A Systematic Review of the Pulmonary Microbiome in Patients with Acute Exacerbation COPD Requiring ICU Admission
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Registration and Literature Search
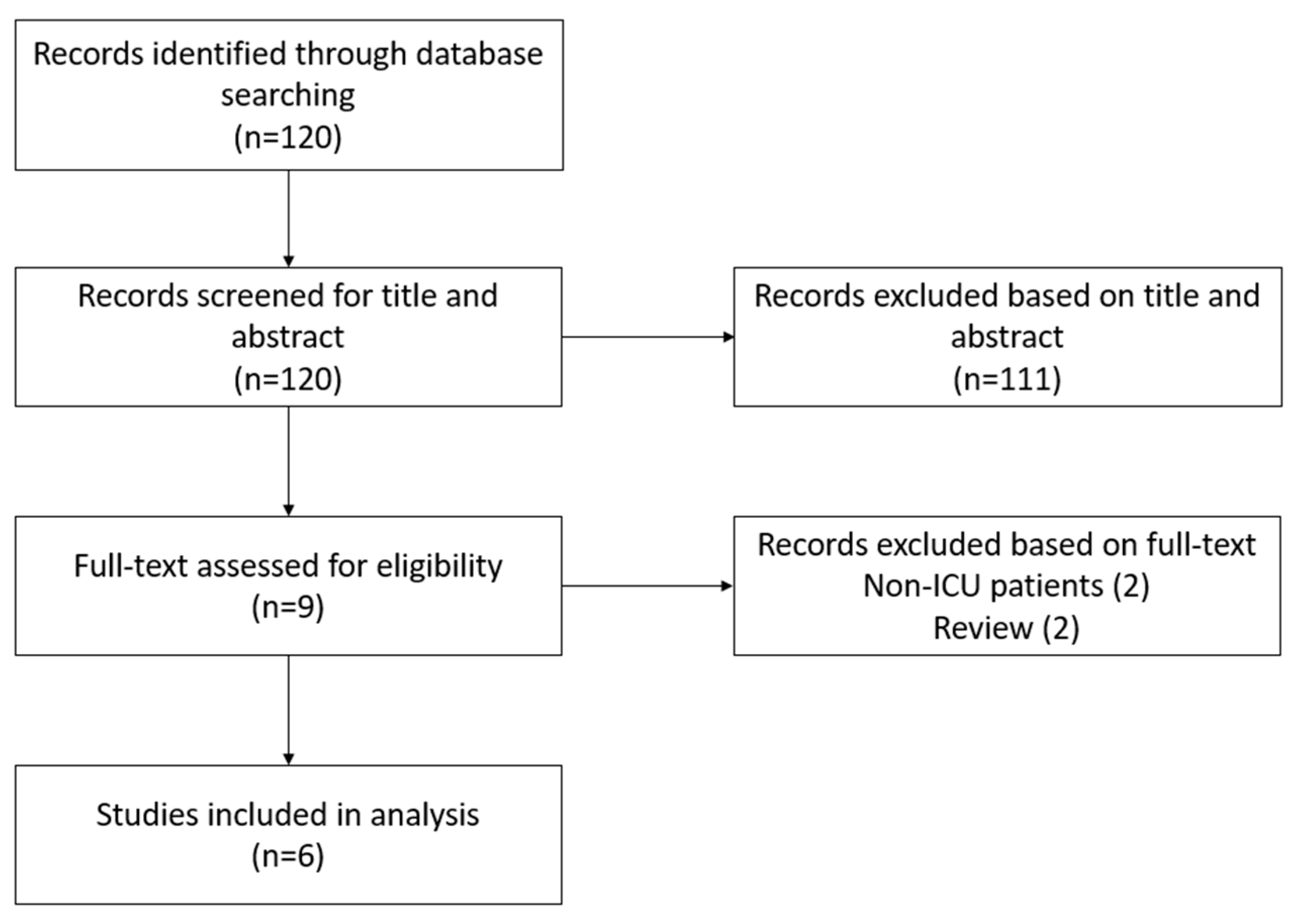
2.2. Selection Criteria and Data Extraction
2.3. Data Collection
3. Results
3.1. Respiratory Microbiome
3.1.1. Bacterial Species
3.1.2. Serological Analysis
3.2. Antimicrobial Therapy
3.2.1. Eradication of Pathogens
3.2.2. Antimicrobial Resistance
3.3. Clinical Outcomes
Mortality
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. Search Query
Appendix B
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | ||
| Study risk of bias assessment | 11 | Specify the methods used to assess the risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study, and whether they worked independently, and if applicable, details of automation tools used in the process. | |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | ||
| 13c | Describe any methods used to tabulate or visually display the results of individual studies and syntheses. | ||
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | ||
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | ||
| 13f | Describe any sensitivity analyses conducted to assess the robustness of the synthesized results. | ||
| Reporting bias assessment | 14 | Describe any methods used to assess the risk of bias due to missing results in a synthesis (arising from reporting biases). | |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | ||
| Study characteristics | 17 | Cite each included study and present its characteristics. | |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was conducted, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | ||
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | ||
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | ||
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | |
| 23b | Discuss any limitations of the evidence included in the review. | ||
| 23c | Discuss any limitations of the review processes used. | ||
| 23d | Discuss the implications of the results for practice, policy, and future research. | ||
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including the register name and registration number, or state that the review was not registered. | |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | ||
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | ||
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | |
| Competing interests | 26 | Declare any competing interests of review authors. | |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | |
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [PubMed]
- Viniol, C.; Vogelmeier, C.F. Exacerbations of COPD. Eur. Respir. Soc. Off. J. Eur. Respir. Soc. 2018, 27, 170103. [Google Scholar]
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar]
- Rangelov, K.; Sethi, S. Role of infections. Clin. Chest Med. 2014, 35, 87–100. [Google Scholar] [PubMed]
- Yagi, K.; Huffnagle, G.B.; Lukacs, N.W.; Asai, N. The Lung Microbiome during Health and Disease. Int. J. Mol. Sci. 2021, 22, 10872. [Google Scholar]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The Microbiome and the Respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [PubMed]
