Abstract
Back ground: Children with epilepsy are affected by several factors, including clinical and social variables. Among these variables, cognitive decline and behavioral disturbances, perceptions of stigma, and fatigue can lead to reductions in quality of life (QOL). Epileptic activities, including seizure severity, frequent seizures, and status epilepticus (SE), have been identified as important predictors of QOL. In addition, the frequency of interictal epileptiform discharges (IEDs) on electroencephalogram (EEG) may also be an important predictor of QOL, because IEDs can lead to cognitive decline and behavioral disturbances. Moreover, frequent seizures and/or IEDs may play a role in emotional mediators, such as stigma and fatigue, in childhood epilepsy. Seizure severity and/or IEDs are, therefore, important QOL-related factors in childhood epilepsy. Seizure severity as a QOL-related factor: Frontal lobe dysfunctions, such as cognitive decline and behavioral disturbances, can result in reduced QOL for both the child and their family. Frontal and prefrontal lobe growth disturbances can be present during active-phase epilepsy in some children with neuropsychological impairments. Recovery from prefrontal lobe growth disturbances may depend on the active seizure period. Children with a shorter active seizure period can recover from disturbances in prefrontal lobe growth more rapidly. In contrast, recovery may be delayed in children with a longer active seizure period. Moreover, frequent seizures can lead to seizure-associated headaches, perceptions of self-stigma and parental stigma, and fatigue. Accordingly, severe seizures can lead to neuropsychological impairments in association with prefrontal lobe growth disturbances in children with epilepsy. EEG abnormalities as QOL-related factors: IEDs on EEG, representing persistent pathological neuronal discharges, may be associated with several pathological aspects. Frontal IEDs can be a risk factor for recurrent seizures, cognitive decline, and behavioral disturbances, and they may also play a role as emotional mediators similar to stigma. In addition, behavioral disturbances may result in the presence of secondary bilateral synchrony (SBS) on EEG. Behavioral disturbances can be improved in association with a reduction in IEDs in children with frontal IEDs and SBS. Therefore, EEG abnormalities, such as frontal IEDs and SBS, can also lead to neuropsychological impairments in children with epilepsy. Therapeutic strategies in children with epilepsy: Seizure severity and IEDs on EEG may be associated with neuropsychological impairments, leading to QOL reduction. Therapeutic management may be desirable to reduce seizures and EEG abnormalities, such as frontal IEDs and SBS, as early as possible to improve QOL in children with epilepsy. During antiseizure medication (ASM) selection and adjustment, physicians should strategize the therapeutic approach to controlling seizures and suppressing EEG abnormalities in children with epilepsy. Among various ASMs, novel ASMs, such as levetiracetam and perampanel, may suppress both clinical seizures and IEDs on EEG; thus, these novel ASMs may represent an important addition to the treatments available for epileptic children presenting with frontal IEDs and SBS.
1. Introduction
Quality of life (QOL) in children with epilepsy seems to be influenced by various factors, including clinical and social variables. Among these variables, cognitive decline and behavioral disturbances, perceptions of stigma, and fatigue can lead to reductions in QOL. These disturbances may be associated with epileptic activities. Thus, seizure control is critical because of the improvement in QOL [1].
Among various epileptic activities, seizure severity, frequent seizures, and the presence of status epilepticus (SE) are regarded as the main QOL-related factors. Seizure clusters may be associated with higher seizure frequency, higher risk of treatment resistance, and lower likelihood of seizure remission, according to a systematic review [2]. In children with seizure clusters, QOL could be lower than that in children with isolated seizures [2]. Seizure clusters may also adversely affect the productivity of patients and their caregivers [2]. In addition, frequent seizures and SE can lead to neuropsychological impairments [3,4]. Accordingly, seizure severity may be associated with a reduction in QOL in children with epilepsy. On the other hand, electroencephalogram (EEG) findings, such as the frequency of interictal epileptiform discharges (IEDs), which are among the epileptic activities, may also be related to cognitive decline and behavioral disturbances [5]. Like frequent seizures, IEDs on EEG could also mediate emotional states, including stigma and fatigue [6]. These findings suggest that epileptic activities, including frequent seizures and the presence of SE and IEDs on EEG, may lead to reduced QOL in children with epilepsy.
On the other hand, stigma is regarded as an important QOL-related factor in epilepsy. The perception of stigma related to the epileptic condition may be very severe and is often under-recognized by clinicians [7]. Stigma has a marked negative impact, not only for children but also their families. Reducing perceptions of stigma is, therefore, necessary for the clinical management of childhood epilepsy.
In addition, fatigue is also associated with reduced QOL in patients with epilepsy. Fatigue may correlate with psychosocial factors, including anxiety, depression, and sleep problems [8]. In addition to these variables, epileptic activities may be associated with fatigue. A previous study showed that frequent seizures can lead to increased fatigue in epileptic patients [9]. Thus, fatigue may also relate to epileptic activities.
