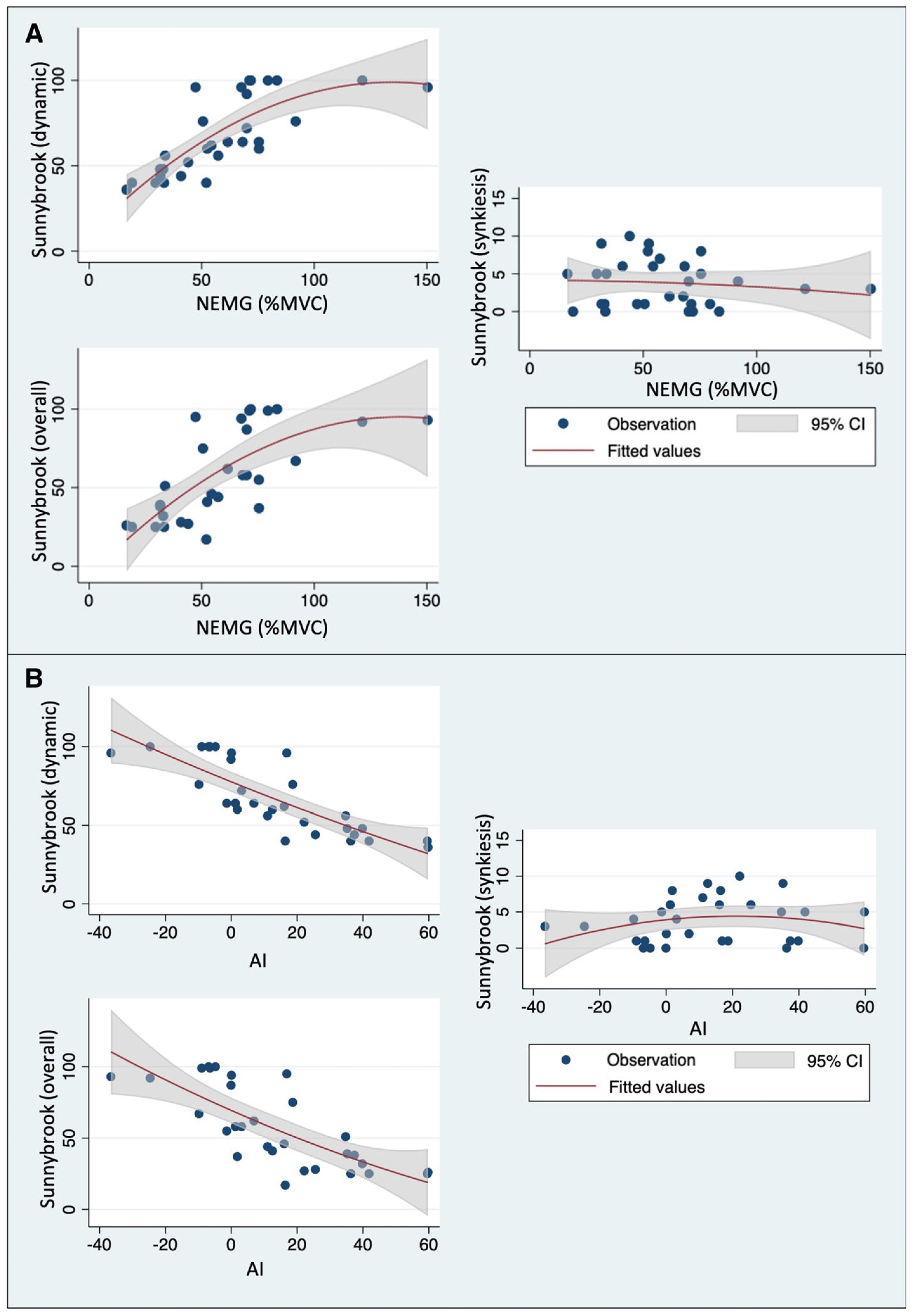
3.3. Correlation between Sunnybrook Scores and Quantitative sEMG Parameters
3.4. Association between Quantitative sEMG Parameters and Clinical Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maniakas, A.; Saliba, I. Microsurgery versus stereotactic radiation for small vestibular schwannomas: A meta-analysis of patients with more than 5 years’ follow-up. Otol. Neurotol. 2012, 33, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Yakkala, V.K.; Mammi, M.; Lamba, N.; Kandikatla, R.; Paliwal, B.; Elshibiny, H.; Corrales, C.E.; Smith, T.R.; Mekary, R.A. Audiovestibular symptoms and facial nerve function comparing microsurgery versus SRS for vestibular schwannomas: A systematic review and meta-analysis. Acta Neurochir. 2022, 164, 3221–3233. [Google Scholar] [CrossRef] [PubMed]
- Hostettler, I.C.; Jayashankar, N.; Bikis, C.; Wanderer, S.; Nevzati, E.; Karuppiah, R.; Waran, V.; Kalbermatten, D.; Mariani, L.; Marbacher, S.; et al. Clinical studies and pre-clinical animal models on facial nerve preservation, reconstruction, and regeneration following cerebellopontine angle tumor surgery-A systematic review and future perspectives. Front. Bioeng. Biotechnol. 2021, 18, 659413. [Google Scholar] [CrossRef] [PubMed]
- Sampath, P.; Holliday, M.J.; Brem, H.; Niparko, J.K.; Long, D.M. Facial nerve injury in acoustic neuroma (vestibular schwannoma) surgery: Etiology and prevention. J. Neurosurg. 1997, 87, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Zanoletti, E.B.E.; Mazzoni, A.; Martini, A.; Abbritti, R.V.; Albertini, R.; Alexandre, E.; Baro, V.; Bartolini, S.; Bernardeschi, D.; Bivona, R.; et al. Surgery of the lateral skull base: A 50-year endeavour. Acta Otorhinolaryngol. Ital. 2019, 39, S1–S146. [Google Scholar] [CrossRef] [PubMed]
- King, T.T.; Sparrow, O.C.; Arias, J.M.; O’Connor, A.F. Repair of facial nerve after removal of cerebellopontine angle tumors: A comparative study. J. Neurosurg. 1993, 78, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Janecka, I.P.; Sekhar, L.N.; Sen, C.N. Facial nerve management in cranial base surgery. Laryngoscope 1993, 103, 291–298. [Google Scholar] [CrossRef]
- Parrino, D.; Franchella, S.; Frigo, A.C.; Mazzoni, A.; Marioni, G.; Zanoletti, E. Facial nerve sacrifice in lateral approaches to the skull base: Simultaneous reconstruction by graft interposition. Am. J. Otolaryngol. 2022, 43, 103210. [Google Scholar] [CrossRef]
