A Comprehensive Clinical Review of Maxillary Sinus Floor Elevation in Patients with Well-Defined Faintly Radiopaque Lesions in the Antrum
Abstract
1. Introduction
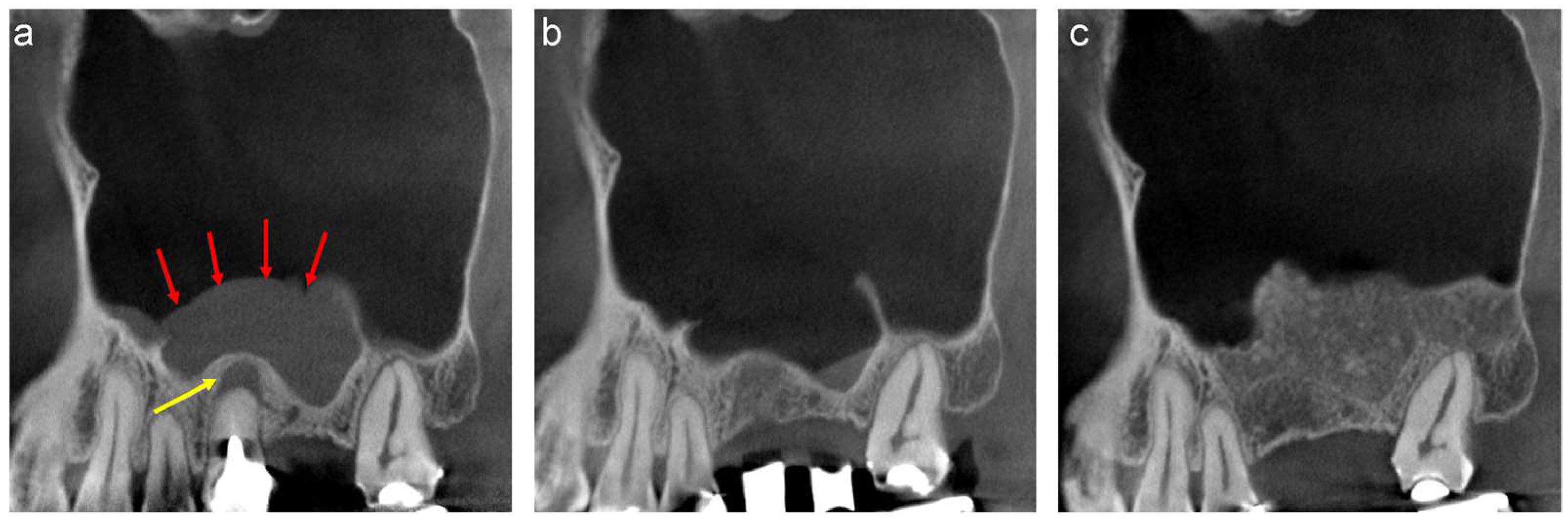
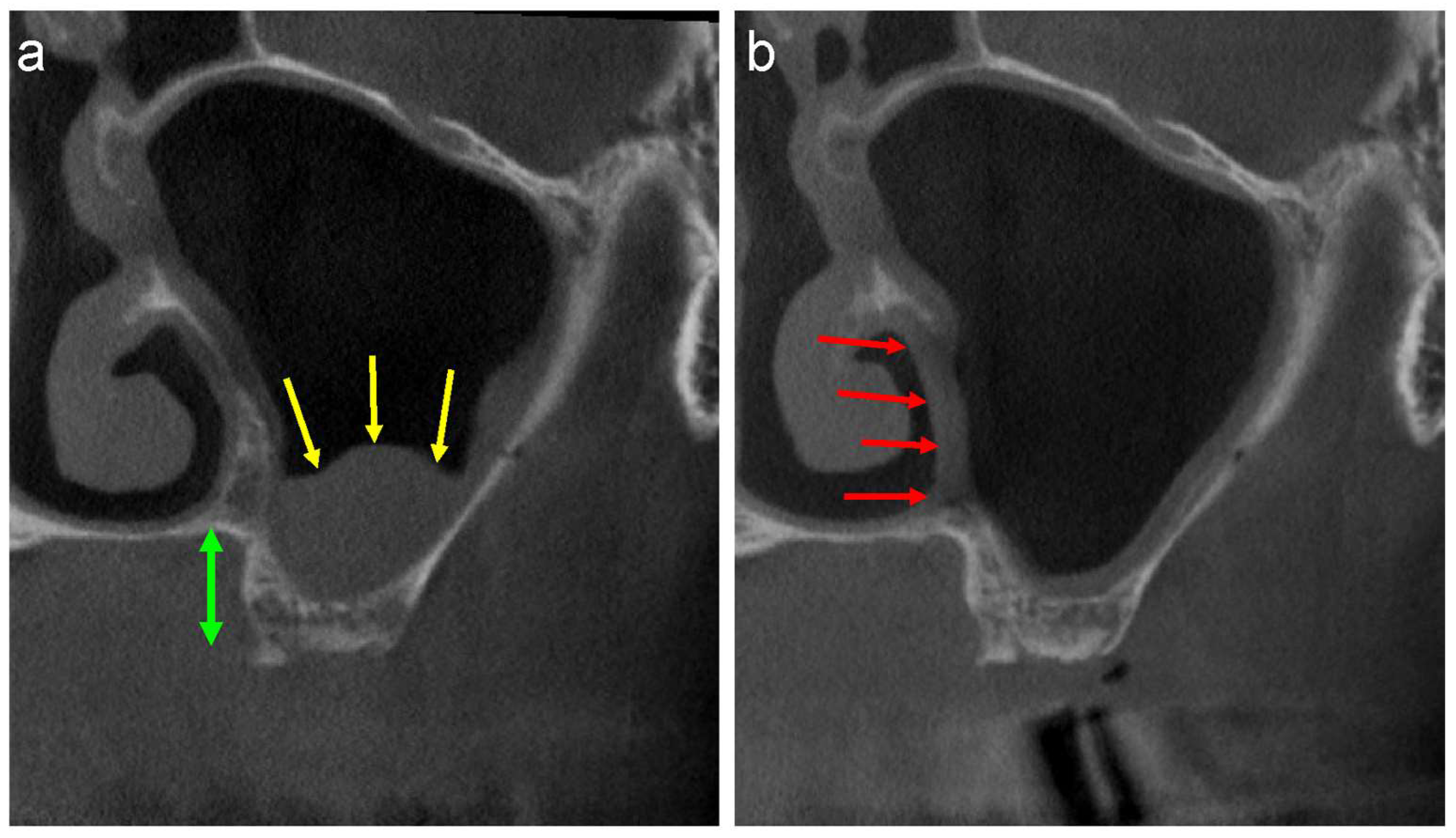
