Clinical Efficacy of 5-Fluorouracil and Bleomycin in Dermatology
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Administration
5. Safety
5.1. Bleomycin
5.2. 5-FU
6. Hypertrophic Scar and Keloid
6.1. Efficacy
6.2. Dosage and Techniques
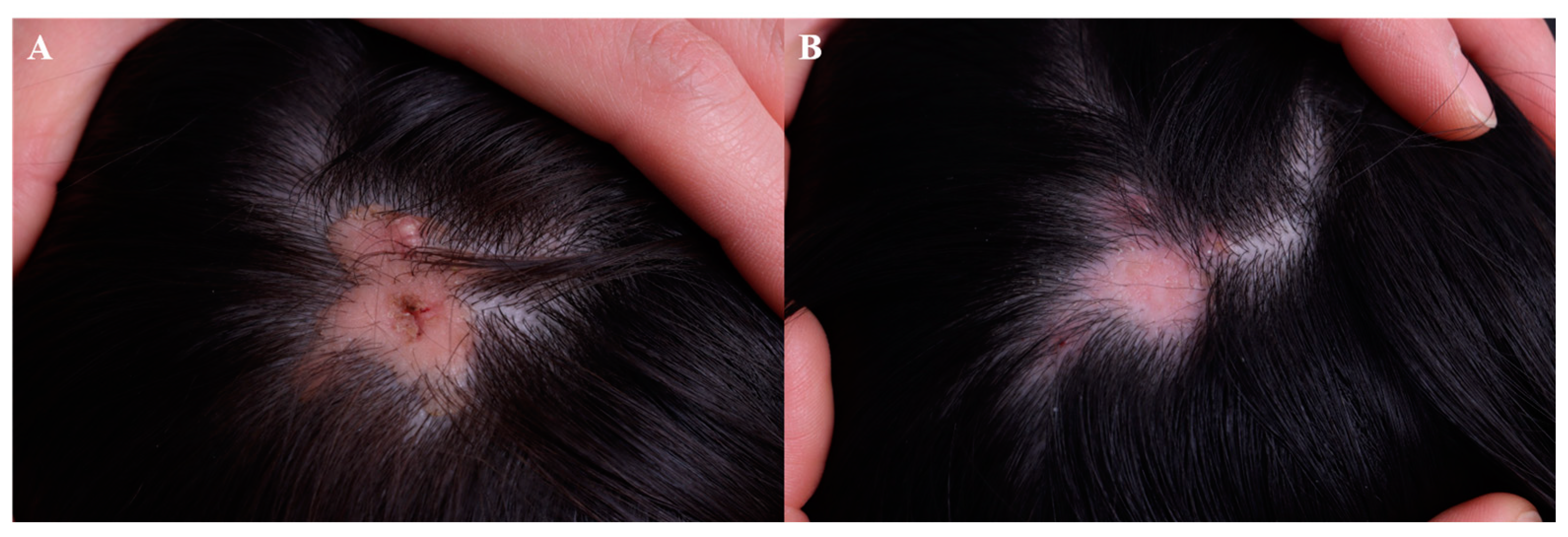
7. Wart
7.1. Efficacy
7.2. Dosages and Techniques
8. Skin Cancer
8.1. Efficacy
8.2. Actinic Keratosis
8.3. Basal Cell Carcinoma
8.4. Squamous Cell Carcinoma and Keratoacanthoma
8.5. Metastatic Melanoma
8.6. Dosages and Techniques
9. Vitiligo
9.1. Efficacy
9.2. Dosage and Techniques
10. Vascular Anomalies
10.1. Efficacy
10.2. Hemangioma
10.3. Vascular Malformation
10.4. Dosage and Techniques
11. Other Indications
12. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bik, L.; Sangers, T.; Greveling, K.; Prens, E.; Haedersdal, M.; van Doorn, M. Efficacy and tolerability of intralesional bleomycin in dermatology: A systematic review. J. Am. Acad. Dermatol. 2020, 83, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Saitta, P.; Krishnamurthy, K.; Brown, L.H. Bleomycin in Dermatology: A Review of Intralesional Applications. Dermatol. Surg. 2008, 34, 1299–1313. [Google Scholar] [CrossRef]
- Searle, T.B.; Al-Niaimi, F.M.; Ali, F.R. 5-Fluorouracil in Dermatology: The Diverse Uses Beyond Malignant and Premalignant Skin Disease. Dermatol. Surg. 2021, 47, e66–e70. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.Y. Clinical applications for topical 5-fluorouracil in the treatment of dermatological disorders. J. Dermatol. Treat. 2009, 20, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Watring, W.G.; Lagasse, L.D. Treatment of vulvar intraepithelial neoplasia (VIN) with local bleomycin. Cancer Clin. Trials 1980, 3, 351–354. [Google Scholar]
- Prince, G.T.; Cameron, M.C.; Fathi, R.; Alkousakis, T. Intralesional and Laser-Assisted 5-Fluorouracil in Dermatologic Disease: A Systematic Review. J. Drugs Dermatol. 2018, 17, 274–280. [Google Scholar]
- Barkat, M.T.; Abdel-Aziz, R.T.A.; Mohamed, M.S. Evaluation of intralesional injection of bleomycin in the treatment of plantar warts: Clinical and dermoscopic evaluation. Int. J. Dermatol. 2018, 57, 1533–1537. [Google Scholar] [CrossRef]
- Kim, W.I.; Kim, S.; Cho, S.W.; Cho, M.K. The efficacy of bleomycin for treating keloid and hypertrophic scar: A systematic review and meta-analysis. J. Cosmet. Dermatol. 2020, 19, 3357–3366. [Google Scholar] [CrossRef]
- Huang, L.; Wong, Y.; Cai, Y.; Lung, I.; Leung, C.; Burd, A. Low-dose 5-fluorouracil induces cell cycle G2 arrest and apoptosis in keloid fibroblasts. Br. J. Dermatol. 2010, 163, 1181–1185. [Google Scholar] [CrossRef]
- Hietanen, K.; Järvinen, T.; Huhtala, H.; Tolonen, T.; Kuokkanen, H.; Kaartinen, I. Treatment of keloid scars with intralesional triamcinolone and 5-fluorouracil injections—A randomized controlled trial. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 4–11. [Google Scholar] [CrossRef]
- Khalid, F.A.; Mehrose, M.Y.; Saleem, M.; Yousaf, M.A.; Mujahid, A.M.; Rehman, S.U.; Ahmad, S.; Tarar, M.N. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: Randomized control trial. Burns 2019, 45, 69–75. [Google Scholar] [CrossRef]
