Association of Fat Mass and Skeletal Muscle Mass with Cardiometabolic Risk Varied in Distinct PCOS Subtypes: A Propensity Score-Matched Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Data Collection and Measurements
2.4. Body Composition Assessment
2.5. Main Outcome and Calculation of the Cardiometabolic Risk Score
2.6. Statistics
3. Results
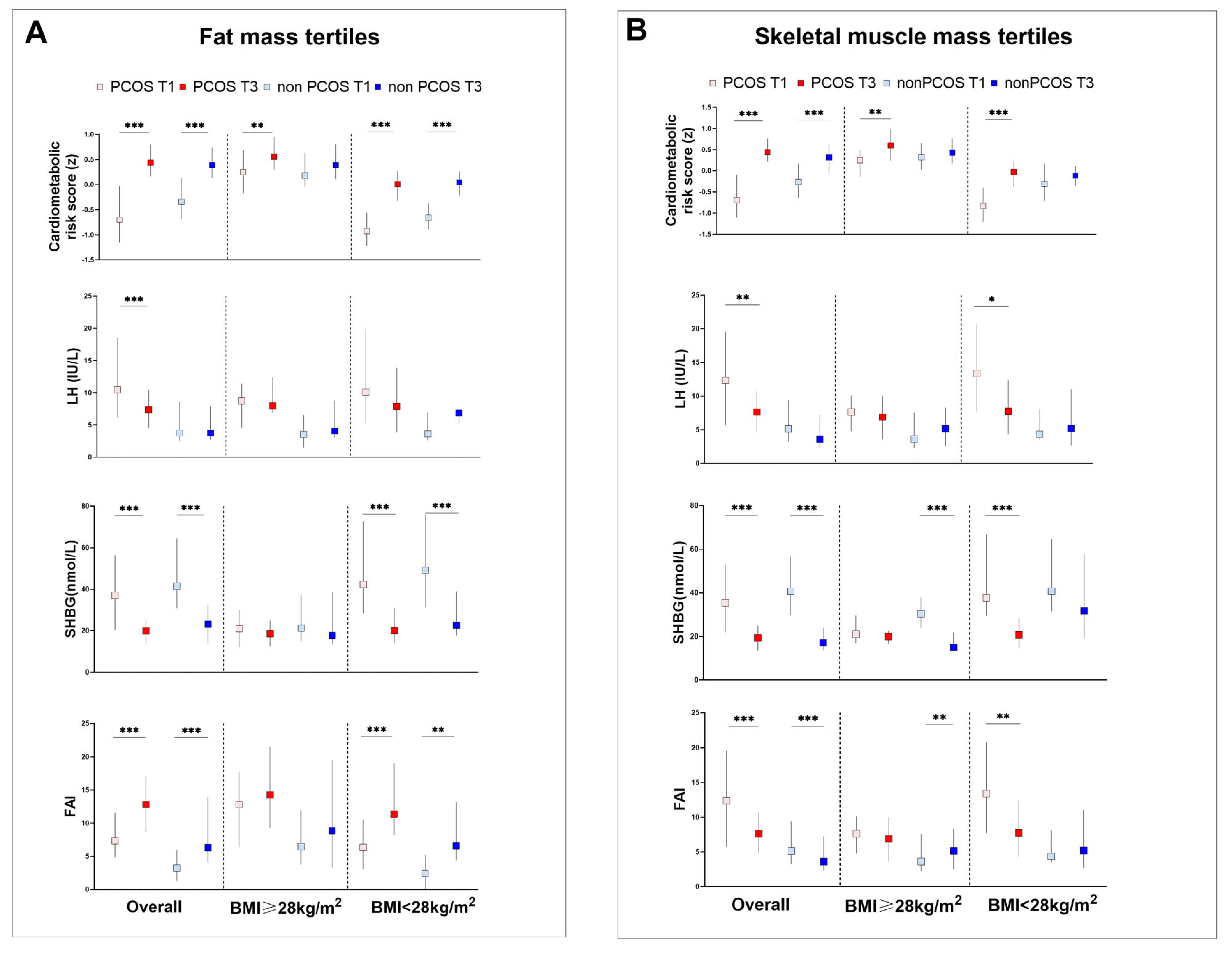
3.1. Characteristics of Body Composite Indices, Anthropometric and Metabolic Measures, and Reproductive Hormones in PCOS and Non-PCOS Women
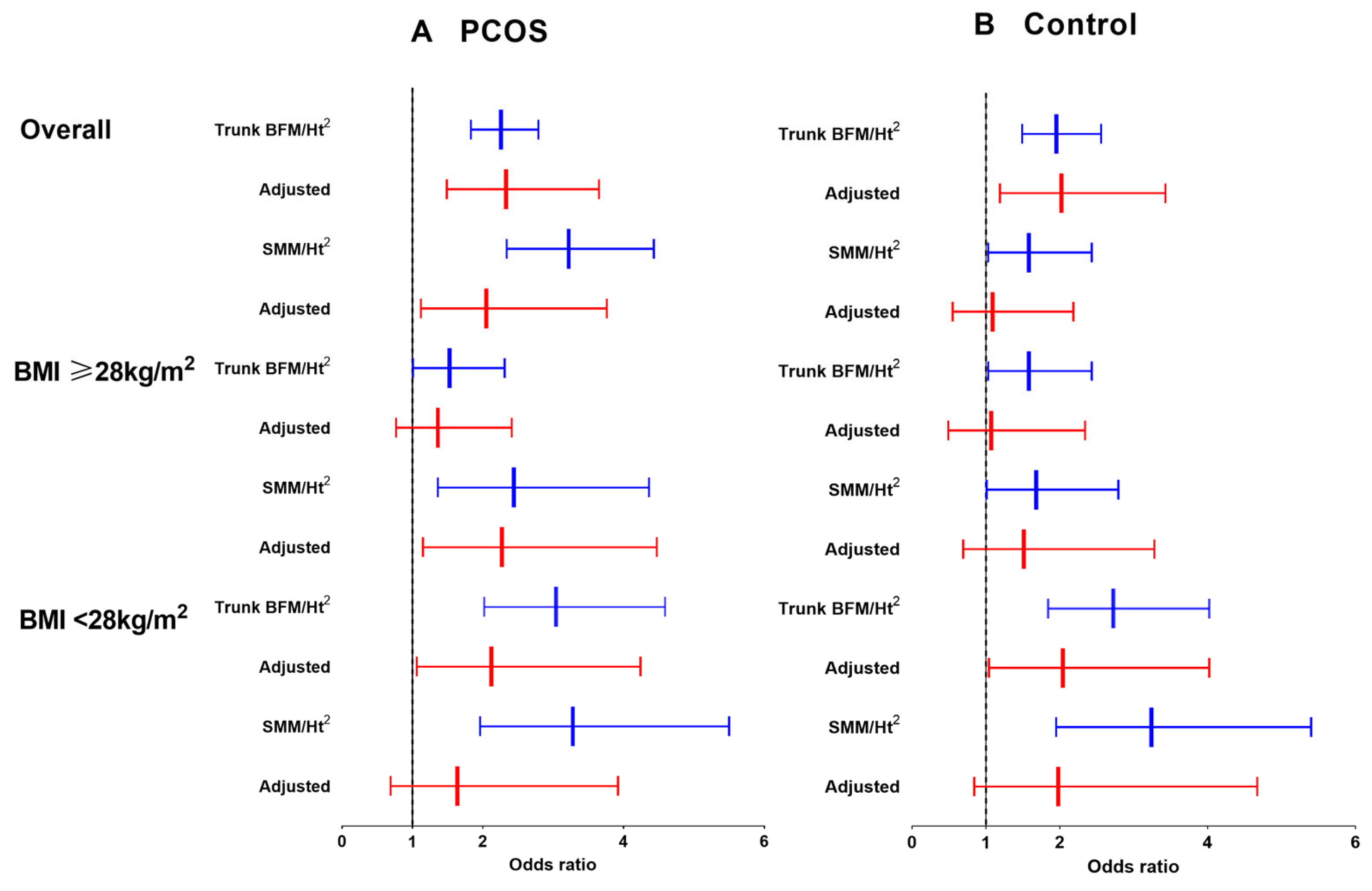
3.2. Association between Trunk BFM/Height2 Levels and Cardiometabolic Risk in PCOS and Controls
3.3. Association between SMM/Height2 Levels and Cardiometabolic Risk in PCOS and Controls
3.4. Body Composition, and Metabolic and Reproductive Hormones in Participants with Obesity and HA
3.5. Characteristics of Anthropometric Measures, Metabolic and Reproductive Hormones and Body Composite Indices in PCOS Subtypes and Control Women
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers. 2016, 2, 16057. [Google Scholar] [CrossRef]
- Van der Ham, K.; Louwers, Y.V.; Laven, J.S.E. Cardiometabolic biomarkers in women with polycystic ovary syndrome. Fertil. Steril. 2022, 117, 887–896. [Google Scholar] [CrossRef]
- Wang, E.T.; Calderon-Margalit, R.; Cedars, M.I.; Daviglus, M.L.; Merkin, S.S.; Schreiner, P.J.; Sternfeld, B.; Wellons, M.; Schwartz, S.M.; Lewis, C.E.; et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet. Gynecol. 2011, 117, 6–13. [Google Scholar] [CrossRef]
- Rotterdam EA-SPcwg. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Carmina, E.; Napoli, N.; Longo, R.A.; Rini, G.B.; Lobo, R.A. Metabolic syndrome in polycystic ovary syndrome (PCOS): Lower prevalence in southern Italy than in the USA and the influence of criteria for the diagnosis of PCOS. Eur. J. Endocrinol. 2006, 154, 141–145. [Google Scholar] [CrossRef][Green Version]
- Hallajzadeh, J.; Khoramdad, M.; Karamzad, N.; Almasi-Hashiani, A.; Janati, A.; Ayubi, E.; Pakzad, R.; Sullman, M.J.M.; Safiri, S. Metabolic syndrome and its components among women with polycystic ovary syndrome: A systematic review and meta-analysis. J. Cardiovasc. Thorac. Res. 2018, 10, 56–69. [Google Scholar] [CrossRef]
- Ehrmann, D.A.; Barnes, R.B.; Rosenfield, R.L.; Cavaghan, M.K.; Imperial, J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 1999, 22, 141–146. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, etiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef]
- Ng, N.Y.H.; Jiang, G.; Cheung, L.P.; Zhang, Y.; Tam, C.H.T.; Luk, A.O.Y.; Quan, J.; Lau, E.S.H.; Yau, T.T.L.; Chan, M.H.M.; et al. Progression of glucose intolerance and cardiometabolic risk factors over a decade in Chinese women with polycystic ovary syndrome: A case–control study. PLoS Med. 2019, 16, e1002953. [Google Scholar] [CrossRef]
- Kahn, C.R. The molecular mechanism of insulin action. Annu. Rev. Med. 1985, 36, 429–451. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signaling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Tosi, F.; Di Sarra, D.; Kaufman, J.M.; Bonin, C.; Moretta, R.; Bonora, E.; Zanolin, E.; Moghetti, P. Total body fat and central fat mass independently predict insulin resistance but not hyperandrogenemia in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2015, 100, 661–669. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Z.; Hu, C.; Sun, F.; Wang, C.; Yuan, H.; Li, Y. Imaging-Based Body Fat Distribution in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 697223. [Google Scholar] [CrossRef]