- Ren, Y.; MacDonald, B.V.; Tawfik, K.O.; Schwartz, M.S.; Friedman, R.A. Clinical predictors of facial nerve outcomes after surgical resection of vestibular schwannoma. Otolaryngol. Head Neck Surg. 2021, 164, 1085–1093. [Google Scholar] [CrossRef]
- Koos, W.T.; Day, J.D.; Matula, C.; Levy, D.I. Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J. Neurosurg. 1998, 88, 506–512. [Google Scholar] [CrossRef]
- Pinkiewicz, M.; Dorobisz, K.; Zatoński, T. A comprehensive approach to facial reanimation: A systematic review. J. Clin. Med. 2022, 11, 2890. [Google Scholar] [CrossRef]
- Fattah, A.Y.; Gurusinghe, A.D.; Gavilan, J.; Hadlock, T.A.; Marcus, J.R.; Marres, H.; Nduka, C.C.; Slattery, W.H.; Snyder-Warwick, A.K. Facial nerve grading instruments: Systematic review of the literature and suggestion for uniformity. Plast. Reconstr. Surg. 2015, 135, 569–579. [Google Scholar] [CrossRef] [PubMed]
- House, J.W.; Brackmann, D.E. Facial nerve grading system. Otolaryngol. Head Neck Surg. 1985, 93, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.G.; Fradet, G.; Nedzelski, J.M. Development of a sensitive clinical facial grading system. Otolaryngol. Head Neck Surg. 1996, 114, 380–386. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Marotta, N.; Agostini, F.; Drago Ferrante, V.; Demeco, A.; Ferrillo, M.; Inzitari, M.T.; Pellegrino, R.; Russo, I.; Ozyemisci Taskiran, O.; et al. A telerehabilitation approach to chronic facial paralysis in the COVID-19 pandemic scenario: What role for electromyography assessment? J. Pers. Med. 2022, 12, 497. [Google Scholar] [CrossRef]
- Ryu, H.-M.; Lee, S.-J.; Park, E.-J.; Kim, S.-G.; Kim, K.H.; Choi, Y.M.; Kim, J.U.; Song, B.Y.; Kim, C.H.; Yoon, H.-M.; et al. Study on the validity of surface electromyography as assessment tools for facial nerve palsy. J. Pharmacopunct. 2018, 21, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Franz, L.; de Filippis, C.; Daloiso, A.; Biancoli, E.; Iannacone, F.P.; Cazzador, D.; Tealdo, G.; Marioni, G.; Nicolai, P.; Zanoletti, E. Facial surface electromyography: A systematic review on the state of the art and current perspectives. Am. J. Otolaryngol. 2023, 45, 104041. [Google Scholar] [CrossRef]
- Franz, L.; Travan, L.; Isola, M.; Marioni, G.; Pozzo, R. Facial muscle activity patterns in clarinet players: A key to understanding facial muscle physiology and dysfunction in musicians. Ann. Otol. Rhinol. Laryngol. 2020, 129, 1078–1087. [Google Scholar] [CrossRef]
