Sutureless Repair for Open Treatment of Inguinal Hernia: Three Techniques in Comparison
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients’ Data
2.3. Outcomes
2.4. Surgical Techniques
2.5. Statistics
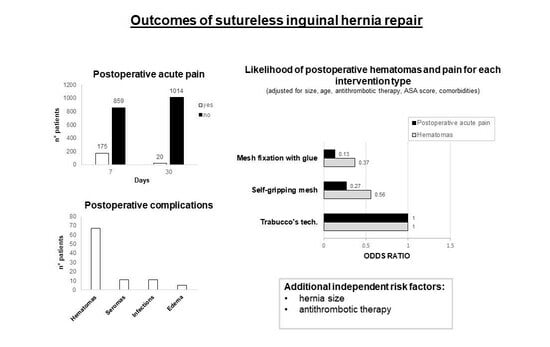
3. Results
3.1. Patients’ Description
3.2. Bivariate Analysis of Postoperative Complications
3.3. Bivariate Analysis of Postoperative Acute Pain and Time to Discharge
3.4. Regression Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campanelli, G.; Canziani, M.; Frattini, F.; Agrusti, S. Inguinal hernia: State of the art. Int. J. Surg. 2008, 6, S26–S28. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Amatucci, C.; Perotti, B.; Zullino, A.; Dezzi, C.; Illuminati, G.; Vietri, F. Outpatient repair for inguinal hernia in elderly patients: Still a challenge? Int. J. Surg. 2014, 12, S4–S7. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Massimi, F.; Lucchese, S.; Grimaldi, S.; Vernaccini, N.; Cirocchi, R.; Sorrenti, S.; Usai, S.; Intini, S.G. Open Surgery for Sportsman’s Hernia: A Retrospective Study. Front. Surg. 2022, 9, 893390. [Google Scholar] [CrossRef]
- Amid, P.K. Lichtenstein tension-free hernioplasty: Its inception, evolution, and principles. Hernia 2004, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alabi, A.; Haladu, N.; Scott, N.W.; Imamura, M.; Ahmed, I.; Ramsay, G.; Brazzelli, M. Mesh fixation techniques for inguinal hernia repair: An overview of systematic reviews of randomised controlled trials. Hernia 2022, 26, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Campanelli, G.; Bruni, P.G.; Cavalli, M.; Morlacchi, A. A Complete Sutureless, Hernia Repair for Primary Inguinal Hernia the Trabucco Repair: A Tribute to Ermanno Trabucco. Surg. Technol. Int. 2016, 28, 141–146. [Google Scholar] [PubMed]
- Colvin, H.S.; Rao, A.; Cavali, M.; Campanelli, G.; Amin, A.I. Glue Versus Suture Fixation of Mesh During Open Repair of Inguinal Hernias: A Systematic Review and Meta-analysis. World J. Surg. 2013, 37, 2282–2292. [Google Scholar] [CrossRef] [PubMed]
- de Goede, B.; Klitsie, P.J.; van Kempen, B.J.; Timmermans, L.; Jeekel, J.; Kazemier, G.; Lange, J.F. Meta-analysis of glue versus sutured mesh fixation for Lichtenstein inguinal hernia repair. Br. J. Surg. 2013, 100, 735–742. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, X.; Gu, Y.; Guo, S. A Meta-Analysis Examining the Use of Fibrin Glue Mesh Fixation versus Suture Mesh Fixation in Open Inguinal Hernia Repair. Dig. Surg. 2014, 31, 444–451. [Google Scholar] [CrossRef]
- Sun, P.; Cheng, X.; Deng, S.; Hu, Q.; Sun, Y.; Zheng, Q. Mesh fixation with glue versus suture for chronic pain and recurrence in Lichtenstein inguinal hernioplasty. Cochrane Database Syst. Rev. 2017, 2, CD010814. [Google Scholar] [CrossRef]
