Long-Term Atrioventricular Block Following Valve Surgery: Electrocardiographic and Surgical Predictors
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Clinical Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Pre-Operative Electrocardiographic Characteristics
3.3. Surgical Characteristics
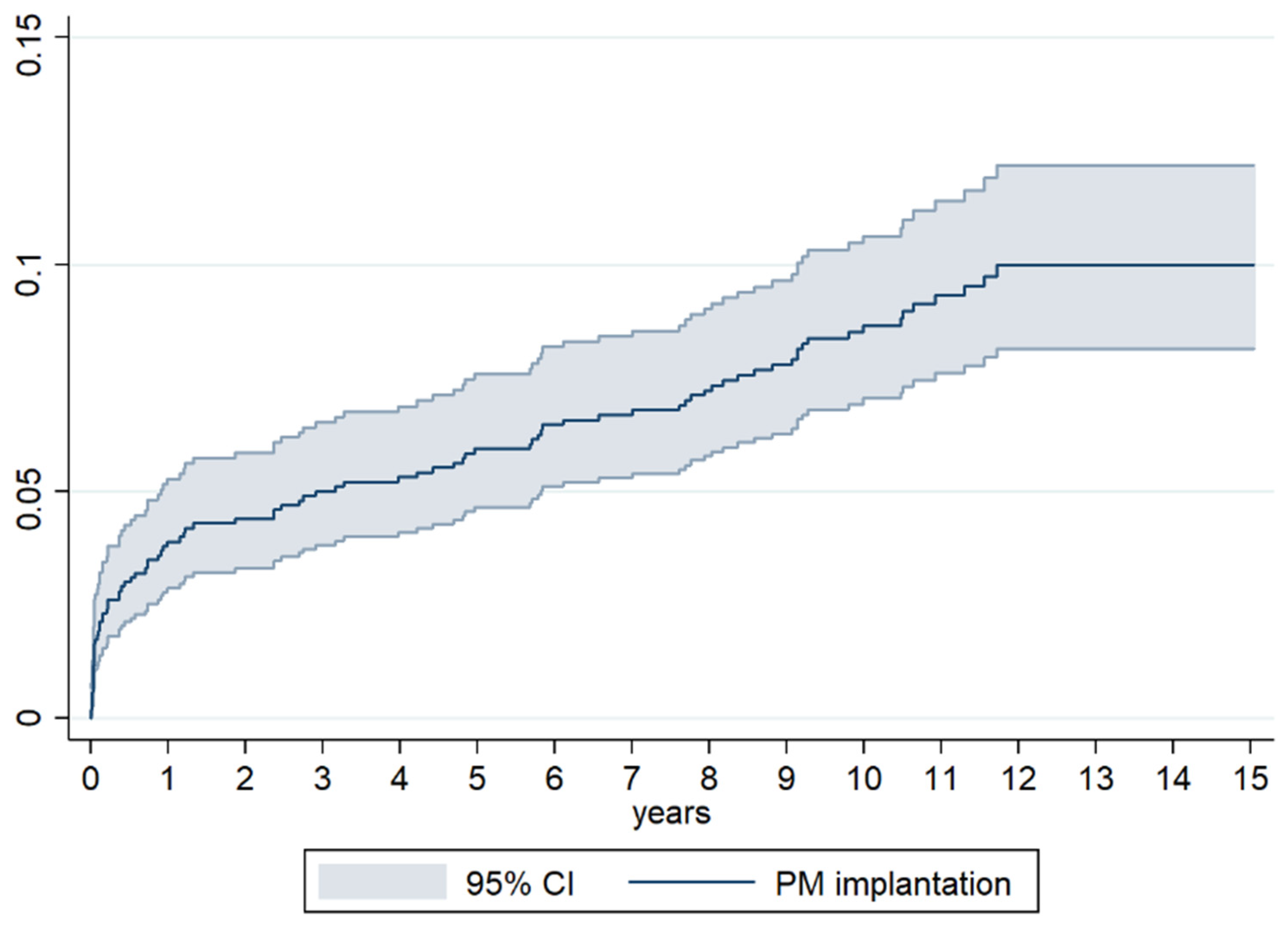
3.4. Incidence of PM Implantation
3.5. Predictors of PM Implantation at Univariate Analysis
3.6. Predictors of PM Implantation at Multivariate Analysis
3.7. Survival
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrari, A.D.L.; Süssenbach, C.P.; Guaragna, J.C.V.D.C.; Piccoli, J.D.C.E.; Gazzoni, G.F.; Ferreira, D.K.; Albuquerque, L.C.; Goldani, M.A. Bloqueio atrioventricular no pós-operatório de cirurgia cardíaca valvar: Incidência, fatores de risco e evolução hospitalar. Brazilian J. Cardiovasc. Surg. 2011, 26, 364–372. [Google Scholar]
- Berdajs, D.; Schurr, U.P.; Wagner, A.; Seifert, B.; Turina, M.I.; Genoni, M. Incidence and pathophysiology of atrioventricular block following mitral valve replacement and ring annuloplasty. Eur. J. Cardio-Thoracic Surg. 2008, 34, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Deeb, G.; Eagle, K.A.; Bruckman, D.; Pelosi, F.; Oral, H.; Sticherling, C.; Baker, R.L.; Chough, S.P.; Wasmer, K.; et al. Complete atrioventricular block after valvular heart surgery and the timing of pacemaker implantation. Am. J. Cardiol. 2001, 87, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Meimoun, P.; Zeghdi, R.; D’attelis, N.; Berrebi, A.; Braunberger, E.; Deloche, A.; Fabiani, J.N.; Carpentier, A. Frequency, predictors, and consequences of atrioventricular block after mitral valve repair. Am. J. Cardiol. 2002, 89, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Durante, A.; Limite, L.R.; Cianflone, D. Postoperative Arrhythmias after Cardiac Surgery: Incidence, Risk Factors, and Therapeutic Management. Cardiol. Res. Pr. 2014, 2014, 615987. [Google Scholar] [CrossRef]
- Leyva, F.; Qiu, T.; McNulty, D.; Evison, F.; Marshall, H.; Gasparini, M. Long-term requirement for pacemaker implantation after cardiac valve replacement surgery. Hear. Rhythm. 2016, 14, 529–534. [Google Scholar] [CrossRef]