- Lapatki, B.G.; Oostenveld, R.; Van Dijk, J.P.; Jonas, I.E.; Zwarts, M.J.; Stegeman, D.F. Optimal placement of bipolar surface EMG electrodes in the face based on single motor unit analysis. Psychophysiology 2010, 47, 299–314. [Google Scholar] [CrossRef]
- Franz, L.; Tealdo, G.; Contro, G.; Bandolin, L.; Carraro, V.; Giacomelli, L.; Alessandrini, L.; Blandamura, S.; Marioni, G. Biological tumor markers (maspin, CD105, nm23-H1) and disease relapse in laryngeal cancer: Cluster analysis. Head Neck 2020, 42, 2129–2136. [Google Scholar] [CrossRef]
- Wenceslau, L.G.; Sassi, F.C.; Magnani, D.M.; Andrade, C.R. Peripheral facial palsy: Muscle activity in different onset times. Codas 2016, 28, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhong, W.; Yang, Z.; Cao, X.; Dai, S.; Huang, X.; Hu, L.; Lan, K.; Li, G.; Yu, H. Comparison of facial muscle activation patterns between healthy and Bell’s palsy subjects using high-density surface electromyography. Front. Hum. Neurosci. 2021, 14, 618985. [Google Scholar] [CrossRef] [PubMed]
- Samii, M.; Matthies, C. Management of 1000 vestibular schwannomas (acoustic neuromas): The facial nerve--preservation and restitution of function. Neurosurgery 1997, 40, 684–694. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O.; Prengel, J.; Cohen, O.; Mäkitie, A.A.; Poorten, V.V.; Ronen, O.; Shaha, A.; Ferlito, A. Pathogenesis, diagnosis and therapy of facial synkinesis: A systematic review and a clinical practice recommendations by the International Head and Neck Scientific Group. Front. Neurol. 2022, 13, 1019554. [Google Scholar] [CrossRef] [PubMed]
- Guntinas-Lichius, O.; Volk, G.F.; Olsen, K.D.; Mäkitie, A.A.; Silver, C.E.; Zafereo, M.E.; Rinaldo, A.; Randolph, G.W.; Simo, R.; Shaha, A.R.; et al. Facial nerve electrodiagnostics for patients with facial palsy: A clinical practice guideline. Eur. Arch. Otorhinolaryngol. 2020, 277, 1855–1874. [Google Scholar] [CrossRef] [PubMed]
- Pavese, C.; Tinelli, C.; Furini, F.; Abbamonte, M.; Giromini, E.; Sala, V.; De Silvestri, A.; Cecini, M.; Toffola, E.D. Validation of the Italian version of the Sunnybrook Facial Grading System. Neurol. Sci. 2013, 34, 457–463. [Google Scholar] [CrossRef]