- Lin, H.; Zhuang, Z.; Ma, T.; Sun, X.; Huang, X.; Liet, Y. A meta-analysis of randomized control trials assessing mesh fixation with glue versus suture in Lichtenstein inguinal hernia repair. Medicine 2018, 97, e0227. [Google Scholar] [CrossRef]
- Zhang, C.; Li, F.; Zhang, H.; Zhong, W.; Shi, D.; Zhaoet, Y. Self-gripping versus sutured mesh for inguinal hernia repair: A systematic review and meta-analysis of current literature. J. Surg. Res. 2013, 185, 653–660. [Google Scholar] [CrossRef]
- Fang, Z.; Zhou, J.; Ren, F.; Liu, D. Self-gripping mesh versus sutured mesh in open inguinal hernia repair: System review and meta-analysis. Am. J. Surg. 2014, 207, 773–781. [Google Scholar] [CrossRef]
- Li, J.; Ji, Z.; Li, Y. The Comparison of Self-Gripping Mesh and Sutured Mesh in Open Inguinal Hernia Repair. Ann. Surg. 2014, 259, 1080–1085. [Google Scholar] [CrossRef]
- Pandanaboyana, S.; Mittapalli, D.; Rao, A.; Prasad, R.; Ahmad, N. Meta-analysis of self-gripping mesh (Progrip) versus sutured mesh in open inguinal hernia repair. Surgeon 2014, 12, 87–93. [Google Scholar] [CrossRef]
- Sajid, M.S.; Farag, S.; Singh, K.K.; Miles, W.F.A. Systematic review and meta-analysis of published randomized controlled trials comparing the role of self-gripping mesh against suture mesh fixation in patients undergoing open inguinal hernia repair. Surgeon 2014, 66, 189–196. [Google Scholar] [CrossRef]
- Ismail, A.; Abushouk, A.I.; Elmaraezy, A.; Abdelkarim, A.H.; Shehata, M.; Abozaid, M.; Ahmed, H.; Negida, A. Self-gripping versus sutured mesh fixation methods for open inguinal hernia repair: A systematic review of clinical trials and observational studies. Surgery 2017, 162, 18–36. [Google Scholar] [CrossRef]
- Molegraaf, M.; Kaufmann, R.; Lange, J. Comparison of self-gripping mesh and sutured mesh in open inguinal hernia repair: A meta-analysis of long-term results. Surgery 2018, 163, 351–360. [Google Scholar] [CrossRef]
- Ladwa, N.; Sajid, M.S.; Sains, P.; Baig, M.K. Suture mesh fixation versus glue mesh fixation in open inguinal hernia repair: A systematic review and meta-analysis. Int. J. Surg. 2013, 11, 128–135. [Google Scholar] [CrossRef]
- Techapongsatorn, S.; Tansawet, A.; Pattanaprateep, O.; Attia, J.; Mckay, G.J.; Thakkinstian, A. Mesh-fixation technique for inguinal hernia repair: Umbrella review. BJS Open 2022, 6, zrac084. [Google Scholar] [CrossRef]
- Negro, P.; Basile, F.; Brescia, A.; Buonanno, G.M.; Campanelli, G.; Canonico, S.; Cavalli, M.; Corrado, G.; Coscarella, G.; Di Lorenzo, N.; et al. Open tension-free Lichtenstein repair of inguinal hernia: Use of fibrin glue versus sutures for mesh fixation. Hernia 2011, 15, 7–14. [Google Scholar] [CrossRef]
