Cardiovascular Effects of Cosmic Radiation and Microgravity
Abstract
1. Introduction
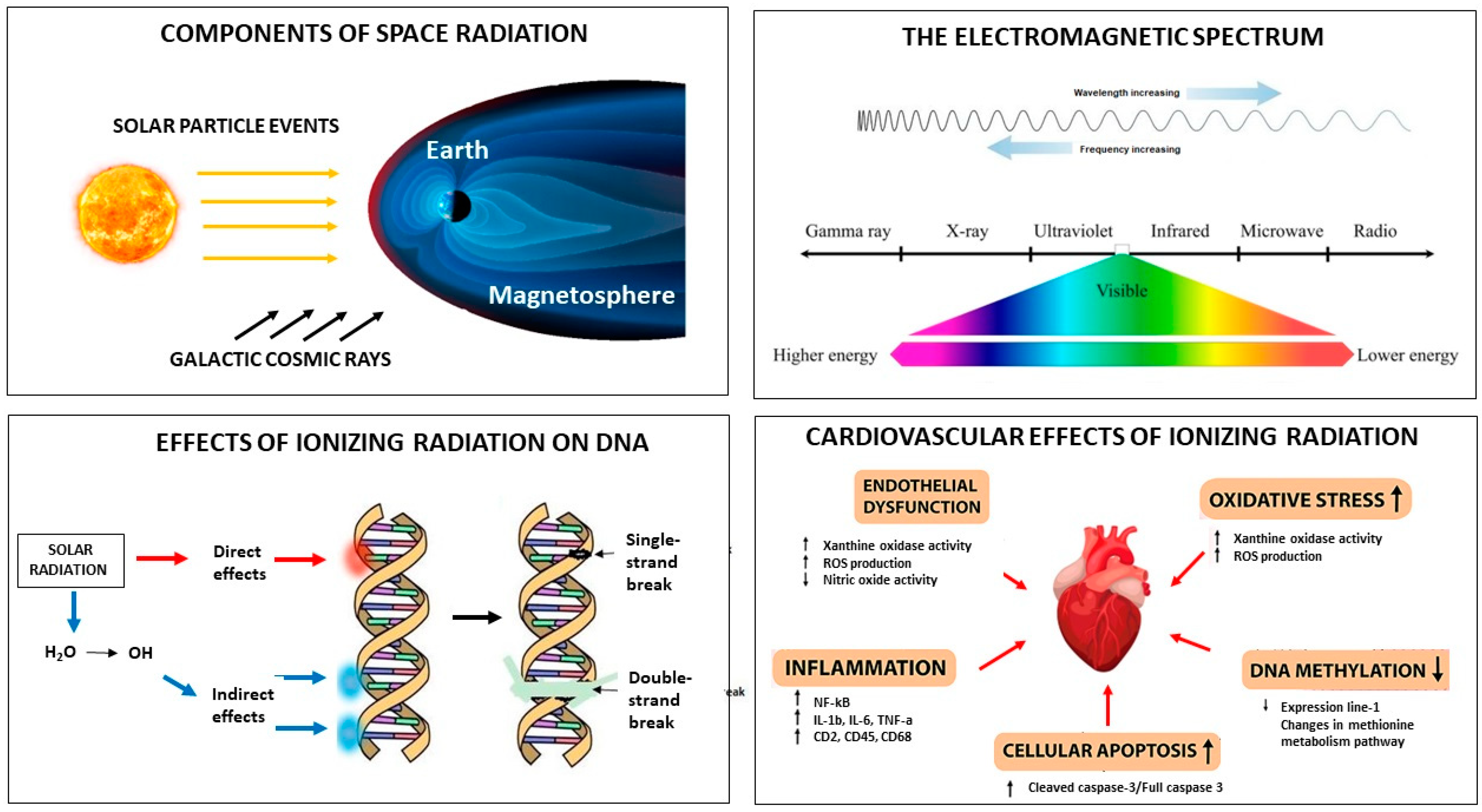
2. Cosmic Radiation
3. Microgravity
4. Therapeutic Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, S. The effects of microgravity and space radiation on cardiovascular health: From low-Earth orbit and beyond. Int. J. Cardiol. Heart Vasc. 2020, 30, 100595. [Google Scholar] [CrossRef] [PubMed]
- Dobney, W.; Mols, L.; Mistry, D.; Tabury, K.; Baselet, B.; Baatout, S. Evaluation of deep space exploration risks and mitigations against radiation and microgravity. Front. Nucl. Med. 2023, 3, 1225034. [Google Scholar] [CrossRef]
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Böttcher, S.; Böhm, E.; et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.M.; Allen, A.R.; Bowles, D.E. Consequences of space radiation on the brain and cardiovascular system. J. Environ. Sci. Health Part C Toxicol. Carcinog. 2021, 39, 180–218. [Google Scholar] [CrossRef] [PubMed]
- Meerman, M.; Bracco Gartner, T.C.L.; Buikema, J.W.; Wu, S.M.; Siddiqi, S.; Bouten, C.V.C.; Grande-Allen, K.J.; Suyker, W.J.L.; Hjortnaes, J. Myocardial disease and long-distance space travel: Solving the radiation problem. Front. Cardiovasc. Med. 2021, 8, 631985. [Google Scholar] [CrossRef] [PubMed]
- Delp, M.D.; Charvat, J.M.; Limoli, C.L.; Globus, R.K.; Ghosh, P. Apollo Lunar astronauts show higher cardiovascular disease mortality: Possible deep space radiation effects on the vascular endothelium. Sci. Rep. 2016, 6, 29901. [Google Scholar] [CrossRef] [PubMed]
- Hughson, R.L.; Helm, A.; Durante, M. Heart in space: Effect of the extraterrestrial environment on the cardiovascular system. Nat. Rev. Cardiol. 2018, 15, 167–180. [Google Scholar] [CrossRef]
- Garikipati, V.N.S.; Arakelyan, A.; Blakely, E.A.; Chang, P.Y.; Truongcao, M.M.; Cimini, M.; Malaredy, V.; Bajpai, A.; Addya, S.; Bisserier, M.; et al. Long-term effects of very low dose particle radiation on gene expression in the heart: Degenerative disease risks. Cells 2021, 10, 387. [Google Scholar] [CrossRef]
- Mitchell, A.; Pimenta, D.; Gill, J.; Ahmad, H.; Bogle, R. Cardiovascular effects of space radiation: Implications for future human deep space exploration. Eur. J. Prev. Cardiol. 2019, 26, 1707–1714. [Google Scholar] [CrossRef]
- Giacinto, O.; Garo, M.L.; Pelliccia, F.; Minati, A.; Chello, M.; Lusini, M. Heart Disease and Microgravity: The Dawn of a New Medical Era? A Narrative Review. Cardiol. Rev. 2023. [Google Scholar] [CrossRef]
- Mednieks, M.I.; Fine, A.S.; Oyama, J.; Philpott, D.E. Cardiac muscle ultrastructure and cyclic AMP reactions to altered gravity conditions. Am. J. Physiol. 1987, 252 Pt 2, R227–R232. [Google Scholar] [CrossRef] [PubMed]