- Viktorsson, S.A.; Orrason, A.W.; Vidisson, K.O.; Gunnarsdottir, A.G.; Johnsen, A.; Helgason, D.; Arnar, D.O.; Geirsson, A.; Gudbjartsson, T. Immediate and long-term need for permanent cardiac pacing following aortic valve replacement. Scand. Cardiovasc. J. 2019, 54, 186–191. [Google Scholar]
- Elahi, M.; Usmaan, K. The bioprosthesis type and size influence the postoperative incidence of permanent pacemaker implantation in patients undergoing aortic valve surgery. J. Interv. Card. Electrophysiol. 2006, 15, 113–118. [Google Scholar] [CrossRef]
- Raza, S.S.; Li, J.; John, R.; Chen, L.Y.; Tholakanahalli, V.N.; Mbai, M.; Adabag, A.S. Long-Term Mortality and Pacing Outcomes of Patients with Permanent Pacemaker Implantation after Cardiac Surgery. Pacing Clin. Electrophysiol. 2011, 34, 331–338. [Google Scholar] [CrossRef]
- Song, J.; Liang, Z.; Wang, Y.; Han, Z.; Ren, X. Incidence of permanent pacemaker implantation after valve replacement surgery. Herz 2020, 46, 109–114. [Google Scholar] [CrossRef]
- Huynh, H.; Dalloul, G.; Ghanbari, H.; Burke, P.; David, M.; Daccarett, M.; Machado, C.; David, S. Permanent Pacemaker Implantation Following Aortic Valve Replacement: Current Prevalence and Clinical Predictors. Pacing Clin. Electrophysiol. 2009, 32, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.W.; Webb, C.R.; Pickard, S.D.; Lehman, J.; Jacobsen, G. The increased need for a permanent pacemaker after reoperative cardiac surgery. J. Thorac. Cardiovasc. Surg. 1998, 116, 74–81. [Google Scholar]
- Gordon, R.S.; Ivanov, J.; Cohen, G.; Ralph-Edwards, A.L. Permanent cardiac pacing after a cardiac operation: Predicting the use of permanent pacemakers. Ann. Thorac. Surg. 1998, 66, 1698–1704. [Google Scholar] [CrossRef]
- DeRose, J.J.; Mancini, D.M.; Chang, H.L.; Argenziano, M.; Dagenais, F.; Ailawadi, G.; Perrault, L.P.; Parides, M.K.; Taddei-Peters, W.C.; Mack, M.J.; et al. Pacemaker Implantation After Mitral Valve Surgery With Atrial Fibrillation Ablation. J. Am. Coll. Cardiol. 2019, 73, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Koplan, B.A.; Stevenson, W.G.; Epstein, L.M.; Aranki, S.F.; Maisel, W.H. Development and validation of a simple risk score to predict the need for permanent pacing after cardiac valve surgery. J. Am. Coll. Cardiol. 2003, 41, 795–801. [Google Scholar] [CrossRef]