2. CT Evaluation
3. Differential Diagnoses of Pseudocyst
3.1. Periodontitis or Periapical Diseases
3.1.1. Case 1
3.1.2. Case 2
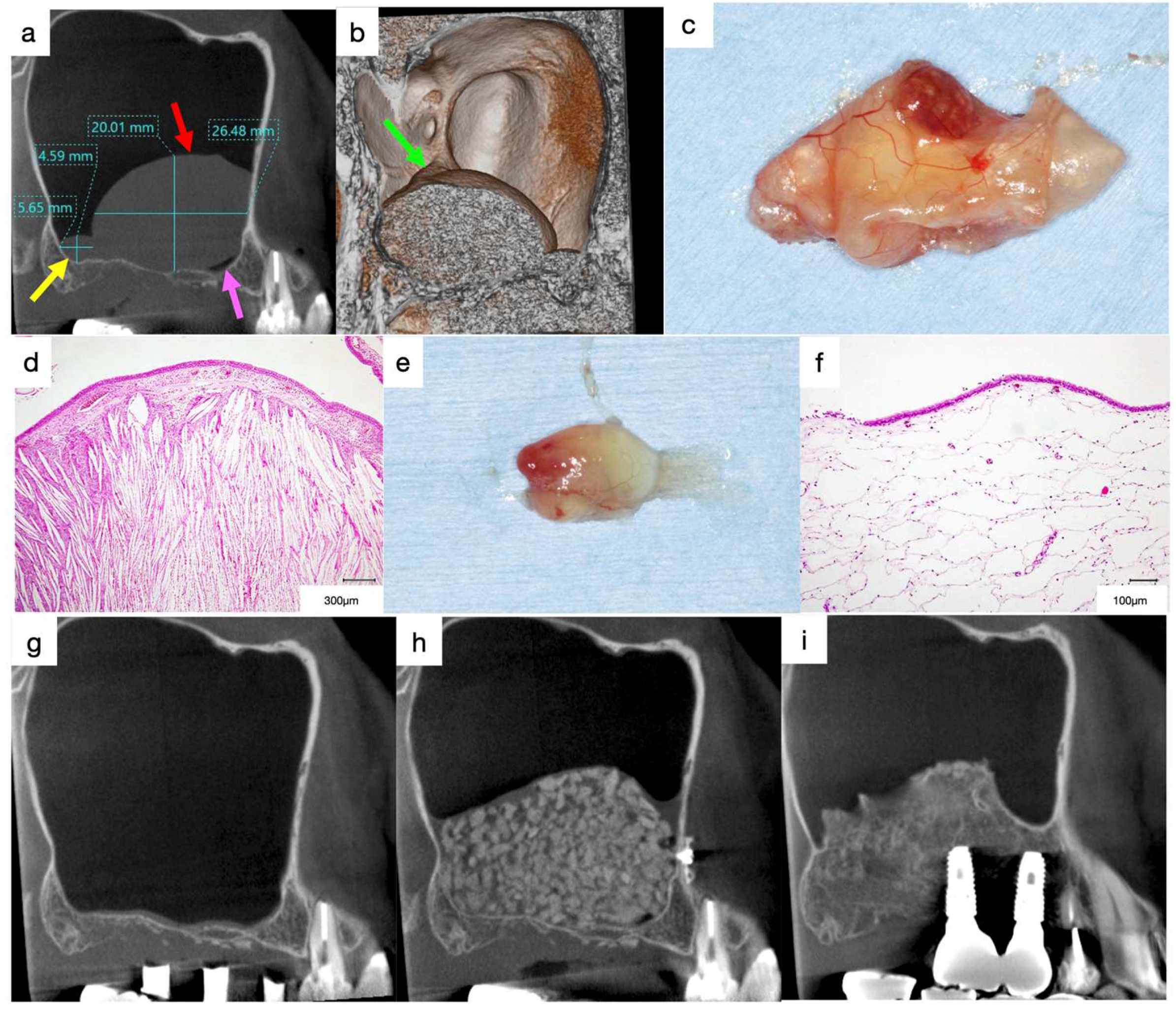
3.2. Lymphangioma
Case 3
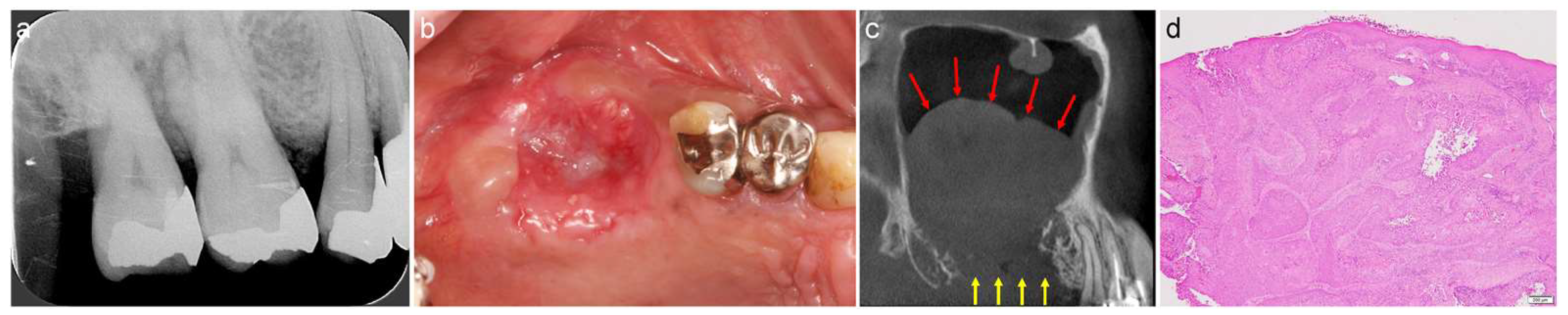