- HerniaSurge Group. International guidelines for groin hernia management. Hernia 2018, 22, 1–165. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Miserez, M.; Alexandre, J.H.; Campanelli, G.; Corcione, F.; Cuccurullo, D.; Pascual, M.H.; Hoeferlin, A.; Kingsnorth, A.N.; Mandala, V.; Palot, J.P.; et al. The European hernia society groin hernia classication: Simple and easy to remember. Hernia 2007, 11, 113–116. [Google Scholar] [CrossRef]
- Koo, C.Y.; Hyder, J.A.; Wanderer, J.P.; Eikermann, M.; Ramachandranet, S.K. A Meta-analysis of the Predictive Accuracy of Postoperative Mortality Using the American Society of Anesthesiologists’ Physical Status Classification System. World J. Surg. 2015, 39, 88–103. [Google Scholar] [CrossRef]
- Douketis, J.D.; Spyropoulos, A.C.; Spencer, F.A.; Mayr, M.; Jaffer, A.K.; Eckman, M.H.; Dunn, A.S.; Kunz, R. Perioperative Management of Antithrombotic Therapy. Chest 2012, 141, e326S–e350S. [Google Scholar] [CrossRef]
- Coda, A.; Lamberti, R.; Martorana, S. Classification of prosthetics used in hernia repair based on weight and biomaterial. Hernia 2012, 16, 9–20. [Google Scholar] [CrossRef]
- Cirocchi, R.; Henry, B.M.; Mercurio, I.; Tomaszewski, K.A.; Palumbo, P.; Stabile, A.; Lancia, M.; Randolph, J. Is it possible to identify the inguinal nerves during hernioplasty? A systematic review of the literature and meta-analysis of cadaveric and surgical studies. Hernia 2019, 23, 569–581. [Google Scholar] [CrossRef]
- Wakasugi, M.; Tei, M.; Suzuki, Y.; Furukawa, K.; Masuzawa, T.; Kishi, K.; Tanemura, M.; Akamatsu, H. Single-incision totally extraperitoneal inguinal hernia repair is feasible and safe in patients on antithrombotic therapy: A single-center experience of 92 procedures. Asian J. Endosc. Surg. 2017, 10, 301–307. [Google Scholar] [CrossRef]
- Ong, W.; Shen, T.; Tan, W.B.; Lomanto, D. Is preoperative withdrawal of aspirin necessary in patients undergoing elective inguinal hernia repair? Surg. Endosc. 2016, 30, 5542–5549. [Google Scholar] [CrossRef]
- Mita, K.; Fujino, K.; Asakawa, H.; Matsuyama, T.; Hayashi, T.; Ito, H. Postoperative bleeding complications after endoscopic inguinal hernia repair in patients receiving anticoagulation agents, antiplatelet agents, or both. Asian J. Endosc. Surg. 2020, 13, 71–76. [Google Scholar] [CrossRef]
- Wakasugi, M.; Akamatsu, H.; Yoshidome, K.; Tori, M.; Ueshima, S.; Omori, T.; Tei, M.; Masuzawa, T.; Iwamoto, T.; Nishida, T. Totally extraperitoneal inguinal hernia repair in patients on antithrombotic therapy: A retrospective analysis. Surg. Today 2013, 43, 942–945. [Google Scholar] [CrossRef]
- Hada, G.; Zhang, S.; Song, Y.; Jaiswar, M.; Xie, Y.; Jian, F.; Lei, W. Safety of Inguinal Hernia Repair in the Elderly with Perioperative Continuation of Antithrombotic Therapy. Visc. Med. 2021, 37, 315–322. [Google Scholar] [CrossRef]