Neuropsychological impairments in children with epilepsy, such as cognitive decline, behavioral disturbances, perception of stigma, and fatigue, that relate to frontal lobe dysfunction can be caused by several factors. Frontal lobe lesions may lead to these disturbances in consonance with lesion location. However, children with no lesions can also present with these disturbances. A previous study showed a negative correlation between the frequency of IEDs on EEG and cognitive function in self-limited epilepsy with centrotemporal spike (SeLECTS) [10]. These disturbances may thus relate to epileptic activities and topography. The frontal lobes mature over a long period and so are easily damaged by various factors. Damage to the frontal regions during childhood interferes with maturational and organizational processes, which can lead to neuropsychological impairments [11]. Results from previous investigations have suggested that severe seizures, as reflected by certain statuses, such as frequent seizures and SE, can impair the developing brain [11,12,13]. In combination with these studies, seizure severity and IEDs on EEG may lead to cognitive, behavioral, and psychological disturbances. Epileptic activities, such as seizure severity and/or IEDs, are important QOL-related factors in childhood epilepsy.
We previously investigated the association between QOL-related factors and epileptic activities. The objective of this review was to assess the association between epileptic activities and QOL-related factors, focusing on cognitive and behavioral disturbances, stigma, and fatigue, according to our research findings. Based on these observations, therapeutic strategies in children with epilepsy are discussed in the following sections.
2. Can Seizure Severity Lead to Reduced QOL in Childhood Epilepsy?
2.1. Seizure Severity and Cognitive/Behavioral Disturbances: Are They Related?
Neuropsychological impairments, such as cognitive decline and behavioral disturbances, reduce QOL in childhood epilepsy and may be induced by frontal lobe dysfunction. In addition, QOL reduction in children can also reduce QOL in the family. Thus, frontal lobe dysfunction can result in reduced QOL for both the child and their family.
The frontal lobes are the largest cortical regions of the brain, comprising approximately 40% of the cerebral cortex. Among these regions, the prefrontal regions involve wide networks [14]. Because of these connections, the prefrontal cortex can receive abundant information from all parts of the cerebrum and can affect information processing in those parts. Prefrontal lobe neurons and glial cells are readily influenced by various factors, so prefrontal lobe functions are regarded as being vulnerable for a long period [15]. Accordingly, severe seizures, such as frequent or prolonged seizures, result in negative effects on prefrontal lobe functions more easily than on other cortical regions [14,15]. Considering these findings, epilepsy associated with prefrontal regions in children may be associated with several neuropsychological impairments in comparison with healthy subjects [3].
In other cerebral regions, seizures can result in memory and learning disturbances, which relate to temporal lobe dysfunctions [16]. Temporal lobe seizures can also lead to behavioral disturbances [17]. However, direct relationships between seizures and temporal or other cerebral lobe functioning have not been fully revealed. Further research is needed to discuss these aspects.
2.1.1. Prefrontal Lobe Growth in Frontal Lobe Epilepsy
Understanding how frontal lobe epilepsy (FLE) impacts the life of patients is important. In a serial three-dimensional (3D) magnetic resonance imaging (MRI) volumetric study, the growth of the frontal and prefrontal lobes in children with drug-responsive FLE without neuropsychological impairments was similar to that in healthy subjects [3]. In contrast, frontal and prefrontal lobe growth disturbances were present during the active epileptic phase in refractory FLE patients with cognitive decline and behavioral disturbances. However, a difference associated with the active seizure period was present. A short active seizure period was associated with prompt growth recovery. In children with a longer active seizure period, the growth disturbance was more severe, and growth recovery took longer [3] (Table 1). Frequent seizures in children with FLE may thus induce prefrontal lobe growth disturbances, which can lead to neuropsychological impairments.

Table 1.
Associations between seizure severity and prefrontal lobe growth disturbances.
2.1.2. Prefrontal Lobe Growth in SeLECTS
SeLECTS is considered a condition free of neurological and psychological impairments. However, children with SeLECTS sometimes present with severe aggravation of epileptic manifestations, cognitive decline, and behavioral disturbances [18]. Frontal and prefrontal lobe volumes and, in particular, the prefrontal-to-frontal lobe volume ratio showed growth disturbances during the active seizure period in patients presenting with atypical evolution [14]. However, differences associated with the active seizure period were present. The active seizure period was shorter in patients with prompt growth recovery. In patients with a longer active seizure period, growth disturbances were more severe, and, again, growth recovery took longer in these patients [14] (Table 1). Seizure severity in SeLECTS may also be associated with prefrontal lobe growth disturbances, again leading to neuropsychological impairments.
2.1.3. Prefrontal Lobe Growth in Self-Limited Epilepsy with Autonomic Seizures
Self-limited epilepsy with autonomic seizures (SeLEAS), which represents Panayiotopoulos syndrome, is generally accepted as lacking neuropsychological impairments. However, cognitive decline and behavioral disturbances may be present in at least some children with SeLEAS. SE can induce cerebral damage to various degrees. In SeLEAS patients, seizures tend to be prolonged, with subsequent focal or focal-to-bilateral tonic–clonic SE [4]. A sequential study using 3D–MRI volumetry showed that frontal and prefrontal lobe growth disturbances were present after episodes of SE in SeLEAS patients presenting with behavioral disturbances. In a patient with only one episode of SE, growth disturbances soon recovered. Conversely, the recovery of growth ratios was delayed in patients with several episodes of SE [4] (Table 1). Moreover, the cognitive scores, as measured using the Wechsler intelligence scale for children, dropped after the SE episodes [4]. The presence of SE in children with SeLEAS may thus induce growth disturbances in the prefrontal lobe, which can lead to neuropsychological impairments.