- Carmina, E.; Bucchieri, S.; Esposito, A.; Del Puente, A.; Mansueto, P.; Orio, F.; Di Fede, G.; Rini, G. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J. Clin. Endocrinol. Metab. 2007, 92, 2500–2505. [Google Scholar] [CrossRef]
- Cascella, T.; Palomba, S.; De Sio, I.; Manguso, F.; Giallauria, F.; De Simone, B.; Tafuri, D.; Lombardi, G.; Colao, A.; Orio, F. Visceral fat is associated with cardiovascular risk in women with polycystic ovary syndrome. Hum. Reprod. 2008, 23, 153–159. [Google Scholar] [CrossRef]
- Penaforte, F.R.; Japur, C.C.; Diez-Garcia, R.W.; Chiarello, P.G. Upper trunk fat assessment and its relationship with metabolic and biochemical variables and body fat in polycystic ovary syndrome. J. Hum. Nutr. Diet. 2011, 24, 39–46. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Gunnarsson, R.; Bjorkman, O.; Olsson, M.; Wahren, J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J. Clin. Investig. 1985, 76, 149–155. [Google Scholar] [CrossRef]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef]
- Kazemi, M.; Pierson, R.A.; Parry, S.A.; Kaviani, M.; Chilibeck, P.D. Obesity, but not hyperandrogenism or insulin resistance, predicts skeletal muscle mass in reproductive-aged women with polycystic ovary syndrome: A systematic review and meta-analysis of 45 observational studies. Obes. Rev. 2021, 22, e13255. [Google Scholar] [CrossRef]
- Hojlund, K.; Glintborg, D.; Andersen, N.R.; Birk, J.B.; Treebak, J.T.; Frosig, C.; Beck-Nielsen, H.; Wojtaszewski, J.F. Impaired insulin-stimulated phosphorylation of Akt and AS160 in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. Diabetes 2008, 57, 357–366. [Google Scholar] [CrossRef]
- Hansen, S.L.; Svendsen, P.F.; Jeppesen, J.F.; Hoeg, L.D.; Andersen, N.R.; Kristensen, J.M.; Nilas, L.; Lundsgaard, A.M.; Wojtaszewski, J.F.P.; Madsbad, S.; et al. Molecular Mechanisms in Skeletal Muscle Underlying Insulin Resistance in Women Who Are Lean with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 1841–1854. [Google Scholar] [CrossRef]
- Kazemi, M.; Jarrett, B.Y.; Parry, S.A.; Thalacker-Mercer, A.E.; Hoeger, K.M.; Spandorfer, S.D.; Lujan, M.E. Osteosarcopenia in Reproductive-Aged Women with Polycystic Ovary Syndrome: A Multicenter Study. J. Clin. Endocrinol. Metab. 2020, 105, e3400–e3414. [Google Scholar] [CrossRef]
- Dolfing, J.G.; Stassen, C.M.; van Haard, P.M.; Wolffenbuttel, B.H.; Schweitzer, D.H. Comparison of MRI-assessed body fat content between lean women with polycystic ovary syndrome (PCOS) and matched controls: Less visceral fat with PCOS. Hum. Reprod. 2011, 26, 1495–1500. [Google Scholar] [CrossRef]
- Fisch, S.C.; Nikou, A.F.; Wright, E.A.; Phan, J.D.; Leung, K.L.; Grogan, T.R.; Abbott, D.H.; Chazenbalk, G.D.; Dumesic, D.A. Precocious subcutaneous abdominal stem cell development to adipocytes in normal-weight women with polycystic ovary syndrome. Fertil. Steril. 2018, 110, 1367–1376. [Google Scholar] [CrossRef]