- Merin, O.; Ilan, M.; Oren, A.; Fink, D.; Deeb, M.; Bitran, D.; Silberman, S. Permanent Pacemaker Implantation Following Cardiac Surgery: Indications and Long-Term Follow-Up. Pacing Clin. Electrophysiol. 2008, 32, 7–12. [Google Scholar] [CrossRef]
- Gaillard, D.; Lespinasse, P.; Vanetti, A. Cardiac Pacing and Valvular Surgery. Pacing Clin. Electrophysiol. 1988, 11, 2142–2148. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Silverman, B.G.; Gross, T.P.; Kaczmarek, R.G.; Hamilton, P.; Hamburger, S. The epidemiology of pacemaker implantation in the United States. Public Health Rep. 1995, 110, 42–46. [Google Scholar]
- Bradshaw, P.J.; Stobie, P.; Knuiman, M.W.; Briffa, T.G.; Hobbs, M.S.T. Trends in the incidence and prevalence of cardiac pacemaker insertions in an ageing population. Open Hear. 2014, 1, e000177. [Google Scholar] [CrossRef]
- Nazif, T.M.; Dizon, J.M.; Hahn, R.T.; Xu, K.; Babaliaros, V.; Douglas, P.S.; El-Chami, M.F.; Herrmann, H.C.; Mack, M.; Makkar, R.R.; et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: The PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc. Interv. 2015, 8, 60–69. [Google Scholar] [PubMed]
- Tarakji, K.G.; Wazni, O.M.; Harb, S.; Hsu, A.; Saliba, W.; Wilkoff, B.L. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: The impact of the infection type and the presence of vegetation on survival. Europace 2014, 16, 1490–1495. [Google Scholar] [CrossRef]
- Lurz, P.; Besler, C.; Schmitz, T.; Bekeredjian, R.; Nickenig, G.; Möllmann, H.; von Bardeleben, R.S.; Schmeisser, A.; Atmowihardjo, I.; Estevez-Loureiro, R.; et al. bRIGHT PAS Principal Investigators. Short-Term Outcomes of Tricuspid Edge-to-Edge Repair in Clinical Practice. J. Am. Coll. Cardiol. 2023, 82, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Weber, M.; Lurz, P.; von Bardeleben, R.S.; Sitges, M.; Sorajja, P.; Hausleiter, J.; Denti, P.; Trochu, J.N.; Näbauer, M.; et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet 2019, 394, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Abraham, W.T.; Lindenfeld, J.; Kar, S.; Grayburn, P.A.