- Dima, E.; Kyriakoudi, A.; Kaponi, M.; Vasileiadis, I.; Stamou, P.; Koutsoukou, A.; Koulouris, N.G.; Rovina, N. The lung microbiome dynamics between stability and exacerbation in chronic obstructive pulmonary disease (COPD): Current perspectives. Respir Med. 2019, 157, 1–6. [Google Scholar] [PubMed]
- Dy, R.; Sethi, S. The lung microbiome and exacerbations of COPD. Curr. Opin. Pulm. Med. 2016, 22, 196–202. [Google Scholar]
- Mammen, M.J.; Sethi, S. COPD and the microbiome. Respirology 2016, 21, 590–599. [Google Scholar]
- Millares, L.; Monso, E. The Microbiome in COPD: Emerging Potential for Microbiome-Targeted Interventions. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1835–1845. [Google Scholar]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [PubMed]
- Wang, Z.; Locantore, N.; Haldar, K.; Ramsheh, M.Y.; Beech, A.S.; Ma, W.; Brown, J.R.; Tal-Singer, R.; Barer, M.R.; Bafadhel, M.; et al. Inflammatory Endotype-associated Airway Microbiome in Chronic Obstructive Pulmonary Disease Clinical Stability and Exacerbations: A Multicohort Longitudinal Analysis. Am. J. Respir. Crit. Care Med. 2021, 203, 1488–1502. [Google Scholar] [PubMed]
- Dicker, A.J.; Huang, J.T.; Lonergan, M.; Keir, H.R.; Fong, C.J.; Tan, B.; Cassidy, A.J.; Finch, S.; Mullerova, H.; Miller, B.E.; et al. The sputum microbiome, airway inflammation, and mortality in chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2021, 147, 158–167. [Google Scholar] [PubMed]
- Wang, H.; Yang, T.; Yu, X.; Chen, Z.; Ran, Y.; Wang, J.; Dai, G.; Deng, H.; Li, X.; Zhu, T. Risk Factors for Length of Hospital Stay in Acute Exacerbation Chronic Obstructive Pulmonary Disease: A Multicenter Cross-Sectional Study. Int. J. Gen. Med. 2022, 15, 3447–3458. [Google Scholar]
- Narayana, J.K.; Aliberti, S.; Mac Aogáin, M.; Jaggi, T.K.; Ali, N.A.; Ivan, F.X.; Cheng, H.S.; Yip, Y.S.; Vos, M.I.; Low, Z.S.; et al. Microbial Dysregulation of the Gut-Lung Axis in Bronchiectasis. Am. J. Respir. Crit. Care Med. 2023, 207, 908–920. [Google Scholar]
- Chiu, Y.C.; Lee, S.W.; Liu, C.W.; Lan, T.Y.; Wu, L.S.H. Relationship between gut microbiota and lung function decline in patients with chronic obstructive pulmonary disease: A 1-year follow-up study. Respir. Res. 2022, 23, 10. [Google Scholar]
- Li, N.; Dai, Z.; Wang, Z.; Deng, Z.; Zhang, J.; Pu, J.; Cao, W.; Pan, T.; Zhou, Y.; Yang, Z.; et al. Gut microbiota dysbiosis contributes to the development of chronic obstructive pulmonary disease. Respir. Res. 2021, 22, 274. [Google Scholar]
- Bowerman, K.L.; Rehman, S.F.; Vaughan, A.; Lachner, N.; Budden, K.F.; Kim, R.Y.; Wood, D.L.A.; Gellatly, S.L.; Shukla, S.D.; Wood, L.G.; et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat. Commun. 2020, 11, 5886. [Google Scholar]
- Zhou, Y.; Liu, M.; Liu, K.; Wu, G.; Tan, Y. Lung microbiota and potential treatment of respiratory diseases. Microb. Pathog. 2023, 181, 106197. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar]