- Mario, F.M.; do Amarante, F.; Toscani, M.K.; Spritzer, P.M. Lean muscle mass in classic or ovulatory PCOS: Association with central obesity and insulin resistance. Exp. Clin. Endocrinol. Diabetes 2012, 120, 511–516. [Google Scholar] [CrossRef]
- Gao, S.; Cheng, Y.; Zhao, L.; Chen, Y.; Liu, Y. The relationships of irisin with bone mineral density and body composition in PCOS patients. Diabetes Metab. Res. Rev. 2016, 32, 421–428. [Google Scholar] [CrossRef]
- Dapas, M.; Lin, F.T.J.; Nadkarni, G.N.; Sisk, R.; Legro, R.S.; Urbanek, M.; Hayes, M.G.; Dunaif, A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 2020, 17, e1003132. [Google Scholar] [CrossRef]
- Zhao, X.; Ni, R.; Li, L.; Mo, Y.; Huang, J.; Huang, M.; Azziz, R.; Yang, D. Defining hirsutism in Chinese women: A cross-sectional study. Fertil. Steril. 2011, 96, 792–796. [Google Scholar] [CrossRef]
- Tan, J.K.; Tang, J.; Fung, K.; Gupta, A.K.; Thomas, D.R.; Sapra, S.; Lynde, C.; Poulin, Y.; Gulliver, W.; Sebaldt, R.J. Development and validation of a comprehensive acne severity scale. J. Cutan. Med. Surg. 2007, 11, 211–216. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Matsuda, M. Reduced time points to calculate the composite index. Diabetes Care 2010, 33, e93. [Google Scholar] [CrossRef]
- Beechy, L.; Galpern, J.; Petrone, A.; Das, S.K. Assessment tools in obesity-psychological measures, diet, activity, and body composition. Physiol. Behav. 2012, 107, 154–171. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Sasso, G.F.; Andreoli, A.; Sorge, R.; Candeloro, N.; Cairella, M. Improved prediction formula for total body water assessment in obese women. Int. J. Obes. Relat. Metab. Disord. 1995, 19, 535–538. [Google Scholar] [PubMed]
- Migueles, J.H.; Cadenas-Sanchez, C.; Lubans, D.R.; Henriksson, P.; Torres-Lopez, L.V.; Rodriguez-Ayllon, M.; Plaza-Florido, A.; Gil-Cosano, J.J.; Henriksson, H.; Escolano-Margarit, M.V.; et al. Effects of an Exercise Program on Cardiometabolic and Mental Health in Children with Overweight or Obesity: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2324839. [Google Scholar] [CrossRef]
- Viitasalo, A.; Lakka, T.A.; Laaksonen, D.E.; Savonen, K.; Lakka, H.M.; Hassinen, M.; Komulainen, P.; Tompuri, T.; Kurl, S.; Laukkanen, J.A.; et al. Validation of metabolic syndrome score by confirmatory factor analysis in children and adults and prediction of cardiometabolic outcomes in adults. Diabetologia 2014, 57, 940–949. [Google Scholar] [CrossRef]
- Armstrong, B.K.W.E.; Saracci, R. Principles of Exposure Measurement in Epidemiology; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Wijndaele, K.; Beunen, G.; Duvigneaud, N.; Matton, L.; Duquet, W.; Thomis, M.; Lefevre, J.; Philippaerts, R.M. A continuous metabolic syndrome risk score: Utility for epidemiological analyses. Diabetes Care 2006, 29, 2329. [Google Scholar] [CrossRef]
- Chen, C.; Lu, F.C.; Department of Disease Control Ministry of Health, P.R.C. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed. Environ. Sci. 2004, 17, 1–36. [Google Scholar]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Sneed, N.M.; Morrison, S.A. Body Composition Methods in Adults with Type 2 Diabetes or at Risk for T2D: A Clinical Review. Curr. Diab Rep. 2021, 21, 14. [Google Scholar] [CrossRef]
- VanItallie, T.B.; Yang, M.U.; Heymsfield, S.B.; Funk, R.C.; Boileau, R.A. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990, 52, 953–959. [Google Scholar] [CrossRef]