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Rinaldi, M.; Kapadia, S.R.; et al. COAPT Investigators. Five-Year Follow-up after Transcatheter Repair of Secondary Mitral Regurgitation. N. Engl. J. Med. 2023, 388, 2037–2048. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. ESC Scientific Document Group. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Biffi, M.; de Zan, G.; Massaro, G.; Angeletti, A.; Martignani, C.; Boriani, G.; Diemberger, I.; Ziacchi, M. Is ventricular sensing always right, when it is left? Clin. Cardiol. 2018, 41, 1238–1245. [Google Scholar] [CrossRef]
- Noheria, A.; van Zyl, M.; Scott, L.R.; Srivathsan, K.; Madhavan, M.; Asirvatham, S.J.; McLeod, C.J. Single-site ventricular pacing via the coronary sinus in patients with tricuspid valve disease. Europace 2018, 20, 636–642. [Google Scholar]
| Variables | Overall (n = 1046) 1 | PM− (n = 955) 1 | PM+ (n = 91) 1 | p-Value 2 |
|---|---|---|---|---|
| Clinical | ||||
| Age, years | 63 (53–72) | 62 (52–71) | 69 (60–74) | <0.001 |
| Sex male | 646/1046 (61.8) | 596/955 (62.4) | 54/91 (55.0) | 0.162 |
| Hypertension | 666/1046 (63.7) | 606/955 (63.5) | 60/91 (65.9) | 0.639 |
| Dyslipidaemia | 346/1046 (33.1) | 312/955 (32.7) | 34/91 (37.4) | 0.363 |
| Diabetes | 921/1045 (11.9) | 108/954 (11.3) | 16/91 (17.6) | 0.078 |
| Smoke | 323/1046 (30.9) | 288/955 (30.2) | 35/91 (38.5) | 0.101 |
| COPD | 82/1046 (7.8) | 70/955 (7.3) | 12/91 (13.2) | 0.041 |
| Creatinine, mg/dL | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) | 1.1 (0.9–1.4) | 0.013 |
| GFR, mL/min/1.73 m2 | 70 (53–89) | 71 (54–90) | 61 (44–80) | <0.001 |
| Echocardiography | ||||
| EF, % | 61 (55–65) | 61 (55–66) | 60 (55–65) | 0.509 |
| EDV, mL | 121 (87–156) | 120 (87–155) | 141 (97–166) | 0.161 |
| ESV, mL | 44 (29–70) | 44 (29–67) | 50 (32–74) | 0.194 |
| Electrocardiography | ||||
| AF/Flutter | 253/1045 (24.2) | 222/954 (23.3) | 31/91 (34.1) | 0.022 |
| PR duration, ms | 170 (160–180) | 170 (160–180) | 180 (168–205) | <0.001 |
| QRS duration, ms | 100 (90–110) | 100 (90–110) | 110 (90–130) | <0.001 |
| PR > 200 ms | 86/793 (10.8) | 70/733 (9.6) | 16/60 (26.7) | <0.001 |