- Ho, C.-H.; Wu, C.-C.; Wu, C.-C.; Tsai, Y.-C. Laparoscopic total extraperitoneal inguinal hernia repair is safe and feasible in patients with continuation of antithrombotics. J. Minimal Access Surg. 2019, 15, 299–304. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Y.; Ruze, R.; Xiong, Y.; Han, H.; Zhan, H.; Wang, M.; Zhang, G. Continuation of low-dose acetylsalicylic acid during perioperative period of laparoscopic inguinal hernia repair is safe: Results of a prospective clinical trial. Hernia 2019, 23, 1141–1148. [Google Scholar] [CrossRef]
- Köckerling, F.; Adolf, D.; Lorenz, R.; Stechemesser, B.; Kuthe, A.; Conze, J.; Lammers, B.; Fortelny, R.; Mayer, F.; Zarras, K.; et al. Perioperative outcome in groin hernia repair: What are the most important influencing factors? Hernia 2022, 26, 201–215. [Google Scholar] [CrossRef]
- Poudel, S.; Miyazaki, K.; Hirano, S. Continuation of antithrombotic therapy increases minor bleeding but does not increase the risk other morbidities in open inguinal hernia repair: A propensity score-matched analysis. Hernia 2020, 24, 857–865. [Google Scholar] [CrossRef]
- Köckerling, F.; Roessing, C.; Adolf, D.; Schug-Pass, C.; Jacob, D. Has endoscopic (TEP, TAPP) or open inguinal hernia repair a higher risk of bleeding in patients with coagulopathy or antithrombotic therapy? Data from the Herniamed Registry. Surg. Endosc. 2016, 30, 2073–2081. [Google Scholar] [CrossRef]
- Alfieri, S.; Amid, P.K.; Campanelli, G.; Izard, G.; Kehlet, H.; Wijsmuller, A.R.; Di Miceli, D.; Doglietto, G.B. International guidelines for prevention and management of post-operative chronic pain following inguinal hernia surgery. Hernia 2011, 15, 239–249. [Google Scholar] [CrossRef]
- Nguyen, D.K.; Amid, P.K.; Chen, D.C. Groin Pain after Inguinal Hernia Repair. Adv. Surg. 2016, 50, 203–220. [Google Scholar] [CrossRef]
- Chen, D.; Bjurstrom, M.; Amid, P.; Nicol, A. Pain control following inguinal herniorrhaphy: Current perspectives. J. Pain Res. 2014, 7, 277–290. [Google Scholar] [CrossRef]
- Hoffmann, H.; Walther, D.; Bittner, R.; Köckerling, F.; Adolf, D.; Kirchhoff, P. Smaller Inguinal Hernias are Independent Risk Factors for Developing Chronic Postoperative Inguinal Pain (CPIP). Ann. Surg. 2020, 271, 756–764. [Google Scholar] [CrossRef]
- Niebuhr, H.; Wegner, F.; Hukauf, M.; Lechner, M.; Fortelny, R.; Bittner, R.; Schug-Pass, C.; Köckerling, F. What are the influencing factors for chronic pain following TAPP inguinal hernia repair: An analysis of 20,004 patients from the Herniamed Registry. Surg. Endosc. 2018, 32, 1971–1983. [Google Scholar] [CrossRef]
- Weyhe, D.; Tabriz, N.; Sahlmann, B.; Uslar, V.N. Risk factors for perioperative complications in inguinal hernia repair—A systematic review. Innov. Surg. Sci. 2017, 2, 47–52. [Google Scholar] [CrossRef]