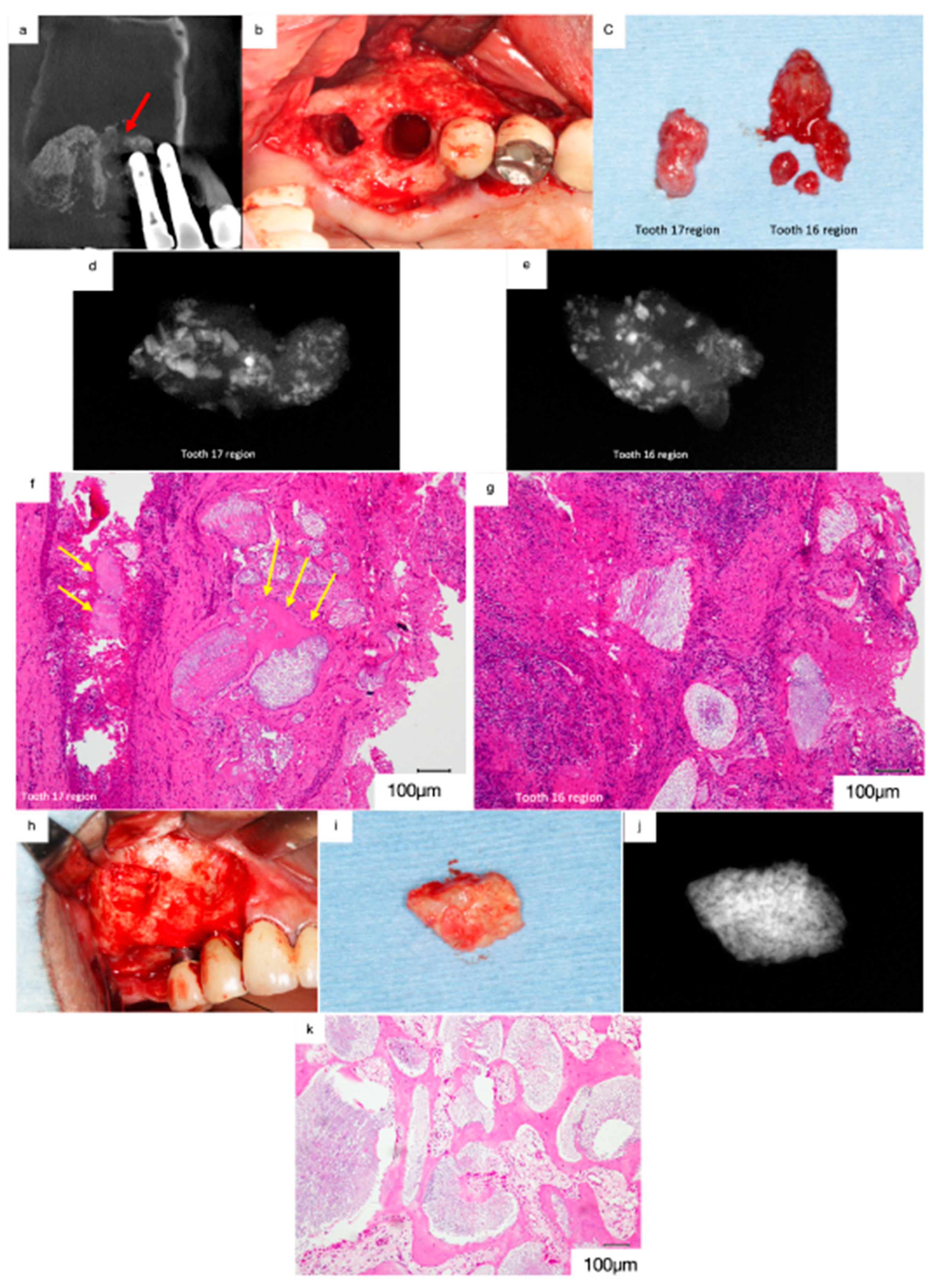
3.3. Gingival Cancer
Case 4
4. Treatment of AP
4.1. Patients and Informed Consent
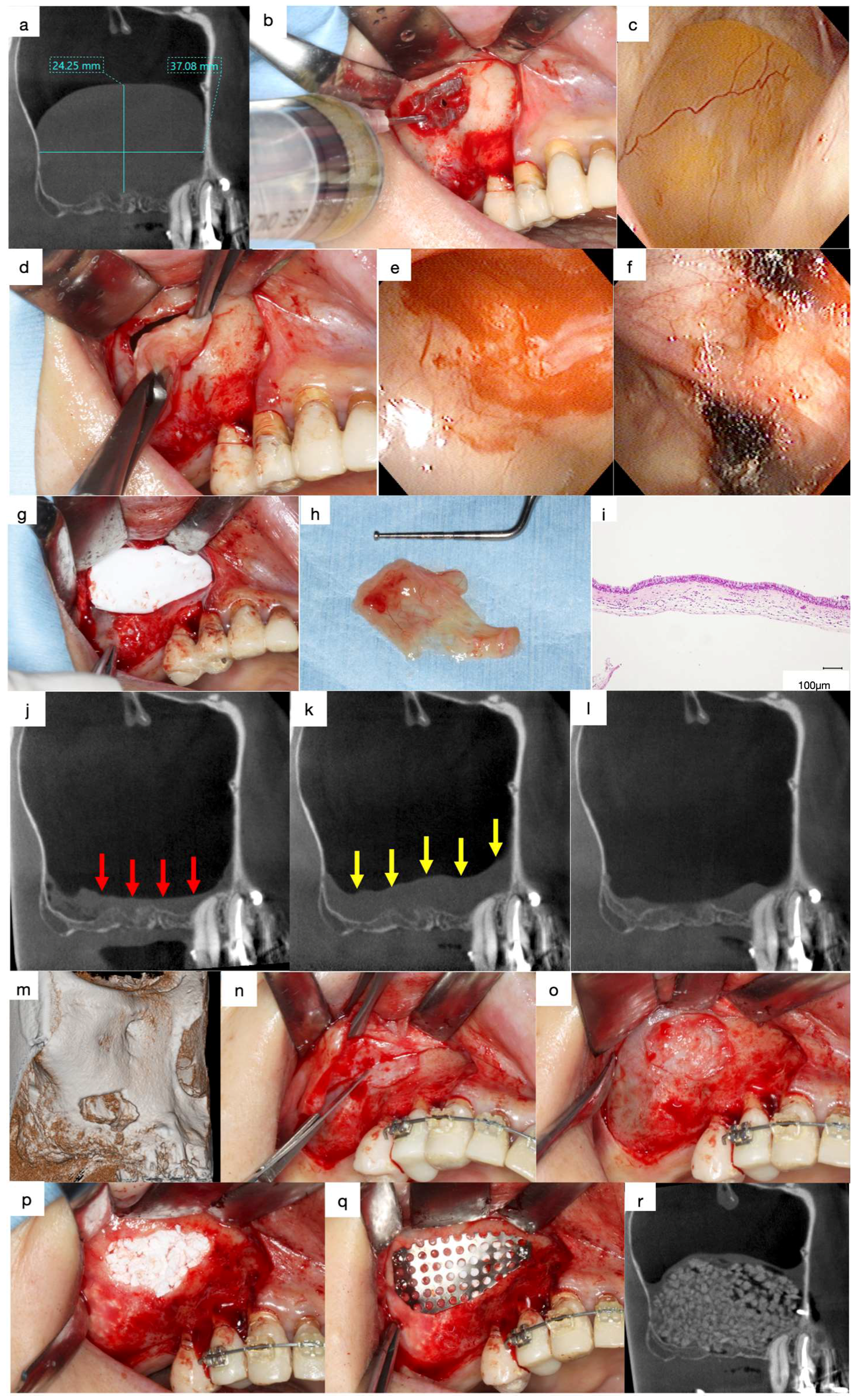
4.2. Enucleation of APs
4.3. Histopathological Findings of APs
4.4. Lateral Approach after Enucleation
5. Chronological CT Evaluation after the Sinus Floor Elevation Using β-TCP Granules Alone
5.1. Before the Placement of Implants
- The β-TCP granules moved upward owing to postoperative swelling of the sinus membrane one week postoperatively. These movements of bone materials determine the final shape of the augmented area (Figure 7b,f). As the weight of the air surrounding the β-TCP granules is comparatively low, it accumulated in the upper portion of the augmented area (Figure 7b, red arrows).