- Philpott, D.E.; Popova, I.A.; Kato, K.; Stevenson, J.; Miquel, J.; Sapp, W. Morphological and biochemical examination of Cosmos 1887 rat heart tissue: Part I—Ultrastructure. FASEB J. 1990, 4, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Fareh, J.; Bayard, B.; Gabrion, J.; Thibault, G.; Oliver, J.; Bouille, C.; Gauquelin, G.; Gharib, C. Cardiac and plasma atrial natriuretic peptide after 9-day hindlimb suspension in rats. J. Appl. Physiol. 1994, 76, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Connor, M.K.; Hood, D.A. Effect of microgravity on the expression of mitochondrial enzymes in rat cardiac and skeletal muscles. J. Appl. Physiol. 1998, 84, 593–598. [Google Scholar] [CrossRef]
- Lwigale, P.Y.; Thurmond, J.E.; Norton, W.N.; Spooner, B.S.; Wiens, D.J. Simulated microgravity and hypergravity attenuate heart tissue development in explant culture. Cells Tissues Organs 2000, 167, 171–183. [Google Scholar] [CrossRef]
- Yu, Z.B.; Zhang, L.F.; Jin, J.P. A proteolytic NH2-terminal truncation of cardiac troponin I that is up-regulated in simulated microgravity. J. Biol. Chem. 2001, 276, 15753–15760. [Google Scholar] [CrossRef]
- Okumura, S.; Tsunematsu, T.; Bai, Y.; Jiao, Q.; Ono, S.; Suzuki, S.; Kurotani, R.; Sato, M.; Minamisawa, S.; Umemura, S.; et al. Type 5 adenylyl cyclase plays a major role in stabilizing heart rate in response to microgravity induced by parabolic flight. J. Appl. Physiol. 2008, 105, 173–179. [Google Scholar] [CrossRef]
- Yin, W.; Liu, J.C.; Fan, R.; Sun, X.Q.; Ma, J.; Feng, N.; Zhang, Q.Y.; Yin, Z.; Zhang, S.M.; Guo, H.T.; et al. Modulation of β-adrenoceptor signaling in the hearts of 4-wk simulated weightlessness rats. J. Appl. Physiol. 2008, 105, 569–574. [Google Scholar] [CrossRef]
- Kwon, O.; Tranter, M.; Jones, W.K.; Sankovic, J.M.; Banerjee, R.K. Differential translocation of nuclear factor-kappaB in a cardiac muscle cell line under gravitational changes. J. Biomech. Eng. 2009, 131, 064503. [Google Scholar] [CrossRef]
- Ito, K.; Kagaya, Y.; Shimokawa, H. Thyroid hormone and chronically unloaded hearts. Vasc. Pharmacol. 2010, 52, 138–141. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, S.-M.; Zhang, Q.-Y.; Fan, R.; Li, J.; Guo, H.-T.; Bi, H.; Wang, Y.-M.; Hu, Y.-Z.; Zheng, Q.-J.; et al. Modulation of intracellular calcium transient in response to beta-adrenoceptor stimulation in the hearts of 4-wk-old rats during simulated weightlessness. J. Appl. Physiol. 2010, 108, 838–844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, H.; Zhang, L.; Xu, P.-T.; Li, Q.; Sheng, J.-J.; Wang, Y.-Y.; Chen, Y.; Zhang, L.-N.; Yu, Z.-B. Nuclear translocation of calpain-2 regulates propensity toward apoptosis in cardiomyocytes of tail-suspended rats. J. Cell Biochem. 2011, 112, 571–580. [Google Scholar] [CrossRef]