- Chen, X.; Yang, D.; Li, L.; Feng, S.; Wang, L. Abnormal glucose tolerance in Chinese women with polycystic ovary syndrome. Hum. Reprod. 2006, 21, 2027–2032. [Google Scholar] [CrossRef]
- Nathan, D.M.; Davidson, M.B.; DeFronzo, R.A.; Heine, R.J.; Henry, R.R.; Pratley, R.; Zinman, B. Impaired fasting glucose and impaired glucose tolerance: Implications for care. Diabetes Care 2007, 30, 753–759. [Google Scholar] [CrossRef]
- Dunaif, A.; Segal, K.R.; Shelley, D.R.; Green, G.; Dobrjansky, A.; Licholai, T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes 1992, 41, 1257–1266. [Google Scholar] [CrossRef]
- Ciaraldi, T.P.; el-Roeiy, A.; Madar, Z.; Reichart, D.; Olefsky, J.M.; Yen, S.S. Cellular mechanisms of insulin resistance in polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 1992, 75, 577–583. [Google Scholar]
- Dunaif, A.; Xia, J.; Book, C.B.; Schenker, E.; Tang, Z. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J. Clin. Investig. 1995, 96, 801–810. [Google Scholar] [CrossRef]
- Dunaif, A.; Wu, X.; Lee, A.; Diamanti-Kandarakis, E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS). Am. J. Physiol. Endocrinol. Metab. 2001, 281, E392–E399. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef]
- Cosar, E.; Ucok, K.; Akgun, L.; Koken, G.; Sahin, F.K.; Arioz, D.T.; Baş, O. Body fat composition and distribution in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2008, 24, 428–432. [Google Scholar] [CrossRef]
- Faloia, E.; Canibus, P.; Gatti, C.; Frezza, F.; Santangelo, M.; Garrapa, G.G.; Boscaro, M. Body composition, fat distribution and metabolic characteristics in lean and obese women with polycystic ovary syndrome. J. Endocrinol. Investig. 2004, 27, 424–429. [Google Scholar] [CrossRef]
- Kirchengast, S.; Huber, J. Body composition characteristics and body fat distribution in lean women with polycystic ovary syndrome. Hum. Reprod. 2001, 16, 1255–1260. [Google Scholar] [CrossRef]
- Jensen, M.D. Role of body fat distribution and the metabolic complications of obesity. J. Clin. Endocrinol. Metab. 2008, 93 (Suppl. S1), S57–S63. [Google Scholar] [CrossRef]
- Bhasin, S.; Woodhouse, L.; Casaburi, R.; Singh, A.B.; Mac, R.P.; Lee, M.; Yarasheski, K.E.; Sinha-Hikim, I.; Dzekov, C.; Dzekov, J.; et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J. Clin. Endocrinol. Metab. 2005, 90, 678–688. [Google Scholar] [CrossRef]
- Dubois, V.; Laurent, M.; Boonen, S.; Vanderschueren, D.; Claessens, F. Androgens and skeletal muscle: Cellular and molecular action mechanisms underlying the anabolic actions. Cell Mol. Life Sci. 2012, 69, 1651–1667. [Google Scholar] [CrossRef]
- Pasquali, R. Obesity and androgens: Facts and perspectives. Fertil. Steril. 2006, 85, 1319–1340. [Google Scholar] [CrossRef]
- Watz, M.E.S.; Tivesten, A.; Ottarsdottir, K.; Li, Y.; Hellgren, M.I.; Lindblad, U.; Daka, B. Sex hormone-binding globulin levels and development of hypertension in middle-aged men and women. J. Hypertens. 2023, 41, 1565–1570. [Google Scholar] [CrossRef]
- Miljkovic, I.; Kuipers, A.L.; Cauley, J.A.; Prasad, T.; Lee, C.G.; Ensrud, K.E.; Cawthon, P.M.; Hoffman, A.R.; Dam, T.T.; Gordon, C.L.; et al. Greater Skeletal Muscle Fat Infiltration Is Associated with Higher All-Cause and Cardiovascular Mortality in Older Men. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 1133–1140. [Google Scholar] [CrossRef]