- Davison, S.P.; Dayan, J.H.; Clemens, M.W.; Sonni, S.; Wang, A.; Crane, A. Efficacy of Intralesional 5-Fluorouracil and Triamcinolone in the Treatment of Keloids. Aesthet. Surg. J. 2009, 29, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, N.; Mollaabasi, F.; Mansouri, P.; Robati, R.M. The Combined Application of Bleomycin and Triamcinolone for Treating Refractory Keloids. Dermatol. Surg. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Kabel, A.M.; Sabry, H.H.; Sorour, N.E.; Moharm, F.M. Comparative study between intralesional injection of bleomycin and 5-fluorouracil in the treatment of keloids and hypertrophic scars. J. Dermatol. Dermatol. Surg. 2016, 20, 32–38. [Google Scholar] [CrossRef]
- Dhar, S.; Rashid, M.; Islam, A.; Bhuiyan, M. Intralesional bleomycin in the treatment of cutaneous warts: A randomized clinical trial comparing it with cryotherapy. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Adalatkhah, H.; Khalilollahi, H.; Amini, N.; Sadeghi-Bazargani, H. Compared therapeutic efficacy between intralesional bleomycin and cryotherapy for common warts: A randomized clinical trial. Dermatol. Online J. 2007, 13, 4. [Google Scholar] [CrossRef]
- Lewis, T.G.; Nydorf, E.D. Intralesional bleomycin for warts: A review. J Drugs Dermatol. 2006, 5, 499–504. [Google Scholar] [PubMed]
- Hodeib, A.A.E.; Al-Sharkawy, B.G.; Hegab, D.S.; Talaat, R.A.Z. A comparative study of intralesional injection of Candida albicans antigen, bleomycin and 5-fluorouracil for treatment of plane warts. J. Dermatol. Treat. 2021, 32, 663–668. [Google Scholar] [CrossRef]
- Yazdanfar, A.; Farshchian, M.; Fereydoonnejad, M.; Farshchian, M. Treatment of common warts with an intralesional mixture of 5-fluorouracil, lidocaine, and epinephrine: A prospective placebo-controlled, double-blind randomized trial. Dermatol. Surg. 2008, 34, 656–659. [Google Scholar] [CrossRef]
- Isçimen, A.; Aydemir, E.H.; Göksügür, N.; Engin, B. Intralesional 5-fluorouracil, lidocaine and epinephrine mixture for the treatment of verrucae: A prospective placebo-controlled, single-blind randomized study. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 455–458. [Google Scholar] [CrossRef]
- Luk, N.M.; Tang, W.Y.M.; Tang, N.L.S.; Chan, S.W.; Wong, J.K.W.; Hon, K.L.E.; Lo, K.K. Topical 5-fluorouracil has no additional benefit in treating common warts with cryotherapy: A single-centre, double-blind, randomized, placebo-controlled trial. Clin. Exp. Dermatol. 2006, 31, 394–397. [Google Scholar] [CrossRef]
- Amer, M.; Diab, N.; Ramadan, A.; Galal, A.; Salem, A. Therapeutic evaluation for intralesional injection of bleomycin sulfate in 143 resistant warts. J. Am. Acad. Dermatol. 1988, 18, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, P.; Frietes-Martinez, A.; Gonzalez, S.; Spugnini, E.P.; Baldi, A. Successful treatment of plantar warts with intralesional bleomycin and electroporation: Pilot prospective study. Dermatol. Pract. Concept. 2017, 7, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Salk, R.S.; Grogan, K.A.; Chang, T.J. Topical 5% 5-fluorouracil cream in the treatment of plantar warts: A prospective, randomized, and controlled clinical study. J. Drugs Dermatol. 2006, 5, 418–424. [Google Scholar]