- Coulson, S.E.; Croxson, G.R.; Adams, R.D.; O’Dwyer, N.J. Reliability of the “Sydney”, “Sunnybrook”, and “House Brackmann” facial grading systems to assess voluntary movement and synkinesis after facial nerve paralysis. Otolaryngol. Head Neck Surg. 2005, 132, 543–549. [Google Scholar] [CrossRef]
- Banks, C.A.; Jowett, N.; Azizzadeh, B.; Beurskens, C.; Bhama, P.; Borschel, G.; Coombs, C.; Coulson, S.; Croxon, G.; Diels, J.; et al. Worldwide testing of the eFACE Facial Nerve Clinician-Graded Scale. Plast. Reconstr. Surg. 2017, 139, 491e–498e. [Google Scholar] [CrossRef]
- VanSwearingen, J.M.; Brach, J.S. The Facial Disability Index: Reliability and validity of a disability assessment instrument for disorders of the facial neuromuscular system. Phys. Ther. 1996, 76, 1288–1298. [Google Scholar] [CrossRef]
- Burres, S.A. Objective grading of facial paralysis. Ann. Otol. Rhinol. Laryngol. 1986, 95, 238–241. [Google Scholar] [CrossRef]
- Murty, G.E.; Diver, J.P.; Kelly, P.J.; O’Donoghue, G.M.; Bradley, P.J. The Nottingham System: Objective assessment of facial nerve function in the clinic. Otolaryngol. Head Neck Surg. 1994, 110, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Bajaj-Luthra, A.; Mueller, T.; Johnson, P.C. Quantitative analysis of facial motion components: Anatomic and nonanatomic motion in normal persons and in patients with complete facial paralysis. Plast. Reconstr. Surg. 1997, 99, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Schumann, N.P.; Bongers, K.; Guntinas-Lichius, O.; Scholle, H.C. Facial muscle activation patterns in healthy male humans: A multi-channel surface EMG study. J. Neurosci. Methods 2010, 187, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Vera-Garcia, F.J.; Moreside, J.M.; McGill, S.M. MVC techniques to normalize trunk muscle EMG in healthy women. J. Electromyogr. Kinesiol. 2010, 20, 10–16. [Google Scholar] [CrossRef]
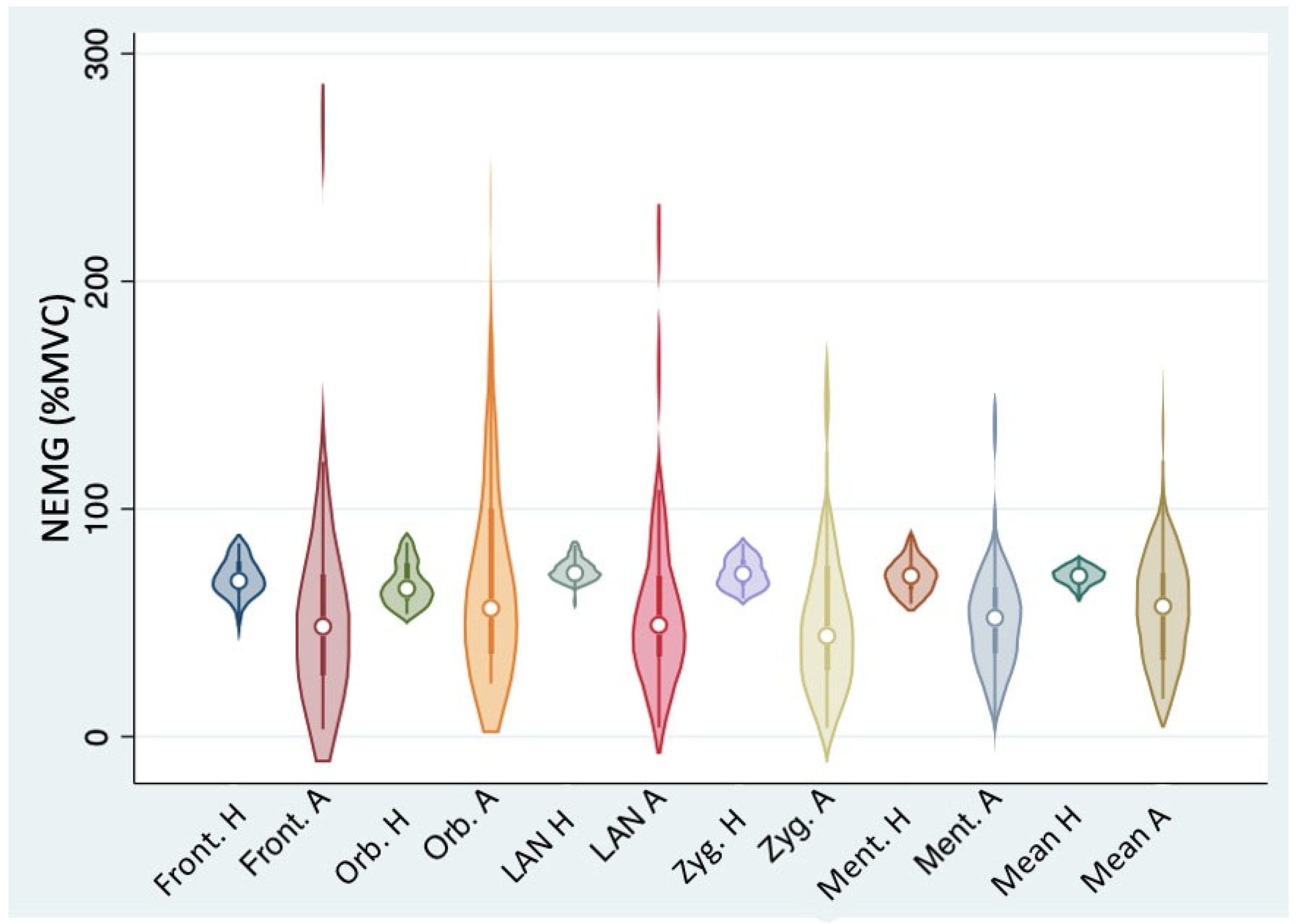
| Muscle | NEMG, Healthy Side (% of the MVC) Mean ± SD | NEMG, Affected Side (% of the MVC) Mean ± SD | Variance Ratio Test p-Value |
|---|---|---|---|
| Frontalis | 68.9 ± 9.3 | 55.2 ± 49.6 | <0.001 |
| Orbicularis oculi | 67.9 ± 10.1 | 73.5 ± 50.6 | <0.001 |
| Levator labii alaeque nasi | 72.7 ± 5.9 | 59.0 ± 43.2 | <0.001 |
| Zygomatic | 72.0 ± 7.0 | 58.5 ± 41.5 | <0.001 |
| Mentalis | 70.6 ± 7.6 | 55.1 ± 29.1 | <0.001 |
| Mean values | 70.5 ± 4.2 | 59.7 ± 28.3 | <0.001 |
| Muscle | Dynamic Sunnybrook Score | Synkinesis Sunnybrook Score | Overall Sunnybrook Score | ||||
|---|---|---|---|---|---|---|---|