- Swelling of the sinus membrane resolved spontaneously three months postoperatively, and a belt-shaped radiolucency (grey zone) can be observed at the interface between the β-TCP granules and the surface of the maxillary sinus (Figure 7c, yellow arrows). This grey zone signifies that the osteoclasts have resorbed the β-TCP granules and the original bone [26], which is an important sign of bone regeneration.
- The radiopacity of the original cortical bone decreases six months postoperatively, whereas that of the grey zone increases (Figure 7d, green arrows).
- A completely radiopaque line (white line) can be observed at the newly formed floor of the maxillary sinus nine months postoperatively (Figure 7e, blue arrows). This white line represents the newly formed bone derived from the periosteum underneath the elevated sinus membrane. The immature bone tissue in the augmented area did not move during drilling for the placement of implants. Thus, the completely white line could be considered an indicator of the implant placement period. There are great differences in the individual ability for bone transformation, so recommending the same period for the placement of implants in a uniform manner may be unsuitable. The β-TCP granules in the central area retained their shape 12 months postoperatively (Figure 7f). Thus, the transformation of the β-TCP granules into bone occurs according to their osseous conductive ability from the bone surface of the maxillary sinus.
5.2. After the Placement of Implants
- Tapered-type implants were placed to achieve initial fixation one year after sinus floor elevation. Small particles of β-TCP granules can be observed around the implant. However, a clear white line and radiopaque line similar to the cortical bone can be observed at the newly formed bottom of the maxillary sinus and bony window, respectively (Figure 8c, green arrows).
- The radiopacity of the augmented area can be divided into two types two years after sinus floor elevation: cortical and cancerous bone. Tiny particles of β-TCP granules can be observed around the implant (Figure 8d).
- The β-TCP granules have disappeared three years after sinus floor elevation, and the augmented area shows cortical and cancerous bone-like radiopacity. The radiopacity of the augmented area is similar to that of the original bone (Figure 8e, yellow arrows). This suggests that new bone tissue has likely formed in the augmented area, mirroring the density of the original bone.
- The radiopacity of the augmented area remained almost unchanged compared with that at three years postoperatively 13 years after sinus floor elevation. Thus, an active bone transformation of the β-TCP granules continues for three years after sinus floor elevation. This results in the regeneration of the critical and cancerous bone. However, the line at the bottom of the maxillary sinus descended slightly due to pneumatization (Figure 8f, red arrows), suggesting that the newly formed bone is natural bone.