2.2. QOL-Related Factors: Headache
Epilepsy and migraine are part of a heterogeneous family of neurological disorders [19]. The prevalence of headache is extremely high, so concomitant headache can be present in many patients with epilepsy. Approximately 35% of epileptic children experienced headaches in association with seizures in our previous study [20]. The frequencies of seizures in children with and without seizure-associated headache were 4.1 and 1.3 times per year, respectively [20] (Table 2). Thus, seizure recurrence can induce headaches in association with seizures, which leads to reduced QOL in children with epilepsy.

Table 2.
Associations between seizure severity and QOL-related factors.
2.3. QOL-Related Factors: Fatigue
Fatigue has a negative impact on QOL in patients with various chronic diseases, including epilepsy [21,22,23]. Our previous study showed that the mean Fatigue Severity Scale scores in epileptic children were significantly higher than those in non-epileptic children [24]. The frequency of seizures was identified as the only significant clinical manifestation associated with fatigue using multiple linear regression analysis. Moreover, children with frequent seizures presented with more severe fatigue [24] (Table 2). Accordingly, frequent seizures can lead to the presence of fatigue in children with epilepsy.
2.4. QOL-Related Factors: Perception of Stigma among Children
The perception of stigma among epileptic patients is a negative psychological issue associated with a reduction in QOL. Stigma may be considered one of the psychological factors that affect QOL, along with seizure factors. Stigma has a negative effect on self-esteem and social status, thus leading to a poor prognosis, including isolation and delayed initiation of treatment for epileptic patients [25]. Frequent seizures could lead to psychosocial impairments in children. Our previous study, which used the Child Stigma Scale, showed that the perceptions of stigma in association with seizure frequency were severe (Table 2) [26]. Thus, stigma has negative effects on social identity in children with epilepsy who experience frequent seizures.
2.5. QOL-Related Factors: Perception of Stigma among Parents
Epilepsy in children can be a risk factor for stress in their parents [27,28,29,30]. Parents of children with intractable epilepsy tend to experience severe anxiety in relation to recurrent seizures, and this parental state of anxiety can lead to poor adaptive function in children [31]. Frequent seizures are, therefore, an important issue with respect to parenting stress [32]. The parents of children with epilepsy showed higher scores on the Parent Stigma Scale than the parents of healthy children [33]. Moreover, greater perceptions of stigma among parents correlated with higher seizure frequency [33] (Table 2). Accordingly, frequent seizures in children with epilepsy can lead to greater perceptions of stigma among parents.
However, the relationship between seizures and stigma has only been analyzed individually without simultaneously considering other biopsychosocial factors; therefore, there may be limitations in the understanding of this relationship.
3. Can EEG Abnormalities Lead to QOL Reduction in Children with Epilepsy?
3.1. Association between IEDs on EEG and Seizure Recurrence
EEG abnormalities, such as IEDs, can be conceptualized as pathological neuronal discharges [34]. This can lead to the fact that IEDs on EEG are associated with seizure recurrence. In SeLECTS, recurrent seizures and prolonged periods of frequent IEDs were correlated [35]. This finding suggests that the occurrence of frequent IEDs and the prolongation of this state may lead to recurrent seizures in SeLECTS. In addition, seizure recurrence may be associated with the location of EEG foci. Our study showed that frontal IEDs induced recurrent seizures after a first unprovoked seizure more often than other EEG foci [36] (Table 3). Thus, the frequency of IEDs and frontal IEDs may be associated with recurrent seizures.

Table 3.
Associations between QOL-related factors and IEDs on EEG.
3.2. Association between IEDs on EEG and Cognitive/Behavioral Disturbances
Cognitive decline and behavioral disturbances are seen more often in children with more severe EEG abnormalities [37,38,39]. Children can exhibit behavioral disturbances in association with IEDs on EEG without clinical seizures [5,40]. These disturbances may be associated with IEDs on EEG.
3.2.1. Association between IEDs and Cognitive/Behavioral Disturbances: SeLECTS
These disturbances were present in relation to a prolonged period of frequent IEDs in SeLECTS [41]. Moreover, these disturbances were also associated with a prolongation of the frontal EEG focus [41] (Table 3). Cognitive function can deteriorate in children with frequent IEDs. In addition, neuropsychological functioning can recover with the normalization of EEG findings [42]. Thus, neuropsychological impairments may be associated with IEDs on EEG in SeLECTS (Table 3). Tassinari et al. reported that the neurophysiological impairments associated with the negative myoclonus or inhibitory seizures seen in atypical SeLECTS suggest the involvement of the frontal cortex, either primarily or secondarily [43]. Moreover, frontal and prefrontal lobe growth disturbances persisted even after seizure disappearance in atypical SeLECTS [14]. These findings suggest that clinical features in atypical SeLECTS may be associated with frontal lobe dysfunctions, which can lead to neuropsychological impairments. In combination with these findings, frequent and frontal IEDs can lead to cognitive decline and behavioral disturbances.
3.2.2. Association between Frontal IEDs and Behavioral Disturbances: ADHD
Frontal IEDs are considered to show various pathogeneses. In children with attention deficit hyperactivity disorder (ADHD) presenting with IEDs, the frequency of IEDs was significantly correlated with the ADHD rating scale (ADHD—RS) score in the frontal IED group, but not in the Rolandic discharge (RD) group [44] (Table 3). In addition, the same study also showed that reductions in IED frequency were significantly correlated with ADHD—RS score reductions in the frontal IED group treated with antiseizure medications (ASMs), but not in the RD group [44] (Table 3). These findings suggest that frontal IEDs can exacerbate behavioral disturbances in children with ADHD.