- Straight, C.R.; Toth, M.J.; Miller, M.S. Current perspectives on obesity and skeletal muscle contractile function in older adults. J. Appl. Physiol. (1985) 2021, 130, 10–16. [Google Scholar] [CrossRef]
- Lu, X.; Yue, J.; Liu, Q.; He, S.; Dong, Y.; Zhang, M.; Qi, Y.; Yang, M.; Zhang, W.; Xu, H.; et al. Thigh muscle fat fraction is independently associated with impaired glucose metabolism in individuals with obesity. Endocr. Connect. 2023, 12, e230248. [Google Scholar] [CrossRef]
- Scott, D.; Joham, A.; Teede, H.; Gibson-Helm, M.; Harrison, C.; Cassar, S.; Hutchison, S.; Ebeling, P.R.; Stepto, N.; de Courten, B. Associations of Vitamin D with Inter- and Intra-Muscular Adipose Tissue and Insulin Resistance in Women with and without Polycystic Ovary Syndrome. Nutrients 2016, 8, 774. [Google Scholar] [CrossRef]
- Tarantino, G.; Sinatti, G.; Citro, V.; Santini, S.J.; Balsano, C. Sarcopenia, a condition shared by various diseases: Can we alleviate or delay the progression? Intern. Emerg. Med. 2023, 18, 1887–1895. [Google Scholar] [CrossRef]
- Kiefer, L.S.; Fabian, J.; Rospleszcz, S.; Lorbeer, R.; Machann, J.; Kraus, M.S.; Fischer, M.; Roemer, F.; Rathmann, W.; Meisinger, C.; et al. Population-based cohort imaging: Skeletal muscle mass by magnetic resonance imaging in correlation to bioelectrical-impedance analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 976–986. [Google Scholar] [CrossRef]
- Lundgren, J.R.; Janus, C.; Jensen, S.B.K.; Juhl, C.R.; Olsen, L.M.; Christensen, R.M.; Svane, M.S.; Bandholm, T.; Bojsen-Møller, K.N.; Blond, M.B.; et al. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N. Engl. J. Med. 2021, 384, 1719–1730. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129 (Suppl. S2), S102–S138. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, Z.; Lu, T.; Shao, X.; Cai, M.; Dilimulati, D.; Gao, X.; Mao, W.; Hu, F.; Su, L.; et al. Effects of a Dulaglutide plus Calorie-Restricted Diet versus a Calorie-Restricted Diet on Visceral Fat and Metabolic Profiles in Women with Polycystic Ovary Syndrome: A Randomized Controlled Trial. Nutrients 2023, 15, 556. [Google Scholar] [CrossRef]
- Hu, L.; Ma, L.; Xia, X.; Ying, T.; Zhou, M.; Zou, S.; Yu, H.; Yin, J. Efficacy of Bariatric Surgery in the Treatment of Women with Obesity and Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2022, 107, e3217–e3229. [Google Scholar] [CrossRef]
- Kogure, G.S.; Silva, R.C.; Miranda-Furtado, C.L.; Ribeiro, V.B.; Pedroso, D.C.C.; Melo, A.S.; Ferriani, R.A.; Reis, R.M.D. Hyperandrogenism Enhances Muscle Strength After Progressive Resistance Training, Independent of Body Composition, in Women with Polycystic Ovary Syndrome. J. Strength Cond. Res. 2018, 32, 2642–2651. [Google Scholar] [CrossRef]

| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| PCOS (n = 245) | Control (n = 156) | p Value (1) | PCOS (n = 141) | Control (n = 141) | p Value (2) | |
| Age (years) | 28 ± 6 | 31 ± 7 | <0.001 | 30 ± 6 | 30 ± 7 | 0.992 |
| BMI (kg/m2) | 26.7 ± 5.7 | 29.1 ± 5.9 | <0.001 | 28.5 ± 5.7 | 28.5 ± 5.3 | 0.941 |
| BMI ≥ 28 kg/m2 (%, n) | 40.0 (98) | 53.8 (84) | 0.007 | 54.6 (77) | 50.4 (71) | 0.474 |