6. Sinus Floor Elevation Using Non-Absorbable Artificial Bone
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Woo, I.; Le, B.T. Maxillary sinus floor elevation: Review of anatomy and two techniques. Implant Dent. 2004, 13, 28–32. [Google Scholar] [CrossRef]
- Mardinger, O.; Manor, I.; Mijiritsky, E.; Hirshberg, A. Maxillary sinus augmentation in the presence of antral pseudocyst: A clinical approach. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Celebi, N.; Gonen, Z.B.; Kilic, E.; Etoz, O.; Alkan, A. Maxillary sinus floor augmentation in patients with maxillary sinus pseudocyst: Case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, e97–e102. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Tang, Y.; Liu, Y.; Chen, F.; Li, D. Maxillary Sinus Floor Elevation Using the Osteotome Technique in the Presence of Antral Pseudocysts: A Retorospective Study with an Average Follow-up of 27 Months. Int. J. Oral Maxillofac. Implant. 2014, 29, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, H.; Arai, Y. Complications of postoperative swelling of the maxillary sinus membrane after sinus floor augmentation. J. Oral Sci. Rehabil. 2015, 1, 26–33. [Google Scholar]
- Menezes, J.D.; Moura, L.B.; Pereira-Filpo, V.A.; Hochuli-Vieira, E. Maxikkary Sinus Mucocele as a Late Complication in Zygomatic-Orbital Complex Fracture. Craniomaxillofac. Trauma Reconstr. 2016, 9, 342–344. [Google Scholar] [CrossRef]
- Yeom, H.G.; Lee, W.; Han, S.I.; Lee, J.H.; Lee, B.D. Mucocele in the maxillary sinus involving the orbit: A report of 2 cases. Imaging Sci. Dent. 2022, 52, 327–332. [Google Scholar] [CrossRef]
- Price, C. Cystic Lymphangioma in the Maxillary Sinus: A Case Report. Br. J. Oral Surg. 1976, 14, 41–46. [Google Scholar] [CrossRef]
- Waheed, S.A.; Zaidan, T.F.; Abdullah, B.H. Odontogenic Cysts and Tumors of Maxilla and Maxillary Sinus (A Clinicopathological Analysis). J. Bagh. Coll. Dentistry 2021, 33, 38–43. [Google Scholar] [CrossRef]
- Martínez-González, J.M.; Barona-Dorado, C.; Arias-Irimia, O.; Martínez-Rodríguez, N.; Fernández-Domínguez, M. Panoramic and tomographic implant studies: Role in the diagnosis of sinus disorders. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e611–e615. [Google Scholar] [CrossRef][Green Version]
- Lofthag-Hansen, S.; Thilander-Klang, A.; Ekestubbe, A.; Helmrot, E.; Gröndahl, K. Calculating effective dose on a cone beam computed tomography device: 3D Accuitomo and 3D Accuitomo FPD. Dentomaxillofac. Radiol. 2008, 37, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Li, G. Patient radiation dose and protection from cone-beam computed tomography. Imaging Sci. Dent. 2013, 43, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Oberli, K.; Bornstein, M.M.; von Arx, T. Periapical surgery and the maxillary sinus: Radiographic parameters for clinical outcome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.W.; Meszaros, E.J. Submucosal Lymphangioma of the Maxillary Sinus. J. Oral Maxillofac. Surg. 2003, 61, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, N.; Malathi, N.; Thamizchelvan, H.; Rajan, S.T. Lymphangioma of the Maxillary Sinus-A Rare Case Report. Oral Maxillofac. Pathol. J. 2013, 4, 372–375. [Google Scholar]
- Rim, B.; Souheil, J.; Amira, K.; Azza, M.; Senda, T.; Ghazi, B. Maxillary’s cystic lymphangioma: A rare location. J. Clin. Med. Images 2023, 3. [Google Scholar] [CrossRef]
- Bobinskas, A.M.; Wiesenfeld, D.; Chandu, A. Influence of the site of origin on the outcome of squamous cell carcinoma of the maxilla-oral versus sinus. Int. J. Oral Maxillofac. Surg. 2014, 43, 137–141. [Google Scholar] [CrossRef]
- Morice, A.; Ostertag, A.; Sahli-Amor, M.; Goudot, P.; Bertolus, C.; Schouman, T. Prognostic factors of gingival-alveolar squamous cell carcinoma of the maxilla. Surg. Oncol. 2016, 25, 263–268. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, H.; Nakajima, Y.; Tanioka, T.; Botticelli, D.; Baba, S. A Reliable Surgical Procedure for Sinus Floor Augmentation with Antral Pseudocysts. Dent. J. 2021, 9, 122. [Google Scholar] [CrossRef]