| Sex | 962 (93.0%) males |
| 72 (7.0%) females | |
| Age range (years) | 18–92 |
| Mean ± SD | 60.4 ± 13.6 |
| Hernia location | 544 (52.6%) L |
| 312 (30.2%) M | |
| 157 (15.2%) LM | |
| 20 (2.0%) n.d. | |
| Hernia size | 104 (10.1%) (≤1 finger) |
| 469 (45.4%) (1–2 fingers) | |
| 327 (31.6%) (≥3 fingers) | |
| 134 (13.0%) n.d. | |
| Comorbidities | 351 (33.9%) cardiovascular diseases |
| 77 (7.4%) respiratory diseases | |
| 69 (6.7%) metabolic diseases | |
| 22 (2.1%) autoimmune diseases | |
| 19 (1.8%) infections | |
| N° comorbidities | 349 (33.8%) (n = 1) |
| 89 (8.6%) (n = 2) | |
| 7 (0.7%) (n = 3) | |
| Antithrombotic therapy | 872 (84.3%) none |
| 121 (11.7%) antiplatelet agents | |
| 41 (4.0%) anticoagulants | |
| ASA score | 880 (85.1%) (1–2) |
| 81 (7.8%) (3) | |
| 73 (6.9%) n.d. | |
| Intervention | 537 (51.9%) Trabucco |
| 312 (30.2%) ProGrip | |
| 185 (17.9%) Tisseel fixation | |
| Mesh weight | 497 (48.1%) Light |
| 537 (51.9%) Standard/Heavy | |
| Time until discharge | 883 (85.3%) (≤12 h) |
| 151 (14.6%) (>12 h) |
| Hematoma | p-Value | Scrotal Edema | p-Value | Seroma | p-Value | Infection | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Age (yrs) median (IQR) | <0.001 | 0.865 | 0.565 | 0.674 | ||||
| No | 61.0 (19.0) | 60.2 (18.0) | 61.0 (19.0) | 61.0 (19) | ||||
| Yes | 70.0 (15.0) | 62.2 (19.0) | 58.0 (17.0) | 68.0 (21.5) | ||||
| Gender | 0.007 | 0.303 | 0.55 | 0.45 | ||||
| F | 0 (0.0%) | 1 (1.4%) | 1 (1.4%) | 0 (0.0%) | ||||
| M | 67 (7.0%) | 4 (0.4%) | 10 (1.0%) | 11 (1.1%) | ||||
| Hernia location | 0.264 | 0.451 | 0.086 | 0.541 | ||||
| L | 37 (6.8%) | 2 (0.4%) | 10 (1.8%) | 5 (0.9%) | ||||
| M | 14 (8.9%) | 3 (1.0%) | 1 (0.6%) | 3 (1.0%) | ||||
| LM | 15 (4.8%) | 0 (0.0%) | 1 (0.6%) | 3 (1.9%) | ||||
| Hernia size | <0.001 | 0.424 | 0.085 | 0.494 | ||||
| 1 | 1 (1.0%) a | 1 (1.0%) | 2 (1.9%) | 1 (1.0%) | ||||
| 2 | 23 (4.9%) a | 3 (0.6%) | 2 (0.4%) | 2 (0.4%) | ||||
| 3 | 35 (10.7%) b | 1 (0.3%) | 6 (1.8%) | 3 (0.9%) | ||||
| CVD | <0.001 | 0.45 | 0.758 | 0.11 | ||||
| No | 30 (4.4%) | 4 (0.6%) | 8 (1.2%) | 10 (1.5%) | ||||
| Yes | 37 (10.5%) | 1 (0.3%) | 3 (0.9%) | 1 (0.3%) | ||||
| Respiratory diseases | 0.333 | 0.679 | 0.195 | 0.575 | ||||
| No | 60 (6.3%) | 5 (0.5%) | 9 (0.9%) | 10 (1.0%) | ||||
| Yes | 7 (9.1%) | 0 (0.0%) | 2 (2.6%) | 1 (1.3%) | ||||
| Metabolic diseases | 0.01 | 0.292 | 0.534 | 0.466 | ||||
| No | 57 (5.9%) | 4 (0.4%) | 10 (1.0%) | 11 (1.1%) | ||||
| Yes | 10 (14.5%) | 1 (1.4%) | 1 (1.4%) | 0 (0.0%) | ||||
| Autoimmune diseases | 0.049 | 0.898 | 0.788 | 0.788 | ||||
| No | 63 (6.2%) | 5 (0.5%) | 11 (1.1%) | 11 (1.1%) | ||||
| Yes | 4 (18.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||||