3.2.3. Association between Frontal IEDs and Behavioral Disturbances: ASD
IEDs in children with autism spectrum disorder (ASD) are frequently located in the frontal region [45]. ASD children with epilepsy with frontal IEDs presented behavioral improvement (reductions in their score according to the Japanese manuals for the Aberrant Behavior Checklist (ABC-J)) in association with EEG improvement (reductions in IED frequency) after ASM treatment [46] (Table 3). This finding suggests that behavioral disturbances can be associated with the frequency of IEDs on EEG and that ASM treatment can lead to both reduced IED frequency and reduced behavioral problems in ASD children with epilepsy with frontal IEDs [46]. Thus, frontal IEDs can lead to an exacerbation of behavioral disturbances in ASD children.
3.2.4. Association between SBS and Behavioral Disturbances: EECSWS
Secondary bilateral synchrony (SBS) on EEG may be associated with cognitive decline and behavioral disturbances. The majority of children with epileptic encephalopathy with continuous spikes and waves during slow sleep (EECSWS), as a representative epileptic syndrome of SBS, present neuropsychological impairments. Our previous study showed that the volumes of frontal and prefrontal lobes were smaller in EECSWS children compared with those in healthy children [47] (Table 3). Moreover, prefrontal lobe growth disturbances were prolonged in children with longer CSWS periods in comparison with those with shorter CSWS periods [47] (Table 3). These findings suggest that persistent, severe abnormalities on EEG may induce prefrontal lobe growth disturbances, which can lead to neuropsychological impairments.
From a therapeutic perspective, the association between the reduction in IED frequency and behavioral improvement after ASM treatment was evaluated in epileptic patients presenting with SBS [48]. Scores for behavioral disturbances measured using the ABC-J were decreased in both EEG responders and EEG non-responders following ASM treatment. Moreover, reductions in ABC-J scores were significantly better in EEG responders than in non-responders. These findings suggest that EEG findings, such as SBS, can lead to behavioral disturbances and that behavioral improvements may be achievable in association with EEG improvement [48] (Table 3). Thus, SBS can lead to behavioral disturbances.
3.3. Association between EEG Abnormalities and Stigma
The emotional state can be influenced by various epileptic activities, including EEG abnormalities. A previous study showed that frontal EEG abnormalities might affect neuropsychological functions [49]. Our previous study indicated an association between EEG abnormalities and perceived stigma [50]. Children with frontal IEDs had higher scores on the Child Stigma Scale than those with IEDs in other regions. This finding suggests that frontal IEDs may be associated with a greater perception of stigma. Thus, frontal IEDs may play the role of emotional mediator in certain factors, such as stigma [50] (Table 3). Prefrontal lobe functions are regarded as being vulnerable for a long period. Thus, frontal IEDs may affect frontal lobe functions, such as neuropsychological states, including stigma. However, the mechanisms underlying the association between frontal IEDs and neuropsychological function are unclear. In addition, the relationship between IEDs on EEG and stigma has only been analyzed individually, without simultaneously considering other biopsychosocial factors. Therefore, the relationships among these factors remain unclear.
4. How Do We Manage the Treatment of Epilepsy in Children?
4.1. Is the Urgent Suppression of Clinical Seizures Needed?
As mentioned above, the presence of frequent seizures and SE can induce growth disturbances in the prefrontal lobe, leading to neuropsychological impairments [3,4] (Figure 1). In addition, recovery from prefrontal lobe growth disturbances may depend on the active seizure period. In several epileptic syndromes, children with a shorter active seizure period can recover from disturbances in prefrontal lobe growth more rapidly. However, such recovery may be delayed in children with a longer active seizure period [3]. These findings support the hypothesized relationship between seizure activities and behavioral disturbances, i.e., “seizure activity per se disrupts behavior”, as suggested by Austin et al. [51]. In addition, SE in children can lead to prefrontal lobe growth disruption. In our volumetric study, more frequent SE episodes led to poorer outcomes [4]. Accordingly, SE can lead to neuropsychological impairments in association with prefrontal lobe growth disruptions (Figure 1). Another study indicated that damage to the frontal regions during childhood can cause deteriorations in neurobehavioral development [52].
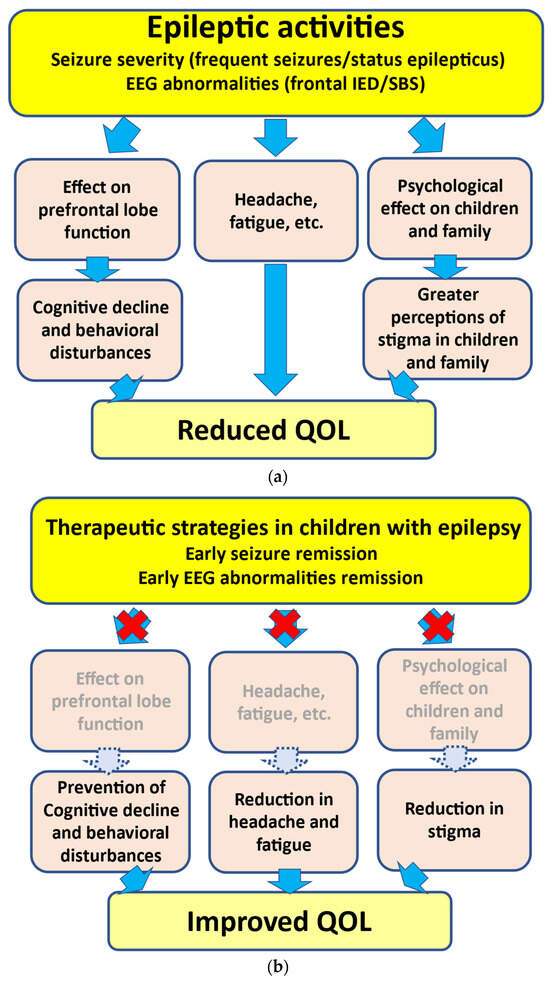
Figure 1.