- IsËik, S.; Koca, R.; Sarici, G.; Altinyazar, H.C. A comparison of a 5% potassium hydroxide solution with a 5-fluorouracil and salicylic acid combination in the treatment of patients with anogenital warts: A randomized, open-label clinical trial. Int. J. Dermatol. 2014, 53, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Good, L.M.; Miller, M.D.; High, W.A. Intralesional agents in the management of cutaneous malignancy: A review. J. Am. Acad. Dermatol. 2011, 64, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Jorizzo, J.; Weiss, J.; Furst, K.; VandePol, C.; Levy, S.F. Effect of a 1-week treatment with 0.5% topical fluorouracil on occurrence of actinic keratosis after cryosurgery: A randomized, vehicle-controlled clinical trial. Arch. Dermatol. 2004, 140, 813–816. [Google Scholar] [CrossRef]
- Krawtchenko, N.; Roewert-Huber, J.; Ulrich, M.; Mann, I.; Sterry, W.; Stockfleth, E. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: A comparison of clinical and his-tological outcomes including 1-year follow-up. Br. J. Dermatol. 2007, 157 (Suppl. S2), 34–40. [Google Scholar] [CrossRef]
- Loven, K.; Stein, L.; Furst, K.; Levy, S. Evaluation of the efficacy and tolerability of 0.5% fluorouracil cream and 5% fluorouracil cream applied to each side of the face in patients with actinic keratosis. Clin. Ther. 2002, 24, 990–1000. [Google Scholar] [CrossRef]
- Miller, B.H.; Shavin, J.S.; Cognetta, A.; Taylor, J.; Salasche, S.; Korey, A.; Orenberg, E.K. Nonsurgical treatment of basal cell carcinomas with intralesional 5-fluorouracil/epinephrine injectable gel. J. Am. Acad. Dermatol. 1997, 36, 72–77. [Google Scholar] [CrossRef]
- Glass, L.F.; Jaroszeski, M.; Gilbert, R.; Reintgen, D.S.; Heller, R. Intralesional bleomycin-mediated electrochemotherapy in 20 patients with basal cell carcinoma. J. Am. Acad. Dermatol. 1997, 37, 596–599. [Google Scholar] [CrossRef]
- Rodríguez-Cuevas, S.; Barroso-Bravo, S.; Almanza-Estrada, J.; Cristóbal-Martínez, L.; González-Rodríguez, E. Electrochemotherapy in Primary and Metastatic Skin Tumors: Phase II Trial Using Intralesional Bleomycin. Arch. Med. Res. 2001, 32, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Pacola, P.R.; Rostey, R.R.L.; Rizzo, F.d.F.A. Chemotherapeutical treatment of basal cell carcinoma with bleomycin via microinfusion of the drug into the skin (MMP®). An. Bras. Dermatol. 2023, 98, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Gyurova, M.S.; Stancheva, M.Z.; Arnaudova, M.N.; Yankova, R.K. Intralesional bleomycin as alternative therapy in the treatment of multiple basal cell carcinomas. Dermatol. Online J. 2006, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Bargman, H.; Hochman, J. Topical treatment of Bowen’s disease with 5-Fluorouracil. J. Cutan. Med. Surg. 2003, 7, 101–105. [Google Scholar] [CrossRef]
- Salim, A.; Leman, J.; McColl, J.; Chapman, R.; Morton, C. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen’s disease. Br. J. Dermatol. 2003, 148, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Metterle, L.; Nelson, C.; Patel, N. Intralesional 5-fluorouracil (FU) as a treatment for nonmelanoma skin cancer (NMSC): A review. J. Am. Acad. Dermatol. 2016, 74, 552–557. [Google Scholar] [CrossRef]
- Maxfield, L.; Shah, M.; Schwartz, C.; Tanner, L.S.; Appel, J. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J. Am. Acad. Dermatol. 2021, 84, 1696–1697. [Google Scholar] [CrossRef]
- Luu, W.; McRae, M.Y. Intralesional 5-fluorouracil as a management for cutaneous squamous cell carcinomas: A rural Australian retrospective case series. Australas. J. Dermatol. 2023, 64, 556–559. [Google Scholar] [CrossRef]