| WC (cm) | 87 ± 14 | 95 ± 16 | <0.001 | 92 ± 14 | 93 ± 14 | 0.551 |
| Metabolic risk factors | ||||||
| Fasting glucose (mmol/L) | 4.74 (4.38, 5.17) | 4.89 (4.62, 5.31) | 0.005 | 4.90 (4.50, 5.32) | 4.85 (4.61, 5.21) | 0.990 |
| 2 h glucose (mmol/L) | 6.98 (5.44, 8.67) | 7.19 (5.95, 8.63) | 0.288 | 7.31 (5.61, 9.07) | 7.05 (5.88, 8.58) | 0.096 |
| Fasting insulin (uIU/mL) | 10.92 (6.13, 18.44) | 11.49 (8.28, 16.19) | 0.378 | 12.55 (7.61, 19.86) | 11.20 (8.17, 16.75) | 0.326 |
| 2 h insulin (uIU/mL) | 79.99 (40.68, 154.11) | 76.92 (44.55, 123.58) | 0.341 | 97.73 (49.54, 171.86) | 76.92 (44.24, 119.14) | 0.006 |
| HOMA-IR | 2.28 (1.27, 3.90) | 2.56 (1.77, 3.77) | 0.151 | 2.86 (1.52, 4.29) | 2.44 (1.61, 3.64) | 0.326 |
| Matsuda index | 3.24 (1.75, 6.49) | 3.19 (1.89, 4.92) | 0.821 | 2.55 (1.48, 4.52) | 3.29 (2.01, 5.24) | 0.020 |
| TG (mmol/L) | 1.20 (0.80, 1.80) | 1.31 (0.88, 2.04) | 0.044 | 1.30 (0.90, 2.08) | 1.26 (0.88, 2.02) | 0.952 |
| TC (mmol/L) | 4.79 ± 0.91 | 4.92 ± 0.83 | 0.158 | 4.84 ± 0.89 | 4.90 ± 0.80 | 0.608 |
| HDL (mmol/L) | 1.28 ± 0.36 | 1.26 ± 0.30 | 0.643 | 1.23 ± 0.33 | 1.28 ± 0.31 | 0.280 |
| LDL (mmol/L) | 2.86 ± 0.82 | 3.00 ± 0.72 | 0.087 | 2.91 ± 0.82 | 2.97 ± 0.70 | 0.529 |
| Cardiometabolic risk score (z) | −0.07 ± 0.71 | 0.08 ± 0.58 | 0.088 | 0.13 ± 0.67 | 0.03 ± 0.56 | 0.236 |
| Reproductive hormone | ||||||
| LH (IU/L) | 8.87 (5.28, 13.97) | 4.14 (2.79, 7.88) | <0.001 | 7.96 (5.28, 13.38) | 4.35 (2.85, 8.44) | <0.001 |
| FSH (IU/L) | 6.47 ± 2.20 | 6.06 ± 3.05 | 0.207 | 6.56 ± 2.22 | 6.14 ± 2.79 | 0.224 |
| LH/FSH | 1.45 (0.88, 2.19) | 0.76 (0.56, 1.30) | <0.001 | 1.45 (0.80, 2.09) | 0.78 (0.56, 1.33) | <0.001 |
| T (nmol/L) | 2.56 ± 0.94 | 1.77 ± 0.65 | <0.001 | 2.52 ± 0.94 | 1.81 ± 0.63 | <0.001 |
| FAI | 10.22 (6.38, 14.94) | 5.36 (2.44, 9.36) | <0.001 | 10.76 (7.25, 14.69) | 5.58 (2.42, 8.44) | <0.001 |
| SHBG (nmol/L) | 22.86 (17.17, 34.90) | 30.43 (17.34, 43.61) | 0.028 | 22.30 (17.49, 31.85) | 30.50 (17.73, 45.07) | 0.012 |
| DHEAS (ng/mL) | 266.93 ± 114.28 | 229.95 ± 98.40 | 0.006 | 259.06 ± 107.98 | 234.91 ± 98.32 | 0.093 |
| A2 (ng/mL) | 3.32 (2.66, 4.33) | 2.40 (1.82, 3.12) | <0.001 | 3.35 (2.50, 4.33) | 2.43 (1.93, 3.15) | <0.001 |
| Body composite indices | ||||||
| Visceral Fat Area (cm2) | 122.80 ± 54.69 | 146.14 ± 51.39 | <0.001 | 138.31 ± 54.48 | 142.95 ± 51.54 | 0.463 |
| Percent Body Fat (%) | 35.66 ± 7.56 | 39.04 ± 6.82 | <0.001 | 37.74 ± 7.02 | 38.65 ± 6.81 | 0.271 |
| Trunk BFM/Ht2 (kg/m2) | 4.81 ± 1.95 | 5.67 ± 1.83 | <0.001 | 5.43 ± 1.92 | 5.53 ± 1.79 | 0.661 |
| SMM/Ht2 (kg/m2) | 9.17 ± 1.26 | 9.55 ± 1.22 | 0.003 | 9.55 ± 1.27 | 9.42 ± 1.11 | 0.368 |
| SMI (kg/m2) | 6.93 ± 0.98 | 7.23 ± 0.97 | <0.001 | 7.21 ± 0.99 | 7.13 ± 0.88 | 0.463 |
| PCOS | Control | |||
|---|---|---|---|---|
| Overall | (n = 245) | p value | (n = 156) | p value |
| Trunk BFM/Ht2 | ||||
| unadjusted | 2.26 (1.83, 2.79) | <0.001 | 1.95 (1.49, 2.56) | <0.001 |
| adjusted | 2.33 (1.49, 3.65) | 0.006 | 2.02 (1.19, 3.43) | 0.009 |
| SMM/Ht2 | ||||
| unadjusted | 3.22 (2.34, 4.43) | <0.001 | 2.07 (1.43, 2.99) | <0.001 |
| adjusted | 2.05 (1.12, 3.76) | 0.020 | 1.09 (0.55, 2.18) | 0.805 |
| BMI ≥ 28 kg/m2 | (n = 98) | (n = 84) | ||
| Trunk BFM/Ht2 | ||||
| unadjusted | 1.53 (1.01, 2.31) | 0.046 | 1.58 (1.03, 2.43) | 0.036 |