| QRS morphology | ||||
| Normal | 835/1046 (79.8) | 794/955 (83.1) | 41/91 (45.1) | <0.001 |
| RBBB | 61/1046 (5.8) | 41/955 (4.3) | 20/91 (22.0) | <0.001 |
| LBBB | 44/1046 (4.2) | 34/955 (3.6) | 10/91 (11.0) | 0.006 |
| LAFB | 42/1046 (4.0) | 34/955 (3.6) | 8/91 (8.8) | 0.121 |
| RBBB and LAFB | 13/1046 (1.2) | 8/955 (0.8) | 5/91 (5.5) | 0.001 |
| Non-specific delay | 32/1046 (3.1) | 27/955 (2.8) | 5/91 (5.5) | 0.158 |
| Incomplete RBBB | 14/1046 (1.3) | 13/955 (1.4) | 1/91 (1.1) | 0.835 |
| Incomplete LBBB | 4/1046 (0.5) | 4/955 (0.4) | 1/91 (1.1) | 0.369 |
| Variables | Overall (n = 1046) 1 | PM− (n = 955) 1 | PM+ (n = 91) 1 | p-Value 2 |
|---|---|---|---|---|
| Indication to surgery | 0.637 | |||
| Valve disease | 822/1046 (78.6) | 746/955 (78.1) | 76/91 (83.5) | |
| AA + Valve disease | 154/1046 (14.7) | 144/955 (15.1) | 10/91 (11.0) | |
| Endocarditis | 34/1046 (3.2) | 31/955 (3.2) | 3/91 (3.3) | |
| Aortic Dissection | 36/1046 (3.4) | 34/955 (3.5) | 2/91 (2.2) | |
| Number of valves treated | ||||
| 1 | 759/1046 (72.6) | 707/955 (74.0) | 52/91 (57.1) | 0.002 |
| 2 | 234/1046 (22.4) | 206/955 (21.6) | 28/91 (30.8) | 0.133 |
| 3 | 53/1046 (5.1) | 42/955 (4.4) | 11/91 (12.1) | 0.001 |
| Valves treated | ||||
| Aortic 3 | 536/1046 (51.2) | 504/955 (52.8) | 32/91 (35.2) | 0.007 |
| Mitral | 228/1046 (21.8) | 207/955 (21.7) | 21/91 (23.1) | 0.757 |
| Aortic and Mitral | 151/1046 (14.4) | 137/955 (14.4) | 14/91 (15.4) | 0.788 |
| Mitral and Tricuspid | 77/1046 (7.4) | 64/955 (6.7) | 13/91 (14.3) | 0.041 |
| Aortic, Mitral, and Tricuspid | 54/1046 (5.2) | 43/955 (4.5) | 11/91 (12.1) | 0.009 |
| Prosthesis | 0.052 | |||
| Mechanical | 726/1046 (69.4) | 671/955 (70.3) | 55/91(60.4) | |
| Biological | 320/1046 (30.6) | 284/955 (29.7) | 37/91 (39.6) | |
| CABG | 126/1046 (12.1) | 111/955 (11.7) | 15/91 (15.4) | 0.306 |
| Maze | 125/1046 (12.0) | 110/955 (11.5) | 15/91 (16.5) | 0.163 |
| Other | 322/1046 (30.8) | 303/955 (31.7) | 19/91 (20.9) | 0.032 |
| Type of Mitral valve intervention 4 | 0.552 | |||
| Mitral replacement | 506/511 (99.0) | 448/452 (99.1) | 58/59 (98.3) | |
| Mitral repair | 5/511 (1.0) | 4/452 (0.9) | 1/59 (1.7) | |