Associations between epileptic activities and reduction in QOL. (a) Epileptic activities including severe seizures (such as frequent seizures and SE) and EEG abnormalities (such as frontal IED and SBS) can lead to neuropsychological impairments. (b) Early seizure and EEG abnormality remission can lead to the prevention of cognitive decline/behavioral disturbances and a reduction in headache, fatigue, and stigma, thus resulting in an improvement in QOL. EEG, electroencephalogram; IED, interictal epileptiform discharge; SBS, secondary bilateral synchrony; QOL, quality of life.
Based on these findings, the therapeutic strategy for childhood epilepsy may require seizure remission as soon as possible to prevent neuropsychological impairments.
4.2. Is the Urgent Suppression of IEDs on EEG Needed?
As inferred from various studies, frequent IEDs and frontal IEDs can lead to neuropsychological impairments [41] (Figure 1). Reductions in IEDs on EEG may be related to behavioral improvements in ADHD/ASD children with frontal IEDs with or without clinical seizures [44,46,53]. Accordingly, frontal IEDs can lead to neurodevelopmental deterioration in ADHD/ASD, and ASM treatment may be effective for both IED reduction and behavioral improvement in children with ADHD/ASD with frontal IEDs.
With respect to EECSWS-related neurodevelopmental deterioration, previous studies have underlined the parallel course of EECSWS and neuropsychological impairments [54]. Neuropsychological impairments may appear concurrently with EEG abnormalities [55] (Figure 1). Moreover, these impairments may improve concurrently with the disappearance of EEG abnormalities rather than clinical seizures. In children with SBS, behavioral improvements can be associated with EEG improvement [48]. These findings suggest that the active phase of “epilepsy”, not only “clinical seizures”, can be a prognostic factor, and the urgent suppression of IEDs, such as SBS, may thus be warranted to prevent neurodevelopmental deterioration in children presenting with SBS [56].
4.3. Therapeutic Strategies in Children with Epilepsy
Based on these findings, the urgent suppression of clinical seizures and EEG abnormalities, such as frontal IEDs and SBS, may be required to prevent neuropsychological impairments. During ASM selection and adjustment, physicians should strategize the therapeutic approach to controlling seizures and suppressing EEG abnormalities in children with epilepsy. Among the various ASMs, novel ASMs, such as levetiracetam and perampanel, may suppress both clinical seizures and IEDs on EEG [48,57,58,59]. These novel ASMs may represent an important addition to the treatments available for epileptic children presenting with frontal IEDs and SBS. However, it remains unclear whether seizure presence or remission are in any way related to environmental factors.
5. Future Perspectives
As observed in these studies, epileptic activities, including seizure severity, frequent seizures, and SE and EEG abnormalities such as frontal IEDs and SBS, can lead to reduced QOL in children with epilepsy. Better control of seizures and EEG abnormality remission may improve QOL in children with epilepsy. However, caution must be taken when generalizing the results. The early seizure and EEG abnormality remission can lead to improvements in behavioral impairments. However, improvements or reductions in other aspects, such as headache, fatigue, and stigma, using ASM treatment have not yet been identified. Studies with a larger sample size are needed to evaluate the correlation between seizure/EEG abnormality remission and improvements in these aspects in children with epilepsy. In addition, the relationship between seizures/IEDs on EEG and stigma has only been analyzed individually, without considering other biopsychosocial factors at the same time. Moreover, the mechanisms of the association between frontal IEDs and neuropsychological functioning are unclear. Therefore, the understanding of these relationships may be limited. Furthermore, there are various factors (i.e., environmental, socioeconomical, and educational factors) other than stigma that affect QOL, along with other clinical factors. However, the associations between these factors and epileptic activities have not been fully investigated. It remains unclear whether seizure presence or remission is in any way related to environmental factors. Further research is needed to clarify these aspects.
6. Conclusions
QOL-related factors, such as cognitive decline and behavioral disturbances, perceptions of stigma, and fatigue, are associated with epileptic activities. These disturbances are not always evident in children with epilepsy. However, severe seizures, such as frequent seizures and SE, and some forms of IEDs can lead to neuropsychological impairments. To prevent these impairments, physicians should focus on controlling seizures and suppressing EEG abnormalities as early as possible. Based on the findings from various studies, therapeutic management may be desirable to achieve seizure remission and EEG abnormalities, such as frontal IED and SBS, as early as possible to improve QOL in children with epilepsy. Among the various ASMs, novel ASMs, such as levetiracetam and perampanel, may suppress both clinical seizures and IEDs on EEG; thus, these novel ASMs may represent an important addition to the treatments available for epileptic children presenting with frontal IEDs and SBS.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
Hideaki Kanemura has received speaking fees from Eisai Co., Ltd.
