Severe Acute Kidney Injury Associated with Transformation of Chronic Myelomonocytic Leukemia into Acute Myeloid Leukemia: A Case Report
Abstract
1. Introduction
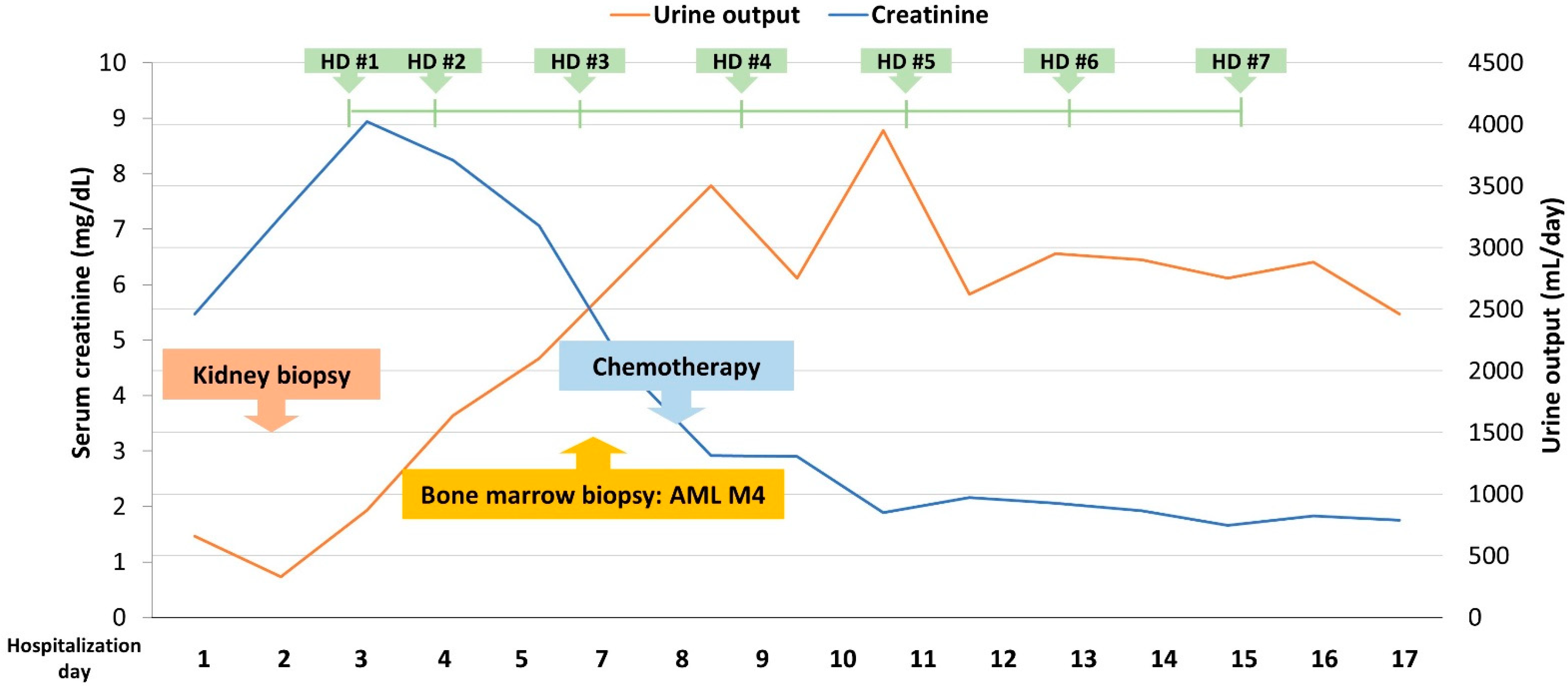
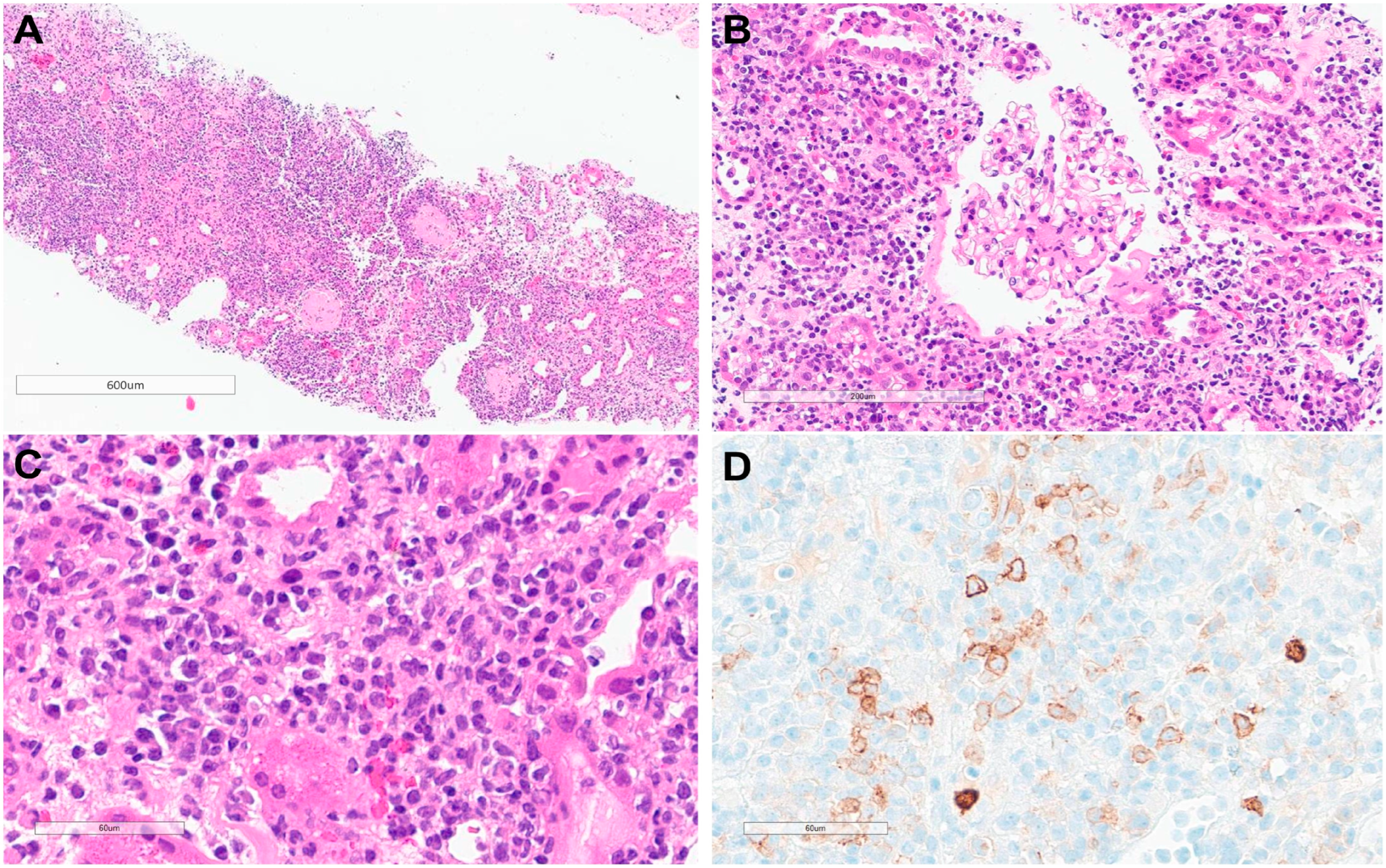
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patnaik, M.M.; Lasho, T. Myelodysplastic syndrome/myeloproliferative neoplasm overlap syndromes: A focused review. Hematol. Am. Soc. Hematol. Educ. Program. 2020, 2020, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Srour, S.A.; Devesa, S.S.; Morton, L.M.; Check, D.P.; Curtis, R.E.; Linet, M.S.; Dores, G.M. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001–2012. Br. J. Haematol. 2016, 174, 382–396. [Google Scholar] [CrossRef]
- Kuendgen, A.; Kasprzak, A.; Germing, U. Hybrid or Mixed Myelodysplastic/Myeloproliferative Disorders–Epidemiological Features and Overview. Front. Oncol. 2021, 11, 778741. [Google Scholar] [CrossRef] [PubMed]
- Neukirchen, J.; Schoonen, W.M.; Strupp, C.; Gattermann, N.; Aul, C.; Haas, R.; Germing, U. Incidence and prevalence of myelodysplastic syndromes: Data from the Düsseldorf MDS-registry. Leuk. Res. 2011, 35, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Elena, C.; Gallì, A.; Such, E.; Meggendorfer, M.; Germing, U.; Rizzo, E.; Cervera, J.; Molteni, E.; Fasan, A.; Schuler, E.; et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood 2016, 128, 1408–1417. [Google Scholar] [CrossRef]
- Derlin, T.; Clauditz, T.S.; Bannas, P. 18F-FDG PET/CT for staging and detection of extramedullary organ involvement in chronic myelomonocytic leukemia. Clin. Nucl. Med. 2014, 39, 811–812. [Google Scholar] [CrossRef]
- Pudasainee, P.; Pyakuryal, B.; Subedi, Y.; Upadhyay, J.; Adhikari, S. Extramedullary Manifestations of Chronic Myelomonocytic Leukemia: Do We Treat like an Acute Myeloid Leukemia? Case Rep. Hematol. 2019, 2019, 8360454. [Google Scholar] [CrossRef]
- Bourantas, K.; Tsiara, S.; Panteli, A.; Milionis, C.; Christou, L. Pleural effusion in chronic myelomonocytic leukemia. Acta Haematol. 1998, 99, 34–37. [Google Scholar] [CrossRef]