| Type of Tricuspid valve intervention 5 | 0.498 | |||
| Tricuspid repair | 92/131 (70.3) | 76/106 (71.6) | 16/25 (64.0) | |
| Tricuspid annuloplasty | 39/131 (29.7) | 30/106 (28.4) | 9/25 (36.0) | |
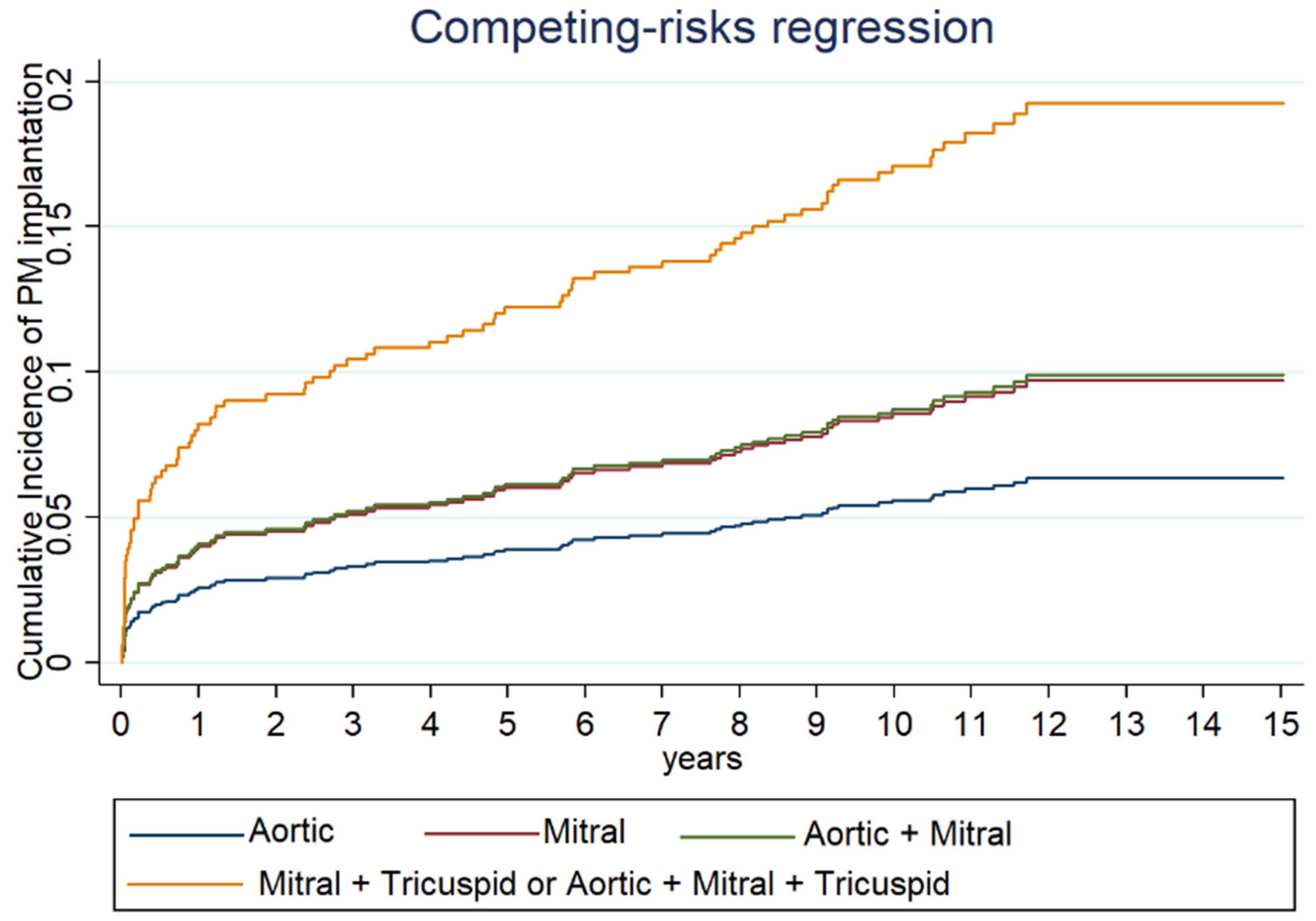
| Valve Surgery | PM Implantation at 1 Month | PM Implantation at 1 Year | PM Implantation at 5 Years | PM Implantation at 10 Years |
|---|---|---|---|---|
| Aortic | 4/530 = 0.8% | 12/523 = 2.3% | 20/499 = 4.0% | 31/382 = 8.1% |
| Mitral | 7/224 = 3.1% | 10/214 = 4.7% | 15/209 = 7.2% | 18/164 = 11.0% |
| Aortic + Mitral | 4/147 = 2.7% | 7/146 = 4.8% | 10/134 = 7.5% | 12/100 = 12.0% |
| Mitral + Tricuspid | 1/75 = 1.3% | 6/71 = 8.5% | 8/65 = 12.3% | 12/52 = 23.1% |
| Aortic + Mitral + Tricuspid | 3/54 = 5.6% | 5/53 = 9.4% | 7/52 = 13.5% | 11/37 = 29.7% |
| Overall | 19/1030 = 1.8% | 40/1007 = 4.0% | 60/959 = 5.6% | 84/735 = 11.4% |
| Variables | Sub-Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age-continuous one-year increase Age-category | 1.03 | 1.01–1.05 | <0.001 |
| <54 years | reference | - | - |
| 54–63 years | 1.5 | 0.7–3.2 | 0.328 |
| 64–72 years | 3.3 | 1.7–6.5 | 0.001 |
| >72 year | 3.1 | 1.6–6.3 | 0.001 |
| QRS morphology | |||
| Normal | reference | - | - |
| RBBB | 7.8 | 4.6–13.3 | <0.001 |
| LBBB | 5.1 | 2.6–10.3 | <0.001 |
| LAFB | 4.3 | 2.0–9.0 | <0.001 |
| RBBB and LAFB | 10.1 | 3.9–26.2 | <0.001 |
| Non-specific delay | 3.4 | 1.4–8.5 | 0.009 |
| Incomplete RBBB | 1.5 | 0.2–11.4 | 0.671 |
| Incomplete LBBB | 4.0 | 0.6–25.1 | 0.136 |
| PR > 200 ms | 3.2 | 1.8–5.7 | <0.001 |
| AF/Flutter | 1.8 | 1.1–2.7 | 0.011 |
| COPD | 1.9 | 1.01–3.4 | 0.047 |
| Echocardiography | |||
| EF, % | 1.0 | 0.97–1.01 | 0.599 |
| EDV, mL | 1.0 | 0.99–1.01 | 0.265 |
| EDS, mL | 1.0 | 0.99–1.01 | 0.316 |
| Number of valve treated | |||
| 1 | reference | - | - |
| 2 | 1.8 | 1.1–2.9 | 0.012 |
| 3 | 3.3 | 1.7–6.3 | <0.001 |
| Type of valve surgery | |||
| Aortic | reference | - | - |
| Mitral | 1.6 | 0.9–2.7 | 0.114 |
| Aortic+Mitral | 1.6 | 0.8–3.0 | 0.150 |
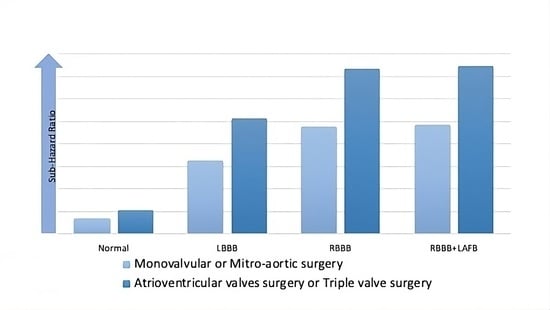
| Aortic + Mitral + Tricuspid or Mitral + Tricuspid | 3.3 | 1.9–5.5 | <0.001 |
| Interventions involving mitral valve vs. other interventions | 2.0 | 1.3–3.1 | 0.001 |