- Vikhlyantsev, I.M.; Okuneva, A.D.; Shpagina, M.D.; Shumilina, Y.V.; Molochkov, N.V.; Salmov, N.N.; Podlubnaya, Z.A. Changes in isoform composition, structure, and functional properties of titin from Mongolian gerbil (Meriones unguiculatus) cardiac muscle after space flight. Biochemistry (Moscow) 2011, 76, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Ogneva, I.V.; Mirzoev, T.M.; Biryukov, N.S.; Veselova, O.M.; Larina, I.M. Structure and functional characteristics of rat’s left ventricle cardiomyocytes under antiorthostatic suspension of various duration and subsequent reloading. J. Biomed. Biotechnol. 2012, 2012, 659869. [Google Scholar] [CrossRef]
- Schwoerer, A.P.; Neef, S.; Broichhausen, I.; Jacubeit, J.; Tiburcy, M.; Wagner, M.; Biermann, D.; Didié, M.; Vettel, C.; Maier, L.S.; et al. Enhanced Ca2+ influx through cardiac L-type Ca2+ channels maintains the systolic Ca2+ transient in early cardiac atrophy induced by mechanical unloading. Pflügers Arch. 2013, 465, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Respress, J.L.; Gershovich, P.M.; Wang, T.; Reynolds, J.O.; Skapura, D.G.; Sutton, J.P.; Miyake, C.Y.; Wehrens, X.H. Long-term simulated microgravity causes cardiac RyR2 phosphorylation and arrhythmias in mice. Int. J. Cardiol. 2014, 176, 994–1000. [Google Scholar] [CrossRef]
- Bederman, I.R.; Lai, N.; Shuster, J.; Henderson, L.; Ewart, S.; Cabrera, M.E. Chronic hindlimb suspension unloading markedly decreases turnover rates of skeletal and cardiac muscle proteins and adipose tissue triglycerides. J. Appl. Physiol. 2015, 119, 16–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, H.; Zhu, H.; Zhang, F.; Dong, X.; Hao, T.; Jiang, X.; Zheng, W.; Zhang, T.; Chen, X.; Wang, P.; et al. Spaceflight Promoted Myocardial Differentiation of Induced Pluripotent Stem Cells: Results from Tianzhou-1 Space Mission. Stem Cells Dev. 2019, 28, 357–360. [Google Scholar] [CrossRef]
- Liang, L.; Yuan, W.; Qu, L.; Li, H.; Zhang, L.; Fan, G.-C.; Peng, T. Administration of losartan preserves cardiomyocyte size and prevents myocardial dysfunction in tail-suspended mice by inhibiting p47phox phosphorylation, NADPH oxidase activation and MuRF1 expression. J. Transl. Med. 2019, 17, 279. [Google Scholar] [CrossRef]
- Loktev, S.S.; Ogneva, I.V. DNA Methylation of Mouse Testes, Cardiac and Lung Tissue During Long-Term Microgravity Simulation. Sci. Rep. 2019, 9, 7974. [Google Scholar] [CrossRef]
- Liu, C.; Zhong, G.; Zhou, Y.; Yang, Y.; Tan, Y.; Li, Y.; Gao, X.; Sun, W.; Li, J.; Jin, X.; et al. Alteration of calcium signalling in cardiomyocyte induced by simulated microgravity and hypergravity. Cell Prolif. 2020, 53, e12783. [Google Scholar] [CrossRef]
- Liang, L.; Li, H.; Cao, T.; Qu, L.; Zhang, L.; Fan, G.-C.; Greer, P.A.; Li, J.; Jones, D.L.; Peng, T. Calpain activation mediates microgravity-induced myocardial abnormalities in mice via p38 and ERK1/2 MAPK pathways. J. Biol. Chem. 2020, 295, 16840–16851. [Google Scholar] [CrossRef]