References
- Terra, V.C.; Furlanetti, L.L.; Nunes, A.A.; Thome, U.; Nishiyama, M.A.; Sakamoto, A.C.; Machado, H.R. Vagus nerve stimulation in pediatric patients: Is it really worthwhile? Epilepsy Behav. 2014, 31, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Szaflarski, J.P.; Choi, E.J.; Wilson, J.C.; Kharawara, S.; Kauer, G.; Hirsch, L.J. A systematic review of seizure clusters: Prevalence, risk factors, burden of disease and treatment patterns. Epilepsy Res. 2021, 177, 106748. [Google Scholar] [CrossRef] [PubMed]
- Kanemura, H.; Sano, F.; Tando, T.; Sugita, K.; Aihara, M. Repeated seizures induce prefrontal growth disturbance in frontal lobe epilepsy. Brain Dev. 2012, 34, 175–180. [Google Scholar] [CrossRef]
- Kanemura, H.; Sano, F.; Ohyama, T.; Aoyagi, K.; Sugita, K.; Aihara, M. Sequential prefrontal lobe volume changes and cognitive dysfunctions in children with Panayiotopoulos syndrome presenting with status epilepticus. Epilepsy Res. 2015, 112, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Binnie, C.D. Cognitive impairment during epileptiform discharges: Is it ever justifiable to treat the EEG? Lancet Neurol. 2003, 2, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, N.; Ozkara, C.; Unsal, P.; Canbeyli, R. A comparative study of health related quality of life, psychological well-being, impact of illness and stigma in epilepsy and migraine. Seizure 2011, 20, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Espinola-Nadurille, M.; Crail-Melendez, D.; Sanchez-Guzman, M.A. Stigma experience of people with epilepsy in Mexico and views of health care providers. Epilepsy Behav. 2014, 32, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, S.; Kahane, P.; Vercueil, L. Fatigue in epilepsy: A prospective inter-ictal and post-ictal survey. Epilepsy Res. 2010, 91, 153–160. [Google Scholar] [CrossRef]
- Hernandez-Ronquillo, L.; Moien-Afshari, F.; Knox, K.; Britz, J.; TellezZenteno, J.F. How to measure fatigue in epilepsy? The validation of three scales for clinical use. Epilepsy Res. 2011, 95, 119–129. [Google Scholar] [CrossRef]
- Weglage, J.; Demsky, A.; Pietsch, M.; Kurlemann, G. Neuropsychological, intellectual, and behavioral findings in patients with centrotemporal spikes with and without seizures. Dev. Med. Child Neurol. 1997, 39, 646–651. [Google Scholar] [CrossRef]
- Hoare, P. The development of psychiatric disorder among school children with epilepsy. Dev. Med. Child Neurol. 1984, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.K.; Risinger, M.W.; Beckett, L.A. Correlates of behavior problems in children with epilepsy. Epilepsia 1992, 33, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.L.; Ben-Ari, Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr. Res. 2001, 49, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Kanemura, H.; Hata, S.; Aoyagi, K.; Sugita, K.; Aihara, M. Serial changes of prefrontal lobe growth in the patients with benign childhood epilepsy with centrotemporal spikes presenting with cognitive impairments/behavioral problems. Brain Dev. 2011, 33, 106–113. [Google Scholar] [CrossRef]
- Sinclair, D.B.; Wheatley, M.; Snyder, T. Frontal lobe epilepsy in childhood. Pediatr. Neurol. 2004, 30, 169–176. [Google Scholar] [CrossRef]
- Williamson, P.D.; Spencer, D.D.; Spencer, S.S.; Novelly, R.A.; Mattson, R.H. Complex partial seizures of frontal lobe origin. Ann. Neurol. 1985, 18, 497–504. [Google Scholar] [CrossRef]
- Engel, J., Jr.; Williamson, P.D.; Wieser, H.G. Mesial temporal lobe epilepsy with hippocampal sclerosis. In Epilepsy. A Comprehensive Textbook, 2nd ed.; Engel, J., Pedley, T., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 2479–2486. [Google Scholar]
- Fejerman, N.; Caraballo, R.H. Early-onset benign childhood occipital epilepsy (Panayiotopoulos type). In Benign Focal Epilepsies in Infancy, Childhood and Adolescence; Fejerman, N., Caraballo, R.H., Eds.; John Libbey Eurotext: Montrouge, France, 2007; pp. 115–144. [Google Scholar]
- Andermann, F. Clinical features of migraine–epilepsy syndrome. In Migraine and Epilepsy; Andermann, F., Lugaresi, E., Eds.; Butterworths: Boston, MA, USA, 1987; pp. 3–30. [Google Scholar]
- Kanemura, H.; Sano, F.; Ishii, S.; Ohyama, T.; Sugita, K.; Aihara, M. Characteristics of headache in children with epilepsy. Seizure 2013, 22, 647–650. [Google Scholar] [CrossRef][Green Version]
- Vercoulen, J.H.; Hommes, O.R.; Swanink, C.M.; Jongen, P.J.; Fennis, J.F.; Galama, J.M.; van der Meer, J.W.M.; Bleijenberg, G. The measurement of fatigue in patients with multiple sclerosis. A multidimensional comparison with chronic fatigue syndrome and healthy subjects. Arch. Neurol. 1996, 53, 642–649. [Google Scholar] [CrossRef]