| Spearman’s ρ | p-Value | Spearman’s ρ | p-Value | Spearman’s ρ | p-Value | ||
| NEMG | Frontalis | 0.7106 | <0.0001 | −0.1712 | 0.3658 | 0.6410 | 0.0001 |
| Orbicularis oculi | 0.4446 | 0.0260 | 0.0465 | 0.8254 | 0.3594 | 0.0776 | |
| Levator labii alaeque nasi | 0.6179 | 0.0003 | 0.0491 | 0.7966 | 0.5225 | 0.0031 | |
| Zygomatic | 0.5081 | 0.0041 | −0.1788 | 0.3445 | 0.4335 | 0.0167 | |
| Mentalis | 0.5919 | 0.0006 | −0.1830 | 0.3329 | 0.5263 | 0.0028 | |
| Mean values | 0.8220 | <0.0001 | −0.1232 | 0.5168 | 0.7231 | <0.0001 | |
| AI | Frontalis | −0.7565 | <0.0001 | 0.2091 | 0.2675 | −0.7073 | 0.0001 |
| Orbicularis oculi | −0.3944 | 0.0511 | −0.0724 | 0.7308 | −0.3290 | 0.1083 | |
| Levator labii alaeque nasi | −0.6331 | 0.0002 | −0.0079 | 0.9672 | −0.5424 | 0.0020 | |
| Zygomatic | −0.5586 | 0.0013 | 0.2129 | 0.2587 | −0.4845 | 0.0067 | |
| Mentalis | −0.6297 | 0.0002 | 0.1747 | 0.3557 | −0.5717 | 0.0010 | |
| Mean values | −0.8640 | <0.0001 | 0.1424 | 0.4527 | −0.7839 | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franz, L.; Marioni, G.; Daloiso, A.; Biancoli, E.; Tealdo, G.; Cazzador, D.; Nicolai, P.; de Filippis, C.; Zanoletti, E. Facial Surface Electromyography: A Novel Approach to Facial Nerve Functional Evaluation after Vestibular Schwannoma Surgery. J. Clin. Med. 2024, 13, 590. https://doi.org/10.3390/jcm13020590
Franz L, Marioni G, Daloiso A, Biancoli E, Tealdo G, Cazzador D, Nicolai P, de Filippis C, Zanoletti E. Facial Surface Electromyography: A Novel Approach to Facial Nerve Functional Evaluation after Vestibular Schwannoma Surgery. Journal of Clinical Medicine. 2024; 13(2):590. https://doi.org/10.3390/jcm13020590
Chicago/Turabian StyleFranz, Leonardo, Gino Marioni, Antonio Daloiso, Elia Biancoli, Giulia Tealdo, Diego Cazzador, Piero Nicolai, Cosimo de Filippis, and Elisabetta Zanoletti. 2024. "Facial Surface Electromyography: A Novel Approach to Facial Nerve Functional Evaluation after Vestibular Schwannoma Surgery" Journal of Clinical Medicine 13, no. 2: 590. https://doi.org/10.3390/jcm13020590
APA StyleFranz, L., Marioni, G., Daloiso, A., Biancoli, E., Tealdo, G., Cazzador, D., Nicolai, P., de Filippis, C., & Zanoletti, E. (2024). Facial Surface Electromyography: A Novel Approach to Facial Nerve Functional Evaluation after Vestibular Schwannoma Surgery. Journal of Clinical Medicine, 13(2), 590. https://doi.org/10.3390/jcm13020590