- Ewig, S.; Soler, N.; Gonzalez, J.; Celis, R.; El-Ebiary, M.; Torres, A. Evaluation of antimicrobial treatment in mechanically ventilated patients with severe chronic obstructive pulmonary disease exacerbations. Crit. Care Med. 2000, 28, 692–697. [Google Scholar] [PubMed]
- Huang, Y.J.; Kim, E.; Cox, M.J.; Brodie, E.L.; Brown, R.; Wiener-Kronish, J.P.; Lynch, S.V.; Mammen, M.J.; Sethi, S.; Sherrard, L.J.; et al. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS J. Integr. Biol. 2010, 14, 9–59. [Google Scholar]
- Soler, N.; Torres, A.; Ewig, S.; Gonzalez, J.; Celis, R.; El-Ebiary, M.; Hernandez, C.; Rodriguez-Roisin, R. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am. J. Respir. Crit. Care Med. 1998, 157 Pt 1, 1498–1505. [Google Scholar]
- Tan, L.; Wang, H.; Li, C.; Pan, Y. 16S rDNA-based metagenomic analysis of dental plaque and lung bacteria in patients with severe acute exacerbations of chronic obstructive pulmonary disease. J. Periodontal Res. 2014, 49, 760–769. [Google Scholar]
- Ferrer, M.; Ioanas, M.; Arancibia, F.; Marco, M.A.; de la Bellacasa, J.P.; Torres, A. Microbial airway colonization is associated with noninvasive ventilation failure in exacerbation of chronic obstructive pulmonary disease. Crit. Care Med. 2005, 33, 2003–2009. [Google Scholar]
- Nseir, S.; Di Pompeo, C.; Cavestri, B.; Jozefowicz, E.; Nyunga, M.; Soubrier, S.; Roussel-Delvallez, M.; Saulnier, F.; Mathieu, D.; Durocher, A. Multiple-drug-resistant bacteria in patients with severe acute exacerbation of chronic obstructive pulmonary disease: Prevalence, risk factors, and outcome. Crit. Care Med. 2006, 34, 2959–2966. [Google Scholar]
- Watson, D.W.; Brandly, C.A. Virulence and pathogenicity. Annu. Rev. Microbiol. 1949, 3, 195–220. [Google Scholar]
- Leitao Filho, F.S.; Alotaibi, N.M.; Ngan, D.; Tam, S.; Yang, J.; Hollander, Z.; Chen, V.; FitzGerald, J.M.; Nislow, C.; Leung, J.M.; et al. Sputum Microbiome Is Associated with 1-Year Mortality after Chronic Obstructive Pulmonary Disease Hospitalizations. Am. J. Respir. Crit. Care Med. 2019, 199, 1205–1213. [Google Scholar]
- Lonergan, M.; Dicker, A.J.; Crichton, M.L.; Keir, H.R.; Van Dyke, M.K.; Mullerova, H.; Miller, B.E.; Tal-Singer, R.; Chalmers, J.D. Blood neutrophil counts are associated with exacerbation frequency mortality in COPD. Respir. Res. 2020, 21, 166. [Google Scholar]
- Kayongo, A.; Bartolomaeus, T.U.P.; Birkner, T.; Markó, L.; Löber, U.; Kigozi, E.; Atugonza, C.; Munana, R.; Mawanda, D.; Sekibira, R.; et al. Sputum Microbiome and Chronic Obstructive Pulmonary Disease in a Rural Ugandan Cohort of Well-Controlled HIV Infection. Microbiol. Spectr. 2023, 11, e0213921. [Google Scholar]
- Su, L.; Qiao, Y.; Luo, J.; Huang, R.; Li, Z.; Zhang, H.; Zhao, H.; Wang, J.; Xiao, Y. Characteristics of the sputum microbiome in COPD exacerbations and correlations between clinical indices. J. Transl. Med. 2022, 20, 76. [Google Scholar] [PubMed]
- Matkovic, Z.; Miravitlles, M. Chronic bronchial infection in COPD. Is there an infective phenotype? Respir. Med. 2013, 107, 10–22. [Google Scholar] [PubMed]
- Murphy, T.F. The role of bacteria in airway inflammation in exacerbations of chronic obstructive pulmonary disease. Curr. Opin. Infect. Dis. 2006, 19, 225–230. [Google Scholar]