- Strupp, C.; Germing, U.; Trommer, I.; Gattermann, N.; Aul, C. Pericardial effusion in chronic myelomonocytic leukemia (CMML): A case report and review of the literature. Leuk. Res. 2000, 24, 1059–1062. [Google Scholar] [CrossRef]
- Kawamoto, K.; Miyoshi, H.; Yoshida, N.; Takizawa, J.; Sone, H.; Ohshima, K. Clinicopathological, cytogenetic, and prognostic analysis of 131 myeloid sarcoma patients. Am. J. Surg. Pathol. 2016, 40, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Hyams, E.S.; Gupta, R.; Melamed, J.; Taneja, S.S.; Shah, O. Renal involvement by chronic myelomonocytic leukemia requiring nephroureterectomy. Rev. Urol. 2009, 11, 33–37. [Google Scholar]
- Belliere, J.; Colombat, M.; Kounde, C.; Recher, C.; Ribes, D.; Huart, A.; Chauveau, D.; Demas, V.; Luquet, I.; Beyne-Rauzy, O.; et al. Kidney Involvement in Patients With Chronic Myelomonocytic Leukemia or BCR-ABL–Negative Myeloproliferative Neoplasms. Kidney Int. Rep. 2020, 6, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Pleyer, L.; Germing, U.; Sperr, W.R.; Linkesch, W.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; Schreder, M.; Pfeilstocker, M.; Lang, A.; et al. Azacitidine in CMML: Matched-pair analyses of daily-life patients reveal modest effects on clinical course and survival. Leuk. Res. 2014, 38, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Wassie, E.A.; Lasho, T.L.; Hanson, C.A.; Ketterling, R.; Tefferi, A. Blast transformation in chronic myelomonocytic leukemia: Risk factors, genetic features, survival, and treatment outcome. Am. J. Hematol. 2015, 90, 411–416. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Parikh, S.A.; Hanson, C.A.; Tefferi, A. Chronic myelomonocytic leukaemia: A concise clinical and pathophysiological review. Br. J. Haematol. 2014, 165, 273–286. [Google Scholar] [CrossRef]
- Fianchi, L.; Quattrone, M.; Criscuolo, M.; Bellesi, S.; Dragonetti, G.; Maraglino, A.M.E.; Bonanni, M.; Chiusolo, P.; Sica, S.; Pagano, L. Extramedullary Involvement in Acute Myeloid Leukemia. A Single Center Ten Years’ Experience. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021030. [Google Scholar] [CrossRef]