| Interventions involving tricuspid valve vs. other interventions | 2.6 | 1.7–4.2 | <0.001 |
| Variables | Sub-Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age-continuous one-year increase | 1.02 | 1.01–1.03 | 0.045 |
| QRS morphology | |||
| Normal | reference | - | - |
| RBBB | 7.0 | 3.9–12.4 | <0.001 |
| LBBB | 4.9 | 2.4–10.1 | <0.001 |
| LAFB | 3.9 | 1.8–8.3 | 0.001 |
| RBBB and LAFB | 7.1 | 2.5–19.8 | <0.001 |
| Non-specific delay | 3.4 | 1.3–8.8 | 0.010 |
| Incomplete RBBB | 1.2 | 0.2–8.5 | 0.890 |
| Incomplete LBBB | 2.7 | 0.5–13.8 | 0.245 |
| Type of valve surgery | |||
| Aortic | reference | - | - |
| Mitral | 1.2 | 0.7–2.2 | 0.522 |
| Aortic + Mitral | 1.5 | 0.8–2.8 | 0.217 |
| Aortic + Mitral + Tricuspid or Mitral + Tricuspid | 2.1 | 1.2–3.8 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, J.; Biffi, M.; Folesani, G.; Di Marco, L.; Martin, S.; Zenesini, C.; Savini, C.; Ziacchi, M.; Diemberger, I.; Martignani, C.; et al. Long-Term Atrioventricular Block Following Valve Surgery: Electrocardiographic and Surgical Predictors. J. Clin. Med. 2024, 13, 538. https://doi.org/10.3390/jcm13020538
Farina J, Biffi M, Folesani G, Di Marco L, Martin S, Zenesini C, Savini C, Ziacchi M, Diemberger I, Martignani C, et al. Long-Term Atrioventricular Block Following Valve Surgery: Electrocardiographic and Surgical Predictors. Journal of Clinical Medicine. 2024; 13(2):538. https://doi.org/10.3390/jcm13020538
Chicago/Turabian StyleFarina, Jacopo, Mauro Biffi, Gianluca Folesani, Luca Di Marco, Sofia Martin, Corrado Zenesini, Carlo Savini, Matteo Ziacchi, Igor Diemberger, Cristian Martignani, and et al. 2024. "Long-Term Atrioventricular Block Following Valve Surgery: Electrocardiographic and Surgical Predictors" Journal of Clinical Medicine 13, no. 2: 538. https://doi.org/10.3390/jcm13020538
APA StyleFarina, J., Biffi, M., Folesani, G., Di Marco, L., Martin, S., Zenesini, C., Savini, C., Ziacchi, M., Diemberger, I., Martignani, C., & Pacini, D. (2024). Long-Term Atrioventricular Block Following Valve Surgery: Electrocardiographic and Surgical Predictors. Journal of Clinical Medicine, 13(2), 538. https://doi.org/10.3390/jcm13020538