- Guarnieri, S.; Morabito, C.; Bevere, M.; Lanuti, P.; Mariggiò, M.A. A Protective Strategy to Counteract the Oxidative Stress Induced by Simulated Microgravity on H9C2 Cardiomyocytes. Oxidative Med. Cell. Longev. 2021, 2021, 9951113. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, L.; Xu, C.; Liu, J.; Fan, Q.; Gai, Y.; Zhao, S.; Wu, X.; Mi, T.; Wang, J.; et al. Comprehensive analysis of transcriptomics and metabolomics to understand tail-suspension-induced myocardial injury in rat. Front. Cardiovasc. Med. 2023, 9, 1074257. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Jones, J.A.; Mason, C.E. Optimizing Human Performance in Extreme Environments through Precision Medicine: From Spaceflight to High-Performance Operations on Earth; Cambridge University Press: Cambridge, UK, 2023; Volume 1, p. e27. [Google Scholar]
- Slaba, T.C.; Bahadori, A.A.; Reddell, B.D.; Singleterry, R.C.; Clowdsley, M.S.; Blattnig, S.R. Optimal shielding thickness for galactic cosmic ray environments. Life Sci. Space Res. 2017, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pal Chowdhury, R.; Stegeman, L.A.; Lund, M.L.; Fry, D.; Madzunkov, S.; Bahadori, A.A. Hybrid methods of radiation shielding against deep-space radiation. Life Sci. Space Res. 2023, 38, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Boerma, M.; Nelson, G.A.; Sridharan, V.; Mao, X.W.; Koturbash, I.; Hauer-Jensen, M. Space radiation and cardiovascular disease risk. World J. Cardiol. 2015, 7, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Bogomolov, V.V.; Kondratenko, S.N.; Kovachevich, I.V.; Repenkova, L.G. Propranolol harmacokinetics and hemodynamic indices in antiorthostatic hypokinesia. Aviakosm Ekol. Med. 2016, 50, 5–10. [Google Scholar]
- Soucy, K.G.; Lim, H.K.; Kim, J.H.; Oh, Y.; Attarzadeh, D.O.; Sevinc, B.; Kuo, M.M.; Shoukas, A.A.; Vazquez, M.E.; Berkowitz, D.E. HZE 56Fe-ion irradiation induces endothelial dysfunction in rat aorta: Role of xanthine oxidase. Radiat. Res. 2011, 176, 474–485. [Google Scholar] [CrossRef]
- Boehm, F.; Edge, R.; Truscott, T.G.; Witt, C. A dramatic effect of oxygen on protection of human cells against gamma-radiation by lycopene. FEBS Lett. 2016, 590, 1086–1093. [Google Scholar] [CrossRef]
- Martens, C.R.; Seals, D.R. Practical alternatives to chronic caloric restriction for optimizing vascular function with ageing. J. Physiol. 2016, 594, 7177–7195. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Method | Results |
|---|---|---|---|
| Mednieks et al. [11] | 1986 | Rat model (retrospective) | Decreased Km cAMP phosphodiesterase |
| Philpott et al. [12] | 1990 | Rat model (retrospective) | Mitochondrial degeneration, decreased ATP, ischemic damage |
| Fareh et al. [13] | 1994 | Rat model (retrospective) | Increased irANP |
| Connor et al. [14] | 1998 | Rat model, spaceflight (retrospective) | Increased MDH, subunit IV, decreased GAPDH |
| Lwigale et al. [15] | 2000 | Avian embryos (retrospective) | Decreased FN and desmosomes |
| Yu et al. [16] | 2001 | Rat model (retrospective) | TM fragments |
| Okumura et al. [17] | 2007 | Rat model (retrospective) | AC expression |