- Canet, E.; Zafrani, L.; Lambert, J.; Thieblemont, C.; Galicier, L.; Schnell, D.; Raffoux, E.; Lengline, E.; Chevret, S.; Darmon, M.; et al. Acute kidney injury in patients with newly diagnosed high-grade hematological malignancies: Impact on remission and survival. PLoS ONE 2013, 8, e55870. [Google Scholar] [CrossRef]
- Lahoti, A.; Kantarjian, H.; Salahudeen, A.K.; Ravandi, F.; Cortes, J.E.; Faderl, S.; O’Brien, S.; Wierda, W.; Mattiuzzi, G.N. Predictors and outcome of acute kidney injury in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome. Cancer 2010, 116, 4063–4068. [Google Scholar] [CrossRef]
- Morschhauser, F.; Wattel, E.; Pagniez, D.; Lovi, V.; Rose, C.; Bauters, F.; Fenaux, P. Glomerular injury in chronic myelomonocytic leukemia. Leuk. Lymphoma 1995, 18, 479–483. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Pierola, A.A.; Vallapureddy, R.; Yalniz, F.F.; Kadia, T.M.; Jabbour, E.J.; Lasho, T.; Hanson, C.A.; Ketterling, R.P.; Kantarjian, H.M.; et al. Blast phase chronic myelomonocytic leukemia: Mayo-MDACC collaborative study of 171 cases. Leukemia 2018, 32, 2512–2518. [Google Scholar] [CrossRef]
- Bera, R.; Chiu, M.-C.; Huang, Y.-J.; Lin, T.-H.; Kuo, M.-C.; Shih, L.-Y. RUNX1 mutations promote leukemogenesis of myeloid malignancies in ASXL1-mutated leukemia. J. Hematol. Oncol. 2019, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Ballo, O.; Eladly, F.; Büttner, S.; Stratmann, J.A.; Rudolf, S.; Brunnberg, U.; Kreisel, E.-M.; Steffen, B.; Wagner, S.; Finkelmeier, F.; et al. Acute kidney injury adversely affects the clinical course of acute myeloid leukemia patients undergoing induction chemotherapy. Ann. Hematol. 2021, 100, 1159–1167. [Google Scholar] [CrossRef]
- Lundberg, W.; Cadman, E.; Finch, S.; Capizzi, R. Renal failure secondary to leukemic infiltration of the kidneys. Am. J. Med. 1977, 62, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Obrador, G.T.; Price, B.; O’Meara, Y.; Salant, D.J. Acute renal failure due to lymphomatous infiltration of the kidneys. J. Am. Soc. Nephrol. 1997, 8, 1348–1354. [Google Scholar] [CrossRef]
- Asano, M.; Hase, H.; Naruse, Y.; Kawada, K.; Kojima, I.; Branch, J.; Kitamura, H.; Shimizu, H. A rare cause of acute kidney injury with chronic myelomonocytic leukemia. CEN Case Rep. 2021, 10, 320–325. [Google Scholar] [CrossRef]