- Dittner, A.J.; Wessely, S.C.; Brown, R.G. The assessment of fatigue: A practical guide for clinicians and researchers. J. Psychosom. Res. 2004, 56, 157–170. [Google Scholar] [CrossRef]
- Christensen, D.; Johnsen, S.P.; Watt, T.; Harder, I.; Kirkevold, M.; Andersen, G. Dimensions of post-stroke fatigue: A two-year follow-up study. Cerebrovasc. Dis. 2008, 26, 134–141. [Google Scholar] [CrossRef]
- Kanemura, H.; Sano, F.; Ohyama, T.; Sugita, K.; Aihara, M. Association between seizure frequency and fatigue levels in children with epilepsy. J. Paediatr. Child Health 2018, 54, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, A.; Baker, G.; Smith, D.; Dewey, M.; Chadwick, D. Measuring the impact of epilepsy: The development of a novel scale. Epilepsy Res. 1993, 16, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kanemura, H.; Sano, F.; Sugita, K.; Aihara, M. Presence of monthly seizures affects perceived stigma in children with epilepsy. J. Pediatr. Epilepsy 2014, 3, 85–92. [Google Scholar]
- Rodenburg, R.; Meijer, A.M.; Dekovic, M.; Aldenkamp, A.P. Parents of children with enduring epilepsy: Predictions of parenting stress and parenting. Epilepsy Behav. 2007, 11, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.C. The impact of a new pediatric epilepsy diagnosis on parents: Parenting stress and activity patterns. Epilepsy Behav. 2008, 13, 169–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiou, H.H.; Hsieh, L.P. Parenting stress in patients of children with epilepsy and asthma. J. Child Neurol. 2008, 23, 301–306. [Google Scholar] [CrossRef]
- Cushner-Weinstein, S.; Dassoulas, K.; Salpekar, J.A.; Henderson, S.E.; Pearl, P.L.; Gaillard, W.D.; Weinstein, S.L. Parenting stress and childhood epilepsy: The impact of depression, learning, and seizure-related factors. Epilepsy Behav. 2008, 13, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kerne, V.; Chapieski, L. Adaptive functioning in pediatric epilepsy: Contributions of seizure-related variables and parental anxiety. Epilepsy Behav. 2015, 43, 48–52. [Google Scholar] [CrossRef]
- Braams, O.; Meekes, J.; Braun, K.; Schappin, R.; van Rijen, P.C.; Hendriks, M.P.H.; Jennekens-Schinkel, A.; van Nieuwenhuizen, O. Parenting stress does not normalize after child’s epilepsy surgery. Epilepsy Behav. 2015, 42, 147–152. [Google Scholar] [CrossRef]
- Kanemura, H.; Sano, F.; Ohyama, T.; Sugita, K.; Aihara, M. Seizure severity in children with epilepsy is associated with their parents’ perception of stigma. Epilepsy Behav. 2016, 63, 42–45. [Google Scholar] [CrossRef]
- Wolff, M.; Weiskopf, N.; Serra, E.; Preissl, H.; Birbaumer, N.; Kraegeloh-Mann, I. Benign partial epilepsy in childhood: Selective cognitive deficits are related to the location of focal spikes determined by combined EEG⁄MEG. Epilepsia 2005, 46, 1661–1667. [Google Scholar] [CrossRef]
- Kanemura, H.; Sano, F.; Ohyama, T.; Sugita, K.; Aihara, M. Sequential EEG characteristics may predict seizure recurrence in rolandic epilepsy. Seizure 2014, 23, 646–650. [Google Scholar] [CrossRef][Green Version]
- Kanemura, H.; Sano, F.; Ohyama, T.; Mizorogi, S.; Sugita, K.; Aihara, M. EEG characteristics predict subsequent epilepsy in children with their first unprovoked seizure. Epilepsy Res. 2015, 115, 58–62. [Google Scholar] [CrossRef]
- Massa, R.; de Saint-Martin, A.; Carcangiu, R.; Rudolf, G.; Seegmuller, C.; Kleitz, C.; Metz–Lutz, M.N.; Hirsch, E.; Marescaux, C. EEG criteria predictive of complicated evolution in idiopathic Rolandic epilepsy. Neurology 2001, 57, 1071–1079. [Google Scholar] [CrossRef]
- Verrotti, A.; Latini, G.; Trotta, D.; Giannuzzi, R.; Cutarella, R.; Morgeseet, G.; Chiarelli, F. Typical and atypical rolandic epilepsy in childhood: A follow-up study. Pediatr. Neurol. 2002, 26, 26–29. [Google Scholar] [CrossRef]
- Vinayan, K.P.; Biji, V.; Thomas, S.V. Educational problems with underlying neuropsychological impairment are common in children with benign epilepsy of childhood with centrotemporal spikes (BECTS). Seizure 2005, 14, 207–212. [Google Scholar] [CrossRef][Green Version]
- Ronen, G.M.; Richards, J.E.; Cunningham, C.; Secord, M. Can sodium valproate improve learning in children with epileptiform bursts but without clinical seizures? Dev. Med. Child Neurol. 2000, 42, 751–755. [Google Scholar] [CrossRef]