| Yin et al. [18] | 2008 | Rat model (retrospective) | SC/cAMP depression |
| Kwon et al. [19] | 2009 | Rat model (retrospective) | Upregulation of NF-kB |
| Ito et al. [20] | 2010 | Rat model (review) | Decreased SERCA2a/PLB ratio |
| Cui et al. [21] | 2010 | Rat model (retrospective) | Ca2+ channel dysfunction |
| Chang et al. [22] | 2011 | Rat model (retrospective) | Calpain induced apoptosis |
| Vykhlyantsev et al. [23] | 2011 | Meriones unguiculatus model, spaceflight (retrospective) | Titin action and structure |
| Ogneva et al. [24] | 2012 | Rat model (retrospective) | Myocardial stiffness/beta, gamma, alfa-actinin-1 and alfa actinin-4 |
| Schwoerer et al. [25] | 2013 | Rat model (retrospective) | Cytosolic and sarcoplasmatic Ca2+ regulation |
| Respress et al. [26] | 2014 | Rat model (retrospective) | RyR2 phosphorilation |
| Bederman et al. [27] | 2015 | Rat model (RCT) | 2H and 18O in protein-bound alanine |
| Li et al. [28] | 2019 | Rat model (review) | Cardiomyocyte differentiation |
| Liang et al. [29] | 2019 | Rat model (retrospective) | NADPH oxidase/ROS |
| Loktev et al. [30] | 2019 | Rat model (RCT) | DNA methylation |
| Liang et al. [29] | 2019 | Rat model (retrospective) | MuRF1 activation |
| Liu et al. [31] | 2020 | Rat model (retrospective) | CaMKII/HDAC4 activation |
| Liang et al. [32] | 2020 | Rat model (retrospective) | Calpain system activation |
| Guarnieri et al. [33] | 2021 | H9C2 rat cardiomyocytes (retrospective) | Actin geometrical aleration |
| Liu et al. [34] | 2023 | Rat model (RCT) | DEG and DEM expression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacinto, O.; Lusini, M.; Sammartini, E.; Minati, A.; Mastroianni, C.; Nenna, A.; Pascarella, G.; Sammartini, D.; Carassiti, M.; Miraldi, F.; et al. Cardiovascular Effects of Cosmic Radiation and Microgravity. J. Clin. Med. 2024, 13, 520. https://doi.org/10.3390/jcm13020520
Giacinto O, Lusini M, Sammartini E, Minati A, Mastroianni C, Nenna A, Pascarella G, Sammartini D, Carassiti M, Miraldi F, et al. Cardiovascular Effects of Cosmic Radiation and Microgravity. Journal of Clinical Medicine. 2024; 13(2):520. https://doi.org/10.3390/jcm13020520
Chicago/Turabian StyleGiacinto, Omar, Mario Lusini, Emanuele Sammartini, Alessandro Minati, Ciro Mastroianni, Antonio Nenna, Giuseppe Pascarella, Davide Sammartini, Massimiliano Carassiti, Fabio Miraldi, and et al. 2024. "Cardiovascular Effects of Cosmic Radiation and Microgravity" Journal of Clinical Medicine 13, no. 2: 520. https://doi.org/10.3390/jcm13020520
APA StyleGiacinto, O., Lusini, M., Sammartini, E., Minati, A., Mastroianni, C., Nenna, A., Pascarella, G., Sammartini, D., Carassiti, M., Miraldi, F., Chello, M., & Pelliccia, F. (2024). Cardiovascular Effects of Cosmic Radiation and Microgravity. Journal of Clinical Medicine, 13(2), 520. https://doi.org/10.3390/jcm13020520