- Chauhan, P.; Gupta, A.; Ora, M.; Agrawal, S.; Nityanand, S. AML with Renal Infiltration Manifesting as Acute Renal Failure, Diagnosed with FDG-PET CT Scan: Case Report. Ann. Hematol. Oncol. 2021, 8, 1368. [Google Scholar] [CrossRef]
- Aratani, S.; Aburakawa, S.; Ryotokuji, T.; Marumo, A.; Sakai, Y.; Inokuchi, K.; Tsuruoka, S. Primary Tumor Infiltration and Severe Acute Kidney Injury in Patients with Acute Myeloblastic Leukemia. J. Nippon. Med. Sch. 2020, 87, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.Y.; Gujadhur, A.; Pham, A.; Lukito, P.; Flanc, R.; Menahem, S. Infiltrative acute myeloid leukaemia as a cause of acute kidney injury. Clin. Kidney J. 2013, 6, 448–449. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-W.; Kim, M.-S.; Kim, Y.-J.; Jung, H.-Y.; Choi, J.-Y.; Cho, J.-H.; Park, S.-H.; Kim, C.-D.; Kim, Y.-L.; Lim, J.-H. Severe Acute Kidney Injury Associated with Transformation of Chronic Myelomonocytic Leukemia into Acute Myeloid Leukemia: A Case Report. J. Clin. Med. 2024, 13, 494. https://doi.org/10.3390/jcm13020494
Lee S-W, Kim M-S, Kim Y-J, Jung H-Y, Choi J-Y, Cho J-H, Park S-H, Kim C-D, Kim Y-L, Lim J-H. Severe Acute Kidney Injury Associated with Transformation of Chronic Myelomonocytic Leukemia into Acute Myeloid Leukemia: A Case Report. Journal of Clinical Medicine. 2024; 13(2):494. https://doi.org/10.3390/jcm13020494
Chicago/Turabian StyleLee, Seong-Wook, Mee-Seon Kim, Yong-Jin Kim, Hee-Yeon Jung, Ji-Young Choi, Jang-Hee Cho, Sun-Hee Park, Chan-Duck Kim, Yong-Lim Kim, and Jeong-Hoon Lim. 2024. "Severe Acute Kidney Injury Associated with Transformation of Chronic Myelomonocytic Leukemia into Acute Myeloid Leukemia: A Case Report" Journal of Clinical Medicine 13, no. 2: 494. https://doi.org/10.3390/jcm13020494
APA StyleLee, S.-W., Kim, M.-S., Kim, Y.-J., Jung, H.-Y., Choi, J.-Y., Cho, J.-H., Park, S.-H., Kim, C.-D., Kim, Y.-L., & Lim, J.-H. (2024). Severe Acute Kidney Injury Associated with Transformation of Chronic Myelomonocytic Leukemia into Acute Myeloid Leukemia: A Case Report. Journal of Clinical Medicine, 13(2), 494. https://doi.org/10.3390/jcm13020494