- Lin, S.H.; Kuo, P.H.; Hsueh, P.R.; Yang, P.C.; Kuo, S.H. Sputum bacteriology in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease in Taiwan with an emphasis on Klebsiella pneumoniae and Pseudomonas aeruginosa. Respirology 2007, 12, 81–87. [Google Scholar]
- Beasley, V.; Joshi, P.V.; Singanayagam, A.; Molyneaux, P.L.; Johnston, S.L.; Mallia, P. Lung microbiology and exacerbations in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 555–569. [Google Scholar]
- Leung, J.M.; Tiew, P.Y.; Mac Aogáin, M.; Budden, K.F.; Yong, V.F.L.; Thomas, S.S.; Pethe, K.; Hansbro, P.M.; Chotirmall, S.H. The role of acute chronic respiratory colonization infections in the pathogenesis of COPD. Respirology 2017, 22, 634–650. [Google Scholar]
- Kan-O, K.; Washio, Y.; Fujimoto, T.; Shiroyama, N.; Nakano, T.; Wakamatsu, K.; Takata, S.; Yoshida, M.; Fujita, M.; Matsumoto, K. Differences in the spectrum of respiratory viruses and detection of human rhinovirus C in exacerbations of adult asthma and chronic obstructive pulmonary disease. Respir. Investig. 2022, 60, 129–136. [Google Scholar]
- Blatter, J.A.; Takahashi, T.; Mittler, B.; Nava, R.G.; Puri, V.; Kreisel, D.; Wang, D. Anellovirus Dynamics Are Associated With Primary Graft Dysfunction in Lung Transplantation. Transplant. Direct 2020, 6, e521. [Google Scholar]
- De Vlaminck, I.; Khush, K.K.; Strehl, C.; Kohli, B.; Luikart, H.; Neff, N.F.; Okamoto, J.; Snyder, T.M.; Cornfield, D.N.; Nicolls, M.R.; et al. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell 2013, 155, 1178–1187. [Google Scholar]
- Keir, H.R.; Contoli, M.; Chalmers, J.D. Inhaled Corticosteroids and the Lung Microbiome in COPD. Biomedicines 2021, 9, 1312. [Google Scholar]
- Ramsheh, M.Y.; Haldar, K.; Esteve-Codina, A.; Purser, L.F.; Richardson, M.; Müller-Quernheim, J.; Greulich, T.; Nowinski, A.; Barta, I.; Stendardo, M.; et al. Lung microbiome composition and bronchial epithelial gene expression in patients with COPD versus healthy individuals: A bacterial 16S rRNA gene sequencing and host transcriptomic analysis. Lancet Microbe 2021, 2, e300-10. [Google Scholar] [PubMed]
- Wang, Z.; Bafadhel, M.; Haldar, K.; Spivak, A.; Mayhew, D.; Miller, B.E.; Tal-Singer, R.; Johnston, S.L.; Ramsheh, M.Y.; Barer, M.R.; et al. Lung microbiome dynamics in COPD exacerbations. Eur. Respir. J. 2016, 47, 1082–1092. [Google Scholar] [PubMed]
- Tiew, P.Y.; Jaggi, T.K.; Chan, L.L.Y.; Chotirmall, S.H. The airway microbiome in COPD, bronchiectasis and bronchiectasis-COPD overlap. Clin. Respir. J. 2021, 15, 123–133. [Google Scholar]
- Flume, P.A.; Chalmers, J.D.; Olivier, K.N. Advances in bronchiectasis: Endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018, 392, 880–890. [Google Scholar]
- Mac Aogáin, M.; Chotirmall, S.H. Microbiology and the Microbiome in Bronchiectasis. Clin. Chest Med. 2022, 43, 23–34. [Google Scholar]
- Richardson, H.; Dicker, A.J.; Barclay, H.; Chalmers, J.D. The microbiome in bronchiectasis. Eur. Respir. Soc. Off. J. Eur. Respir. Soc. 2019, 28. [Google Scholar]
- Dicker, A.J.; Lonergan, M.; Keir, H.R.; Smith, A.H.; Pollock, J.; Finch, S.; Cassidy, A.J.; Huang, J.T.J.; Chalmers, J.D. The sputum microbiome and clinical outcomes in patients with bronchiectasis: A prospective observational study. Lancet Respir. Med. 2021, 9, 885–896. [Google Scholar]