| Infectious diseases | 0.629 | 0.911 | 0.815 | 0.815 | ||||
| No | 67 (6.6%) | 5 (0.5%) | 11 (1.1%) | 11 (1.1%) | ||||
| Yes | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||||
| Comorbidities | <0.001 | 1 | 1 | 0.359 | ||||
| 0 | 23 (3.9%) a | 3 (0.5%) | 6 (1.0%) | 9 (1.5%) | ||||
| 1 | 30 (8.6%) b | 2 (0.6%) | 4 (1.1%) | 2 (0.6%) | ||||
| 2 | 12 (13.5%) b | 0 (0.0%) | 1 (1.1%) | 0 (0.0%) | ||||
| 3 | 2 (28.6%) b | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||||
| ASA score | <0.001 | 0.643 | 0.622 | 0.622 | ||||
| 1–2 | 48 (5.5%) | 5 (0.6%) | 10 (1.1%) | 10 (1.1%) | ||||
| 3 | 14 (17.3%) | 0 (0.0%) | 1 (1.2%) | 1 (1.2%) | ||||
| Therapy | <0.001 | 0.574 | 0.055 | 1 | ||||
| None | 37 (4.2%) a | 4 (0.5%) | 7 (0.8%) a | 10 (1.1%) | ||||
| Antiplatelets | 24 (19.8%) b | 1 (0.8%) | 4 (3.3%) b | 1 (0.8%) | ||||
| Anticoagulants | 6 (14.6%) b | 0 (0.0%) | 0 (0.0%) a,b | 0 (0.0%) | ||||
| Intervention | 0.066 | 0.12 | 0.126 | 0.004 | ||||
| ProGrip | 14 (4.5%) | 0 (0.0%) | 4 (1.3%) | 0 (0.0%) a | ||||
| Trabucco | 44 (8.2%) | 5 (0.9%) | 3 (0.6%) | 11 (2.0%) b | ||||
| Tisseel | 9 (4.9%) | 0 (0.0%) | 4 (2.2%) | 0 (0.0%) a,b | ||||
| Mesh | 0.02 | 0.037 | 0.131 | 0.001 | ||||
| Light | 23 (4.6%) | 0 (0.0%) | 8 (1.6%) | 0 (0.0%) | ||||
| Standard/Heavy | 44 (8.2%) | 5 (0.9%) | 3 (0.6%) | 11 (2.0%) |
| Acute Pain | p-Value | Chronic Pain | p-Value | Time until Discharge | p-Value | |
|---|---|---|---|---|---|---|
| >12 h | ||||||
| Age: median (IQR) | 0.669 | 0.831 | <0.001 | |||
| No | 61 (21.0) | 57.5 (24.7) | 59.4 (18.0) | |||
| Yes | 61 (19.0) | 61 (15.7) | 64.5 (19.5) | |||
| Gender | 0.631 | 0.545 | 0.863 | |||
| F | 14 (19.4%) | 0 (0.0%) | 11 (15.3%) | |||
| M | 167 (17.4%) | 9 (1.0%) | 140 (14.6%) | |||
| Hernia location | 0.441 | 0.193 | 0.456 | |||
| L | 106 (19.5%) | 5 (1.0%) | 89 (16.4%) | |||
| M | 48 (15.4%) | 1 (0.3%) | 43 (13.8%) | |||
| LM | 27 (17.2%) | 3 (1.9%) | 19 (12.1%) | |||
| Hernia size | <0.001 | 1 | 0.009 | |||
| 1 | 32 (30.8%) a | 1 (1.2%) | 10 (9.6%) a,b | |||
| 2 | 98 (20.9%) a | 5 (1.2%) | 60 (12.8%) b | |||
| 3 | 45 (13.8%) b | 3 (1.0%) | 64 (19.6%) a | |||
| Cardiovascular diseases | 0.3 | 0.644 | 0.033 | |||
| No | 126 (18.4%) | 6 (0.9%) | 88 (12.9%) | |||
| Yes | 55 (15.7%) | 3 (0.9%) | 63 (17.9%) | |||
| Respiratory diseases | 0.534 | 0.146 | 0.017 | |||
| No | 170 (17.8%) | 7 (0.8%) | 132 (13.8%) | |||
| Yes | 11 (14.3%) | 2 (2.7%) | 19 (24.7%) | |||
| Metabolic diseases | 0.412 | 0.545 | 0.292 | |||
| No | 172 (17.8%) | 9 (1.0%) | 138 (14.3%) | |||
| Yes | 9 (13.0%) | 0 (0.0%) | 13 (18.8%) | |||
| Autoimmune diseases | 0.783 | 0.818 | 0.549 | |||
| No | 178 (17.6%) | 9 (1.0%) | 147 (14.5%) | |||
| Yes | 3 (13.6%) | 0 (0.0%) | 4 (18.2%) | |||