| adjusted | 1.36 (0.77, 2.41) | 0.293 | 1.07 (0.49, 2.34) | 0.858 |
| SMM/Ht2 | ||||
| unadjusted | 2.44 (1.36, 4.36) | 0.003 | 1.68 (1.01, 2.79) | 0.047 |
| adjusted | 2.27 (1.15, 4.47) | 0.018 | 1.51 (0.69, 3.28) | 0.301 |
| BMI < 28 kg/m2 | (n = 147) | (n = 72) | ||
| Trunk BFM/Ht2 | ||||
| unadjusted | 3.04 (2.02, 4.59) | <0.001 | 2.72 (1.84, 4.02) | <0.001 |
| adjusted | 2.12 (1.06, 4.24) | 0.034 | 2.04 (1.04, 4.02) | 0.039 |
| SMM/Ht2 | ||||
| unadjusted | 3.28 (1.96, 5.50) | <0.001 | 3.24 (1.95, 5.40) | <0.001 |
| adjusted | 1.64 (0.69, 3.92) | 0.267 | 1.98 (0.84, 4.67) | 0.118 |
| p-value for interaction * | ||||
| BMI * Trunk BFM/Ht2 | <0.001 | <0.001 | ||
| BMI * SMM/Ht2 | <0.001 | <0.001 | ||
| PCOS (n = 77) | Control (n = 26) | p Value | |
|---|---|---|---|
| BMI (kg/m2) | 32.4 (30.1, 34.8) | 33.5 (31.1, 37.8) | 0.109 |
| WC (cm) | 100 (90, 103) | 108 (96, 115) | 0.019 |
| Fasting glucose (mmol/L) | 5.09 (4.64, 5.51) | 5.10 (4.70, 5.58) | 0.651 |
| 2 h glucose (mmol/L) | 7.95 (6.66, 9.50) | 8.47 (6.90, 10.13) | 0.413 |
| Fasting insulin (uIU/mL) | 18.60 (13.60, 24. 23) | 14.80 (11.49, 19.90) | 0.130 |
| 2 h insulin (uIU/mL) | 129.26 (78.29, 251.78) | 135.9 (75.66, 158.75) | 0.456 |
| TG (mmol/L) | 1.60 (1.06, 2.30) | 1.91 (1.45, 2.65) | 0.114 |
| HDL (mmol/L) | 1.08 (0.98, 1.25) | 1.13 (1.05, 1.27) | 0.272 |
| LDL (mmol/L) | 3.04 (2.63, 3.67) | 3.15 (2.70, 4.00) | 0.350 |
| Cardiometabolic risk score | 0.49 (0.26, 0.83) | 0.43 (0.31, 0.80) | 0.754 |
| LH (IU/L) | 7.96 (5.43, 11.46) | 5.33 (3.59, 7.50) | 0.013 |
| LH/FSH | 1.39 (0.96, 1.65) | 1.00 (0.67, 1.52) | 0.122 |
| T (nmol/L) | 2.53 (2.08, 2.97) | 2.38 (1.70, 2.80) | 0.122 |
| FAI | 13.95 (10.58, 18.25) | 14.80 (9.92, 23.80) | 0.325 |
| SHBG (nmol/L) | 17.95 (12.08, 23.58) | 13.79 (10.50, 16.96) | 0.006 |
| DHEAS (ng/mL) | 242.23 (189.00, 306.64) | 295.37 (228.84, 374.37) | 0.064 |
| A2 (ng/mL) | 3.50 (2.78, 4.32) | 3.49 (6.37, 4.40) | 0.510 |
| Trunk BFM/Ht2 (kg/m2) | 6.86 (6.00, 7.75) | 7.10 (6.37, 8.13) | 0.182 |
| SMM/Ht2 (kg/m2) | 10.74 (9.83, 11.36) | 10.21 (9.72, 10.78) | 0.051 |
| Metabolic PCOS (n = 57) | Control (n = 57) | p Value (1) | Reproductive PCOS (n = 48) | Control (n = 48) | p Value (2) | |
|---|---|---|---|---|---|---|
| Age (years) | 28 ± 7 | 27 ± 6 | 0.299 | 29 ± 5 | 29 ± 6 | 0.692 |
| BMI (kg/m2) | 30.1 ± 4.8 | 28.3 ± 7.7 | 0.084 | 24.8 ± 4.56 | 25.0 ± 4.6 | 0.815 |
| WC (cm) | 100 ± 14 | 94 ± 22 | 0.240 | 84 ± 14 | 85 ± 15 | 0.688 |
| Metabolic variables | ||||||
| Fasting glucose (mmol/L) | 4.96 (4.36, 5.40) | 4.71 (4.54, 5.19) | 0.304 | 4.60 (4.16, 4.95) | 4.81 (4.45, 5.15) | 0.029 |
| 2 h glucose (mmol/L) | 7.46 (6.18, 8.79) | 6.78 (5.45, 7.97) | 0.066 | 5.83 (4.74, 7.47) | 6.85 (5.47, 8.21) | 0.095 |
| Fasting insulin (uIU/mL) | 19.25 (13.64, 24.36) | 10.82 (6.32, 14.80) | <0.001 | 6.50 (4.17, 11.77) | 8.26 (5.21, 12.76) | 0.259 |
| 2 h insulin (uIU/mL) | 145.16 (102.05, 233.48) | 58.93 (38.19, 96.48) | <0.001 | 51.51 (25.83, 105.76) | 57.66 (37.44, 92.32) | 0.412 |
| HOMA-IR | 4.20 (3.20, 5.36) | 2.56 (1.35, 3.50) | <0.001 | 1.36 (0.88, 2.35) | 1.73 (1.05, 2.86) | 0.159 |
| Matsuda index | 1.83 (1.20, 2.53) | 3.53 (2.26, 5.97) | <0.001 | 5.99 (2.92, 9.92) | 4.08 (2.56, 7.73) | 0.145 |