- Morse, L.G.; Kendrick, C.; Hooper, D.; Ward, H.; Parry, E. Treatment of squamous cell carcinoma with intralesional 5-Fluorouracil. Dermatol. Surg. 2003, 29, 1150–1153; discussion 1153. [Google Scholar]
- Leonard, A.L.; Hanke, C.W. Treatment of giant keratoacanthoma with intralesional 5-fluorouracil. J. Drugs Dermatol. 2006, 5, 454–456. [Google Scholar]
- Byrne, C.M.; Thompson, J.F.; Johnston, H.; Hersey, P.; Quinn, M.J.; Michael Hughes, T.; McCarthy, W.H. Treatment of metastatic mel-anoma using electroporation therapy with bleomycin (electrochemotherapy). Melanoma Res. 2005, 15, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Gaudy, C.; Richard, M.-A.; Folchetti, G.; Bonerandi, J.J.; Grob, J.-J. Randomized Controlled Study of Electrochemotherapy in the Local Treatment of Skin Metastases of Melanoma. J. Cutan. Med. Surg. 2006, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Hamada, T. Topically Administered Fluorouracil in Vitiligo. Arch. Dermatol. 1983, 119, 722–727. [Google Scholar] [CrossRef]
- Adil, M.; Zahra, F.T.; Amin, S.S.; Mohtashim, M.; Bansal, R.; Khan, H.Q. Efficacy of topical 5% 5-fluorouracil with needling versus 5% 5-fluorouracil alone in stable vitiligo: A randomized controlled study. J. Cutan. Aesthet. Surg. 2020, 13, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.; Salah, M.; Samy, N.; Rabie, A.; Farrag, A.R. Effect of Topical 5-Fluorouracil Alone versus Its Combination with Erbium:YAG (2940 nm) Laser in Treatment of Vitiligo. Clin. Cosmet. Investig. Dermatol. 2020, 13, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zohdy, H.A.; Hussein, M.S. Intradermal injection of Fluorouracil versus triamcinolone in localized vitiligo treatment. J. Cosmet. Dermatol. 2019, 18, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Tiwari, P.; Sharma, S.; Kumar, R.; Singh, O. Role of intralesional bleomycin and intralesional triamcinolone therapy in residual haemangioma following propranolol. Int. J. Oral Maxillofac. Surg. 2018, 47, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Horbach, S.E.R.; Rigter, I.M.; Smitt, J.H.S.; Reekers, J.A.; Spuls, P.I.; van der Horst, C.M.A.M. Intralesional Bleomycin Injections for Vascular Malformations: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2016, 137, 244–256. [Google Scholar] [CrossRef]
- Lee, S.; Cho, S.H.; Lee, J.D.; Kim, H.S. Venous malformation of the glans penis successfully treated with intralesional bleomycin injection. Dermatol Ther. 2019, 32, e13083. [Google Scholar] [CrossRef]
- Lee, W.J.; Lee, S.M.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Moon, K.C. Efficacy of intralesional bleomycin for the treatment of plantar hard corns. Int. J. Dermatol. 2014, 53, e572–e577. [Google Scholar] [CrossRef]
- Figueroa, S.; Gennaro, A.R. Intralesional bleomycin injection in treatment of condyloma acuminatum. Dis. Colon Rectum 1980, 23, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Gharavi, N.; Diehl, J.; Soriano, T. Cutaneous Sarcoidosis Successfully Treated with Intralesional 5-Fluorouracil. Dermatol. Surg. 2015, 41, 1082–1085. [Google Scholar] [CrossRef]
- Dayan, S.H.; Arkins, J.P.; Brindise, R. Soft Tissue Fillers and Biofilms. Fac. Plast. Surg. 2011, 27, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bondi, E.E.; Clark, W.H., Jr.; Elder, D.; Guerry, D., 4th; Greene, M.H. Topical chemotherapy of dysplastic melanocytic nevi with 5% fluorouracil. Arch. Dermatol. 1981, 117, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Chang, M.W.; Shwayder, T. Topical tretinoin and 5-fluorouracil in the treatment of linear verrucous epidermal nevus. J. Am. Acad. Dermatol. 2000, 43, 129–132. [Google Scholar] [CrossRef]