| Infectious diseases | 0.759 | 0.15 | 0.179 | |||
| No | 177(17.4%) | 8 (0.9%) | 146 (14.4%) | |||
| Yes | 4 (21.1%) | 1 (5.9%) | 5 (26.3%) | |||
| Comorbidities | 0.343 | 0.452 | <0.001 | |||
| 0 | 111 (18.8%) | 4 (0.7%) | 66 (11.3%) a | |||
| 1 | 59 (16.9%) | 5 (1.6%) | 63 (18.1%) b | |||
| 2 | 11 (12.4%) | 0 (0.0%) | 18 (20.2%) a,b | |||
| 3 | 0 (0.0%) | 0 (0.0%) | 4 (57.1%) b | |||
| ASA score | 0.172 | 0.495 | 0.037 | |||
| 1–2 | 133 (15.1%) | 8 (1.0%) | 132 (15.0%) | |||
| 3 | 16 (19.8%) | 0 (0.0%) | 19 (23.5%) | |||
| Therapy | 0.904 | 0.122 | <0.001 | |||
| None | 155 (17.8%) | 6 (0.7%) | 97 (11.1%) a | |||
| Antiplatelets | 20 (16.5%) | 3 (2.8%) | 31 (25.6%) b | |||
| Anticoagulants | 6 (14.6%) | 0 (0.0%) | 23 (56.1%) c | |||
| Intervention | <0.001 | 0.162 | <0.001 | |||
| ProGrip | 31 (9.9%) a | 4 (1.4%) | 0 (0.0%) a | |||
| Tisseel | 10 (5.4%) a | 3 (1.7%) | 64 (34.6%) b | |||
| Trabucco | 140 (26.1%) b | 2 (0.4%) | 87 (16.2%) c | |||
| Mesh | <0.001 | 0.105 | 0.135 | |||
| Light | 41 (8.2%) | 7 (1.5%) | 64 (12.9%) | |||
| Standard/Heavy | 140 (26.1%) | 2 (0.4%) | 87 (16.2%) | |||
| Hematoma | <0.001 | 0.076 | 0.473 | |||
| No | 148 (15.3%) | 7 (0.8%) | 139 (14.4%) | |||
| Yes | 33 (49.3%) | 2 (4.0%) | 12 (17.9%) | |||
| Scrotal edema | <0.001 | 0.453 | ||||
| No | 176 (17.1%) | - | 151 (14.7%) | |||
| Yes | 5 (100%) | 0 (0.0%) | ||||
| Seroma | 0.109 | 0.073 | 0.668 | |||
| No | 177 (17.3%) | 8 (0.8%) | 149 (14.6%) | |||
| Yes | 4 (36.4%) | 1 (12.5%) | 2 (18.2%) | |||
| Infection | 0.7 | 0.901 | 0.506 | |||
| No | 180 (17.6%) | 9 (1.0%) | 150 (14.7%) | |||
| Yes | 1 (9.1%) | 1 (0.0%) | 1 (9.1%) |
| Logistic Regression Including Interventions | Logistic Regression Including Mesh Weight | |||
|---|---|---|---|---|
| Hematomas | Hematomas | |||
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Intervention | ||||
| Trabucco | 1 | |||
| ProGrip | 0.56 (0.27–1.14) | 0.109 | - | - |
| Tisseel | 0.37 (0.16–0.84) | 0.018 | ||
| Mesh | ||||
| Light | - | - | 1 | |
| Standard/Heavy | 2.14 (1.17–3.92) | 0.014 | ||
| Age | 1.02 (0.99–1.05) | 0.221 | 1.02 (0.99–1.05) | 0.252 |
| Cardiovascular diseases | ||||
| No | 1 | 1 | ||
| Yes | 1.28 (0.65–2.52) | 0.474 | 1.30 (0.66–2.55) | 0.451 |
| Autoimmune diseases | ||||
| No | 1 | |||
| Yes | 0.83 (0.10–6.99) | 0.866 | - | - |
| Hernia size | ||||
| 1 | 1 | 1 | ||
| 2 | 5.17 (0.67–39.72) | 0.115 | 4.97 (0.65–38.17) | 0.123 |
| 3 | 9.92 (1.30–75.85) | 0.027 | 9.51 (1.25–72.55) | 0.03 |
| Therapy | ||||
| None | 1 | 1 | ||
| Antiplatelets | 4.95 (2.45–10.00) | <0.001 | 4.69 (2.35-9.33) | <0.001 |
| Anticoagulants | 1.72 (0.50–5.92) | 0.39 | 1.63 (0.48-5.56) | 0.436 |
| ASA score | ||||
| 1–2 | 1 | 1 | ||
| 3 | 0.81 (0.37–2.17) | 0.807 | 0.95 (0.39–2.27) | 0.902 |