- Kanemura, H.; Sano, F.; Aoyagi, K.; Sugita, K.; Aihara, M. Do sequential EEG changes predict atypical clinical features in rolandic epilepsy? Dev. Med. Child Neurol. 2012, 54, 912–917. [Google Scholar] [CrossRef]
- Baglietto, M.G.; Battaglia, F.M.; Nobili, L.; Tortorelli, S.; De Negri, E.; Calevoet, M.G.; Veneselli, E. Neuropsychological disorders related to interictal epileptic discharges during sleep in benign epilepsy of childhood with centrotemporal or Rolandic spikes. Dev. Med. Child Neurol. 2001, 43, 407–412. [Google Scholar] [CrossRef]
- Tassinari, C.A.; Rubboli, G.; Shibasaki, H. Neurophysiology of positive and negative myoclonus. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 181–195. [Google Scholar] [CrossRef]
- Kanemura, H.; Sano, F.; Tando, T.; Hosaka, H.; Sugita, K.; Aihara, M. EEG improvements with antiepileptic drug treatment can show a high correlation with behavior recovery in children with ADHD. Epilepsy Behav. 2013, 27, 443–448. [Google Scholar] [CrossRef]
- Kanemura, H.; Sano, F.; Tando, T.; Sugita, K.; Aihara, M. Can EEG characteristics predict development of epilepsy in autistic children? Eur. J. Paediatr. Neurol. 2013, 17, 232–237. [Google Scholar] [CrossRef]
- Kanemura, H.; Sano, F.; Hoshino, H.; Aihara, M. Efficacy of perampanel in epilepsy patients with autism spectrum disorder. Epilepsy Res. 2021, 170, 106550. [Google Scholar] [CrossRef]
- Kanemura, H.; Aihara, M. Sequential prefrontal lobe volume changes in epileptic patients with continuous spikes and waves during slow sleep. In Epilepsy in Childen—Clinical and Social Aspects; Gadze, Z.P., Ed.; INTECH: Rijeka, Croatia, 2011; pp. 13–24. [Google Scholar]
- Kanemura, H.; Sano, F.; Hoshino, H.; Takayama, K.; Aihara, M. Effects of perampanel on secondary bilateral synchrony and behavioral problems in adolescents with epilepsy showing insufficient response with levetiracetam. Seizure 2020, 80, 131–137. [Google Scholar] [CrossRef]
- Coan, J.A.; Allen, J.J.B. The state and trait nature of frontal EEG asymmetry in emotion. In The Asymmetry Brain; Hugdahl, K., Davidson, R.J., Eds.; MIT Press: Cambridge, MA, USA, 2003; pp. 565–615. [Google Scholar]
- Kanemura, H.; Sano, F.; Ohyama, T.; Sugita, K.; Aihara, M. Correlation between perceived stigma and EEG paroxysmal abnormality in childhood epilepsy. Epilepsy Behav. 2015, 52, 44–48. [Google Scholar] [CrossRef]
- Austin, J.K.; Dunn, D.W.; Caffrey, H.M.; Perkins, S.M.; Harezlak, J.; Rose, D.F. Recurrent seizures and behavior problems in children with first recognized seizures: A prospective study. Epilepsia 2002, 43, 1564–1573. [Google Scholar] [CrossRef]
- Aihara, M.; Aoyagi, K.; Goldberg, E.; Nakazawa, S. Age shifts frontal cortical control in a cognitive bias task from right to left: Part I. Neuropsychological study. Brain Dev. 2003, 25, 555–559. [Google Scholar] [CrossRef]
- Kanemura, H.; Sano, F.; Ohyama, T.; Sugita, K.; Aihara, M. Effect of levetiracetam on behavioral problems in pervasive developmental disorder children with epilepsy. Eur. J. Paediatr. Neurol. 2014, 18, 482–488. [Google Scholar] [CrossRef]
- De Negri, M.; Baglietto, M.G.; Battaglia, F.M.; Gaggero, R.; Pessagno, A.; Recanati, L. Treatment of electrical status epilepticus by short diazepam (DZP) cycles after DZP rectal bolus test. Brain Dev. 1995, 17, 330–333. [Google Scholar] [CrossRef]
- Morikawa, T.; Seino, M.; Yagi, K. Long-term outcome of four children with continuous spike-waves during sleep. In Epileptic Syndromes in Infancy, Childhood and Adolescence, 2nd ed.; Roger, J., Bureau, M., Dravet, C.H., Dreifuss, F.E., Perret, A., Wolf, P., Eds.; John Libbey: London, UK, 1992; pp. 257–266. [Google Scholar]
- Kanemura, H.; Aihara, M. Neurobiological effects of CSWS on brain growth: A magnetic resonance imaging volumetric study. J. Pediatr. Epilepsy 2012, 1, 187–193. [Google Scholar]
- Stodieck, S.; Steinhoff, B.J.; Kolmsee, S.; Van Rijckevorsel, K. Effect of levetiracetam in patients with epilepsy and interictal epileptiform discharges. Seizure 2001, 10, 583–587. [Google Scholar] [CrossRef][Green Version]
- Kanemura, H.; Sano, F.; Ohyama, T.; Aihara, M. Efficacy of levetiracetam for reducing rolandic discharges in comparison with carbamazepine and valproate sodium in rolandic epilepsy. Seizure 2018, 62, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Ido, K.; Ohgoh, M.; Hanada, T. Mode of seizure inhibition by sodium channel blockers, an SV2A ligand, and an AMPA receptor antagonist in a rat amygdala kindling model. Epilepsy Res. 2019, 154, 42–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).