| TG (mmol/L) | 1.40 (1.06, 1.90) | 1.05 (0.80, 1.83) | 0.015 | 1.00 (0.78, 1.60) | 1.00 (0.80, 1.92) | 0.679 |
| TC (mmol/L) | 5.03 ± 0.82 | 4.77 ± 0.80 | 0.123 | 4.88 ± 0.93 | 4.81 ± 0.76 | 0.690 |
| HDL (mmol/L) | 1.10 ± 0.20 | 1.30 ± 0.31 | <0.001 | 1.41 ± 0.40 | 1.30 ± 0.28 | 0.137 |
| LDL (mmol/L) | 3.14 ± 0.73 | 2.84 ± 0.70 | 0.039 | 2.85 ± 0.76 | 2.86 ± 0.69 | 0.943 |
| Cardiometabolic risk score | 0.39 ± 0.41 | −0.15 ± 0.58 | <0.001 | −0.40 ± 0.64 | −0.20 ± 0.53 | 0.109 |
| Reproductive hormone | ||||||
| LH (IU/L) | 8.72 (7.00, 11.75) | 4.14 (3.14, 7.52) | <0.001 | 12.35 (8.31, 18.70) | 4.11 (2.88, 7.10) | <0.001 |
| FSH (IU/L) | 5.90 ± 1.65 | 5.16 ± 2.20 | 0.517 | 7.25 ± 1.82 | 5.97 ± 2.71 | 0.012 |
| T (nmol/L) | 2.71 (2.22, 3.08) | 2.05 (1.40, 2.46) | <0.001 | 2.24 (1.74, 2.24) | 1.90 ± 0.66 | 0.002 |
| FAI | 14.66 (11.82, 18.89) | 4.48 (2.44, 9.88) | <0.001 | 7.03 (4.82, 9.28) | 3.28 (0.63, 6.28) | <0.001 |
| SHBG (nmol/L) | 18.05 (12.93, 21.87) | 35.23 (17.32, 56.51) | <0.001 | 32.53 (22.46, 52.53) | 39.14 (27.06, 60.52) | 0.152 |
| DHEAS (ng/mL) | 293.65 ± 127.04 | 261.39 ± 98.07 | 0.193 | 229.52 ± 118.15 | 241.07 ± 93.88 | 0.648 |
| A2 (ng/mL) | 3.78 (3.12, 4.60) | 2.68 (2.35, 3.64) | <0.001 | 3.99(2.97, 4.94) | 2.65 (2.14, 3.42) | <0.001 |
| Body composite indices | ||||||
| Trunk BFM/Ht2 (kg/m2) | 6.10 ± 1.57 | 5.29 ± 2.31 | 0.032 | 4.32 ± 1.78 | 4.33 ± 1.66 | 0.968 |
| SMM/Ht2 (kg/m2) | 9.98 ± 1.06 | 9.39 ± 1.38 | 0.012 | 8.70 ± 1.02 | 8.80 ± 0.94 | 0.613 |
| Metabolic PCOS | Reproductive PCOS | |||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Trunk BFM/Ht2 | ||||
| model 1 | 1.67 (1.14, 2.44) | 0.008 | 2.34 (1.54, 3.57) | <0.001 |
| model 2 | 1.24 (0.77, 2.00) | 0.381 | 2.37 (1.33, 4.21) | 0.003 |
| SMM/Ht2 | ||||
| model 1 | 3.14 (1.52, 6.49) | 0.002 | 2.77 (1.50, 5.11) | <0.001 |
| model 2 | 2.63 (1.13, 6.16) | 0.026 | 1.31 (0.59, 2.90) | 0.513 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Yue, J.; Lu, N.; Li, S.; Zheng, J.; Huang, R.; Jiang, Y.; Shan, C.; Liu, W.; Ma, J.; et al. Association of Fat Mass and Skeletal Muscle Mass with Cardiometabolic Risk Varied in Distinct PCOS Subtypes: A Propensity Score-Matched Case-Control Study. J. Clin. Med. 2024, 13, 483. https://doi.org/10.3390/jcm13020483
Cai J, Yue J, Lu N, Li S, Zheng J, Huang R, Jiang Y, Shan C, Liu W, Ma J, et al. Association of Fat Mass and Skeletal Muscle Mass with Cardiometabolic Risk Varied in Distinct PCOS Subtypes: A Propensity Score-Matched Case-Control Study. Journal of Clinical Medicine. 2024; 13(2):483. https://doi.org/10.3390/jcm13020483
Chicago/Turabian StyleCai, Jie, Jiang Yue, Nan Lu, Shengxian Li, Jun Zheng, Rong Huang, Yihong Jiang, Chang Shan, Wei Liu, Jing Ma, and et al. 2024. "Association of Fat Mass and Skeletal Muscle Mass with Cardiometabolic Risk Varied in Distinct PCOS Subtypes: A Propensity Score-Matched Case-Control Study" Journal of Clinical Medicine 13, no. 2: 483. https://doi.org/10.3390/jcm13020483
APA StyleCai, J., Yue, J., Lu, N., Li, S., Zheng, J., Huang, R., Jiang, Y., Shan, C., Liu, W., Ma, J., & Wang, L. (2024). Association of Fat Mass and Skeletal Muscle Mass with Cardiometabolic Risk Varied in Distinct PCOS Subtypes: A Propensity Score-Matched Case-Control Study. Journal of Clinical Medicine, 13(2), 483. https://doi.org/10.3390/jcm13020483