- Lu, D.; Li, C.; Zhong, Z.; Abudouaini, M.; Amar, A.; Wu, H.; Wei, X. Changes in the airway microbiome in patients with bronchiectasis. Medicine 2023, 102, e36519. [Google Scholar]
- Grochowalska, A.; Kozioł-Montewka, M.; Sobieszczańska, A. Analysis of Acinetobacter baumannii resistance patterns in patients with chronic obstructive pulmonary disease (COPD) in terms of choice of effective empiric antibiotic therapy. Ann. Agric. Environ. Med. AAEM 2017, 24, 307–311. [Google Scholar]
- Clavo-Sánchez, A.J.; Girón-González, J.A.; López-Prieto, D.; Canueto-Quintero, J.; Sánchez-Porto, A.; Vergara-Campos, A.; Marín-Casanova, P.; Córdoba-Dona, J.A. Multivariate analysis of risk factors for infection due to penicillin-resistant and multidrug-resistant Streptococcus pneumoniae: A multicenter study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1997, 24, 1052–1059. [Google Scholar]
- Doern, G.V.; Brueggemann, A.B.; Pierce, G.; Hogan, T.; Holley, H.P.J.; Rauch, A. Prevalence of antimicrobial resistance among 723 outpatient clinical isolates of Moraxella catarrhalis in the United States in 1994 and 1995: Results of a 30-center national surveillance study. Antimicrob. Agents Chemother. 1996, 40, 2884–2886. [Google Scholar] [PubMed]
- Mussema, A.; Beyene, G.; Gashaw, M. Bacterial Isolates and Antibacterial Resistance Patterns in a Patient with Acute Exacerbation of Chronic Obstructive Pulmonary Disease in a Tertiary Teaching Hospital, Southwest Ethiopia. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 9709253. [Google Scholar]
- Nseir, S.; Ader, F.; Lubret, R.; Marquette, C.H. Pathophysiology of airway colonization in critically ill COPD patient. Curr. Drug Targets 2011, 12, 514–520. [Google Scholar]
- Qi, X.; Qu, H.; Yang, D.; Zhou, L.; He, Y.W.; Yu, Y.; Qu, J.; Liu, J. Lower respiratory tract microbial composition was diversified in Pseudomonas aeruginosa ventilator-associated pneumonia patients. Respir. Res. 2018, 19, 139. [Google Scholar]
| Author | Year | Study Type | Sample Size | Inclusion Criteria | Exclusion Criteria | Study Design | Outcomes |
|---|---|---|---|---|---|---|---|
| Ewig et al., Spain [22] | 2000 | Prospective cohort | 50 | Diagnosed with an exacerbation COPD | Clinical or radiographic evidence of bronchiectasis | TBAS, PSB, and BAL within 24 h of mechanical ventilation | PPM |
| Severe respiratory failure requiring mechanical ventilation | Infiltrates on chest radiograph | Sampling repeated after 72 h | Anti-microbial resistance patterns | ||||
| No hospital admission last 3 months before trial | Severe immunosuppression, malignancies, and coagulopathies | Paired blood serum samples | Clinical outcomes related to PPM | ||||
| No prior antimicrobial treatment within 4 weeks before admission | |||||||
| Huang et al., USA [23] | 2010 | Retrospective cohort | 8 | Diagnosed with an exacerbation COPD | Not specified | Retrospectively 16S rRNA analysis of BAL samples | Bacterial taxa in aspirate |
| Mechanically ventilated patients | Clinical outcome related to PPM | ||||||