- Busaba, N.Y.; Salman, S.D. Maxillary sinus mucoceles: Clinical presentation and long-term result of endoscopic surgical treatment. Laryngoscope 1999, 109, 1446–1449. [Google Scholar] [CrossRef]
- Seno, S.; Ogawa, T.; Shibayama, M.; Ogawa, F.; Fukui, J.; Owaki, S.; Suzuki, M.; Shimizu, T. Endoscopic sinus surgery for the odontogenic maxillary cysts. Rhinology 2009, 47, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.G. Pseudocysts and retention cysts of the maxillary sinus. Oral Surg. 1984, 58, 561–567. [Google Scholar] [CrossRef]
- Liu, Y.; Takagi, M. Pedunculated Pseudocyst in the maxillary Sinus: Report of a case. Oral Med. Pathol. 1999, 4, 75–78. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H.; Torre, A.; Eguia, A. Are mucous retention cysts and pseudocysts in the maxillary sinus a risk factor for dental implants? A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2021, 26, e276–e283. [Google Scholar] [CrossRef]
- Gardner, D.G.; Gullane, P. Mucocele of the maxillary sinus. Oral Surg. Oral Med. Oral Pathol. 1986, 62, 538–543. [Google Scholar] [CrossRef]
- Matsunaga, A.; Takami, M.; Irié, T.; Mishima, K.; Inagaki, K.; Kamijo, R. Microscopic study on resorption of β-TCP phosphate materials by osteoclasts. Cytotechnology 2015, 67, 727–732. [Google Scholar] [CrossRef]
- Lutz, R.; Berger-Fink, S.; Stockmann, P.; Neukam, F.W.; Schlegel, K.A. Sinus floor augmentation with autogenous bone vs. a bovine-derived xenograft—A 5-year retrospective study. Clin. Oral Implant. Res. 2015, 26, 644–648. [Google Scholar] [CrossRef]
- Canellas, J.V.D.S.; Drugos, L.; Ritto, F.G.; Fischer, R.G.; Medeiros, P.J.D. Xenograft materials in maxillary sinus floor elevation surgery: A systematic review with network meta-analyses. Br. J. Oral Maxillofac. Surg. 2021, 59, 742–751. [Google Scholar] [CrossRef]
- Simensen, A.N.; Bøe, O.E.; Berg, E.; Leknes, K.N. Patient knowledge and expectations prior to receiving implant-supported restorations. Int. J. Oral Maxillofac. Implant. 2015, 30, 41–47. [Google Scholar] [CrossRef]
- Park, W.B.; Han, J.Y.; Kang, K.L. Long-Term comparison of survival and marginal bone of implants with and without sinus augmentation in maxillary molars within the same patients: A 5.8- to 22-year retrospective study. J. Clin. Med. 2021, 10, 1360. [Google Scholar] [CrossRef]
- Kim, S.B.; Yun, P.Y.; Kim, Y.K. Clinical evaluation of sinus bone graft in patients with mucous retention cyst. Maxillofac. Plast Reconstr. Surg. 2016, 38, 35. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosaka, Y.; Nosaka, H.; Munakata, M.; Sanda, M. A Comprehensive Clinical Review of Maxillary Sinus Floor Elevation in Patients with Well-Defined Faintly Radiopaque Lesions in the Antrum. J. Clin. Med. 2024, 13, 332. https://doi.org/10.3390/jcm13020332
Nosaka Y, Nosaka H, Munakata M, Sanda M. A Comprehensive Clinical Review of Maxillary Sinus Floor Elevation in Patients with Well-Defined Faintly Radiopaque Lesions in the Antrum. Journal of Clinical Medicine. 2024; 13(2):332. https://doi.org/10.3390/jcm13020332
Chicago/Turabian StyleNosaka, Yasuhiro, Hitomi Nosaka, Motohiro Munakata, and Minoru Sanda. 2024. "A Comprehensive Clinical Review of Maxillary Sinus Floor Elevation in Patients with Well-Defined Faintly Radiopaque Lesions in the Antrum" Journal of Clinical Medicine 13, no. 2: 332. https://doi.org/10.3390/jcm13020332
APA StyleNosaka, Y., Nosaka, H., Munakata, M., & Sanda, M. (2024). A Comprehensive Clinical Review of Maxillary Sinus Floor Elevation in Patients with Well-Defined Faintly Radiopaque Lesions in the Antrum. Journal of Clinical Medicine, 13(2), 332. https://doi.org/10.3390/jcm13020332