| Logistic Regression Including Interventions | Logistic Regression Including Mesh Weight | |||
|---|---|---|---|---|
| Postoperative Acute Pain | Postoperative Acute Pain | |||
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Intervention | ||||
| Trabucco | 1 | |||
| ProGrip | 0.27 (0.18–0.42) | <0.001 | - | - |
| Tisseel | 0.13 (0.07–0.27) | <0.001 | ||
| Mesh | ||||
| Light | - | - | 1 | |
| Standard/Heavy | 4.56 (3.07–6.76) | <0.001 | ||
| Hernia size | ||||
| 1 | 1 | 1 | ||
| 2 | 0.63 (0.38–1.03) | 0.066 | 0.60 (0.36–0.98) | 0.043 |
| 3 | 0.31 (0.17–0.55) | <0.001 | 0.29 (0.16–0.53) | <0.001 |
| Hematoma | ||||
| No | 1 | 1 | ||
| Yes | 7.55 (4.12–13.86) | <0.001 | 7.47 (4.08–13.61) | <0.001 |
| Time until Discharge > 12 h | ||
|---|---|---|
| Odds Ratio (95% CI) | p-Value | |
| Mesh | ||
| Light | 1 | |
| Standard/Heavy | 1.47 (0.98–2.22) | 0.065 |
| Age | 1.02 (1.00–1.04) | 0.014 |
| Hernia size | ||
| 1 | 0.48 (0.22–1.01) | 0.053 |
| 2 | 0.64 (0.43–0.97) | 0.035 |
| 3 | 1 | |
| Cardiovascular diseases | ||
| No | 1 | |
| Yes | 0.63 (0.40–1.00) | 0.053 |
| Respiratory diseases | ||
| No | 1 | |
| Yes | 1.49 (0.79–2.82) | 0.221 |
| Therapy | ||
| None | 1 | |
| Antiplatelets | 2.38 (1.38–4.10) | 0.002 |
| Anticoagulants | 8.53 (3.77–19.27) | <0.001 |
| ASA score | ||
| 1–2 | 1 | |
| 3 | 0.48 (0.22–1.05) | 0.065 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldini, E.; Lori, E.; Morini, C.; Palla, L.; Coletta, D.; De Luca, G.M.; Giraudo, G.; Intini, S.G.; Perotti, B.; Sorge, A.; et al. Sutureless Repair for Open Treatment of Inguinal Hernia: Three Techniques in Comparison. J. Clin. Med. 2024, 13, 589. https://doi.org/10.3390/jcm13020589
Baldini E, Lori E, Morini C, Palla L, Coletta D, De Luca GM, Giraudo G, Intini SG, Perotti B, Sorge A, et al. Sutureless Repair for Open Treatment of Inguinal Hernia: Three Techniques in Comparison. Journal of Clinical Medicine. 2024; 13(2):589. https://doi.org/10.3390/jcm13020589
Chicago/Turabian StyleBaldini, Enke, Eleonora Lori, Carola Morini, Luigi Palla, Diego Coletta, Giuseppe M. De Luca, Giorgio Giraudo, Sergio G. Intini, Bruno Perotti, Angelo Sorge, and et al. 2024. "Sutureless Repair for Open Treatment of Inguinal Hernia: Three Techniques in Comparison" Journal of Clinical Medicine 13, no. 2: 589. https://doi.org/10.3390/jcm13020589
APA StyleBaldini, E., Lori, E., Morini, C., Palla, L., Coletta, D., De Luca, G. M., Giraudo, G., Intini, S. G., Perotti, B., Sorge, A., Sozio, G., Arganini, M., Beltrami, E., Pironi, D., Ranalli, M., Saviano, C., Patriti, A., Usai, S., Vernaccini, N., ... Palumbo, P. (2024). Sutureless Repair for Open Treatment of Inguinal Hernia: Three Techniques in Comparison. Journal of Clinical Medicine, 13(2), 589. https://doi.org/10.3390/jcm13020589