| enrolled in parent study of Pseudomonas aeruginosa in intubated patients | |||||||
| Soler et al., Spain [24] | 1998 | Prospective cohort | 50 | Diagnosed with an exacerbation COPD | Clinical radiographic evidence of bronchiectasis | Pharyngeal swab, TBAS, BAL within 24 h of mechanical ventilation | PPM |
| Mechanical ventilation for hypercapnic respiratory failure | Severe immunosuppression, malignancies, and coagulopathies | Sampling repeated after 72 h | Clinical outcomes related to PPM | ||||
| No hospital admission last 3 months before trial | Paired blood serum samples | Anti-microbial resistance patterns | |||||
| No prior antimicrobial treatment within 4 weeks before admission | |||||||
| Tan et al., China [25] | 2014 | Prospective cohort | 53 | Acute exacerbation COPD requiring mechanical ventilation | Mild and moderate COPD | Dental plaque and TBAS on first day of admission to ICU | Comparison of bacterial species in plaques and tracheal aspirate |
| S. pneumoniae, P. aeruginosa or K. pneumoniae in tracheal aspirate. | Pregnancy or breast feeding | Pathogenic bacterial load | |||||
| Periodontal therapy or antibiotics in last 3 months prior to trial | |||||||
| Antibiotic treatment in last 3 months prior to trial | |||||||
| Ferrer et al., Spain [26] | 2005 | Prospective cohort | 137 | Clinical symptoms of exacerbation of COPD | Pneumonia and other causes of pulmonary infiltrates | Sputum of patients undergoing NIV within 24 h and after 3 days | PPM |
| Prior exacerbation of hospitalization in the previous 2 months | TBAS of intubated patients within 24 h and after 3 days | NIV success rate related to PPM | |||||
| Prior hospital stay longer than 24 h during current admission | |||||||
| Tracheotomy | |||||||
| Nseir et al., France [27] | 2006 | Prospective cohort | 857 | Acute exacerbation COPD requiring mechanical ventilation > 48 h | Hospitalization > 24 h prior to intubation | Tracheal aspirates at admission to ICU | MDR |
| Patients intubated > 24 h | Clinical outcomes related to MDR | ||||||
| Evidence of bronchiectasis | Risk factors for MDR bacteria |
| Author | PPMs (%) |
|---|---|
| Ewig et al., 2000 [22] | 56 |
| Soler et al., 1998 [24] | 72 |
| Nseir et al., 2006 [27] | 30 |
| Ferrer et al., 2005 [26] | 69 * |
| Prevalence of Pathogens Categorized Per Study (%) | |||||
|---|---|---|---|---|---|
| Bacterial Species | Ewig et al. [22] | Soler et al. [24] | Tan et al. * [25] | Ferrer et al. ** [26] | Nseir et al. [27] |
| Acetobacter europaeus | 2 | ||||
| Acinetobacter baumannii | 5 | 0 | 9 | ||
| Aggregatibacter actinomycetemcomitans | 3 | ||||
| Arabidopsis thaliana | 1 | ||||
| Bacillus subtilis | 1 | ||||
| Candida spp. | 6 | ||||
| Capnocytophaga sputigena | 6 | ||||
| Chlamydia pneumoniae | 13 | ||||
| Chlamydia psittaci | 2 | ||||
| Chryseobacterium meningosepticum | 3 | ||||
| Corynebacterium spp. | 3 | ||||
| Coxiella burnetii | 2 | ||||
| Enterobacter cloacae | 4 | 3 | 6 | 1 | |
| Enterococcus faecalis | 8 | ||||
| Escherichia coli | 2 | 0 | 4 | 1 | |
| Haemophilus influenzae | 23 | 17 | 3 | 21 | 17 |
| Klebsiella pneumoniae | 8 | 1 | |||
| Moraxella catarrhalis | 9 | 6 | 4 | 9 | |