- Nelson, B.R.; Kolansky, G.; Gillard, M.; Ratner, D.; Johnson, T.M. Management of linear verrucous epidermal nevus with topical 5-fluorouracil and tretinoin. J. Am. Acad. Dermatol. 1994, 30 Pt 1, 287–288. [Google Scholar] [CrossRef]
- Lindberg, M.R.; DiLorenzo, A.; DeSimone, J.A. Cutaneous T-Cell Lymphoma Treatment: Case Series of Combination Therapy with Intralesional Injections of 5-Fluorouracil and Topical Imiquimod. Cutis 2023, 111, E19–E25. [Google Scholar] [CrossRef]






| Indications | Mechanisms of Action | Bleomycin (Injection 0.5–1.0 U/mL) | 5-FU (Topical or Injection 50 mg/mL) | Recommended Interval |
|---|---|---|---|---|
| Hypertrophic scar | Induces apoptosis of keratinocytes and inhibits collagen synthesis | Intralesionally into the mid-dermis | 2–4 weeks | |
| Wart | Blocks the cell cycle and cleaves DNA in viruses | Intralesionally into the superficial dermis | 2–4 weeks | |
| Skin cancer | Blocks the cell cycle and disrupts DNA synthesis | For patients who are unable to undergo surgery | Once daily for topicals, spaced 1–2 weeks apart for injection | |
| Actinic keratosis | Scarce data | Topical 5-FU with cryotherapy | ||
| Basal cell carcinoma | 0.5 to 1.5 U/mL Bleomycin ECT is recommended | Topical 5-FU (limited to superficial BCC 2) | ||
| Squamous cell carcinoma | Scarce data | Intralesional 5-FU | ||
| Metastatic melanoma | 0.5 to 1.5 U/mL bleomycin ECT is recommended | Scarce data | ||
| Vitiligo | 5-FU: induces the proliferation of melanocytes in hair follicles | Scarce data | For patients with stable disease, topical agents following dermabrasion or intralesional injection | Once daily for topicals, space 2 weeks apart for injection |
| Vascular anomalies | Bleomycin: its sclerosing and antineoplastic effect, which induces apoptosis of the immature cells | 0.5 mg/mL 0.5–1 mg/kg (under 1 year) Maximum 15 mg (aged 1 and ove) | 2–4 weeks | |
| Hemangioma | For patients resistant to propranolol and vascular laser | Scarce data | ||
| Vascular malformation | For lymphatic and venous malformation | |||
| Other indications | Corn/Callus Condyloma acuminatum | Cutaneous sarcoidosis Refractory inflammatory nodule Dysplastic nevus Cutaneous T-cell lymphoma | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Woo, Y.R.; Cho, S.H.; Lee, J.D.; Kim, H.S. Clinical Efficacy of 5-Fluorouracil and Bleomycin in Dermatology. J. Clin. Med. 2024, 13, 335. https://doi.org/10.3390/jcm13020335
Kim S, Woo YR, Cho SH, Lee JD, Kim HS. Clinical Efficacy of 5-Fluorouracil and Bleomycin in Dermatology. Journal of Clinical Medicine. 2024; 13(2):335. https://doi.org/10.3390/jcm13020335
Chicago/Turabian StyleKim, Suyeon, Yu Ri Woo, Sang Hyun Cho, Jeong Deuk Lee, and Hei Sung Kim. 2024. "Clinical Efficacy of 5-Fluorouracil and Bleomycin in Dermatology" Journal of Clinical Medicine 13, no. 2: 335. https://doi.org/10.3390/jcm13020335
APA StyleKim, S., Woo, Y. R., Cho, S. H., Lee, J. D., & Kim, H. S. (2024). Clinical Efficacy of 5-Fluorouracil and Bleomycin in Dermatology. Journal of Clinical Medicine, 13(2), 335. https://doi.org/10.3390/jcm13020335