| Morganella morganii | 0.9 | ||||
| Neisseria spp. | 2 | ||||
| Peptostreptococcus | 10 | ||||
| Porphyromonas gingivalis | 7 | ||||
| Proteus mirabilis | 2 | 2 | 4 | 3 | |
| Pseudomonas aeruginosa | 13 | 14 | 8 | 16 | 10 |
| Pseudomonas fluorescens | 4 | - | |||
| Saccharomyces cerevisiae | 2 | ||||
| Serratia marcescens | 2 | 2 | 3 | 2 | |
| Staphylococcus aureus | 3 | 2 | 9 | ||
| Methicillin resistant staphylococcus aureus (MRSA) | 4 | 6 | |||
| Staphylococcus epidermidis | 13 | ||||
| Stenotrophomonas maltophilia | 4 | 3 | 3 | ||
| Streptococcus group F | 2 | ||||
| Streptococcus mitis | 2 | ||||
| Streptococcus oralis | 14 | ||||
| Streptococcus pneumoniae | 9 | 6 | 12 | 16 | 20 |
| Streptococcus vividans | 20 | ||||
| Tannerella forsythis | 4 | ||||
| Treponema denticola | 6 | ||||
| Total bacteria isolated in tracheal samples | 53 | 64 | 289 | 51 | 304 |
| Author | PPM (%) | Study Group | Duration of Mechanical Ventilation (Days (SD)) | Duration of ICU Stay (Days (SD)) | Nosocomial Infection (%) | Mortality (%) |
|---|---|---|---|---|---|---|
| Ewig et al., 2000 [22] | 56 | PPM with appropriate antibiotics | 7.6 (7.6) | 9.4 (7.1) | 6 | 6 |
| PPM with inappropriate antibiotics | 6.4 (4.8) | 8.3 (4.9) | ||||
| Soler et al., 1998 [24] | 72 | With PPM | 7.4 (6.7) | 9.2 (6.7) | 6 | 6 |
| Without PPM | 9.6 (6.5) | 10.9 (6.5) | ||||
| Nseir et al., 2006 [27] | 30 | With PPM | 10 (11) | 15 (14) | 30 | |
| Without PPM | 7 (9) | 12 (11) | 24 | |||
| Ferrer et al., 2005 [26] | 69 * | IMV | 8.1 (7.2) | 10.1 (7.9) | 22 | 18 |
| NIV-failure | 2.2 (1.0) | 13.6 (10.7) | 41 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Bie, S.; Haaksma, M.E.; Vermin, B.; van Assema, H.; van Gorp, E.C.M.; Langerak, T.; Endeman, H.; Snijders, D.; van den Akker, J.P.C.; van Houten, M.A.; et al. A Systematic Review of the Pulmonary Microbiome in Patients with Acute Exacerbation COPD Requiring ICU Admission. J. Clin. Med. 2024, 13, 472. https://doi.org/10.3390/jcm13020472
van der Bie S, Haaksma ME, Vermin B, van Assema H, van Gorp ECM, Langerak T, Endeman H, Snijders D, van den Akker JPC, van Houten MA, et al. A Systematic Review of the Pulmonary Microbiome in Patients with Acute Exacerbation COPD Requiring ICU Admission. Journal of Clinical Medicine. 2024; 13(2):472. https://doi.org/10.3390/jcm13020472
Chicago/Turabian Stylevan der Bie, Sjoerd, Mark E. Haaksma, Ben Vermin, Hidde van Assema, Eric C. M. van Gorp, Thomas Langerak, Henrik Endeman, Dominic Snijders, Johannes P. C. van den Akker, Marlies A. van Houten, and et al. 2024. "A Systematic Review of the Pulmonary Microbiome in Patients with Acute Exacerbation COPD Requiring ICU Admission" Journal of Clinical Medicine 13, no. 2: 472. https://doi.org/10.3390/jcm13020472
APA Stylevan der Bie, S., Haaksma, M. E., Vermin, B., van Assema, H., van Gorp, E. C. M., Langerak, T., Endeman, H., Snijders, D., van den Akker, J. P. C., van Houten, M. A., van Lelyveld, S. F. L., & Goeijenbier, M. (2024). A Systematic Review of the Pulmonary Microbiome in Patients with Acute Exacerbation COPD Requiring ICU Admission. Journal of Clinical Medicine, 13(2), 472. https://doi.org/10.3390/jcm13020472




