Abstract
N-acetyl-L-cysteine (NAC) was initially introduced as a treatment for mucus reduction and widely used for chronic respiratory conditions associated with mucus overproduction. However, the mechanism of action for NAC extends beyond its mucolytic activity and is complex and multifaceted. Contrary to other mucoactive drugs, NAC has been found to exhibit antioxidant, anti-infective, and anti-inflammatory activity in pre-clinical and clinical reports. These properties have sparked interest in its potential for treating chronic lung diseases, including chronic obstructive pulmonary disease (COPD), bronchiectasis (BE), cystic fibrosis (CF), and idiopathic pulmonary fibrosis (IPF), which are associated with oxidative stress, increased levels of glutathione and inflammation. NAC’s anti-inflammatory activity is noteworthy, and it is not solely secondary to its antioxidant capabilities. In ex vivo models of COPD exacerbation, the anti-inflammatory effects have been observed even at very low doses, especially with prolonged treatment. The mechanism involves the inhibition of the activation of NF-kB and neurokinin A production, resulting in a reduction in interleukin-6 production, a cytokine abundantly present in the sputum and breath condensate of patients with COPD and correlates with the number of exacerbations. The unique combination of mucolytic, antioxidant, anti-infective, and anti-inflammatory properties positions NAC as a safe, cost-effective, and efficacious therapy for a plethora of respiratory conditions.
Keywords:
N-acetyl-L-cysteine; NAC; mucolytic; antioxidant; anti-inflammatory; NKA; IL-6; COPD; chronic respiratory disease 1. Introduction
N-acetyl-L-cysteine (NAC) was first introduced in 1965 primarily for its role in promoting mucolysis [1] and has been the preferred treatment for paracetamol intoxication since the mid-1970s [2]. Its intravenous formulation for this purpose is recognized on the WHO’s list of Essential Medicines [3].
The excessive production and secretion of airway mucus have significant importance in the pathophysiology and clinical manifestations of different respiratory diseases, including acute and chronic bronchitis (CB), chronic obstructive pulmonary disease (COPD), bronchiectasis, and cystic fibrosis (CF) [4,5].
Recent evidence indicates that oxidative stress and inflammation contribute to the development of these pathologies, showing a close correlation with clinical outcomes and triggering the overproduction of mucus by glands and goblet cells [6,7,8,9]. Under diverse stress conditions, such as infection, pathogenic factors, smoking, and oxidative stress, epithelial cells in the airway release pro-secretory molecules, leading to hypertrophy of secretory cells and hyperplasia of goblet cells, with concomitant overproduction of mucus [5,6,7,8].
Conditions like chronic bronchitis, bronchiectasis, CF, and COPD are associated with impairment of ciliary function and/or airway mucus hypersecretion [6,7,10,11].
COPD is characterized by airflow limitation, oxidative stress, and airway inflammation [12]. It is a persistent and advancing disease linked to a heightened inflammatory response in the respiratory tract triggered by gases, noxious particles, or cigarette smoke, which results in the injury of cells lining the airway and mucus hypersecretion [13]. In particular, the over-secretion of components of mucus MUC5AC and MUC5B in the respiratory tract, coupled with epithelial damage, leads to the shedding of ciliated cells, hyperplasia of goblet cells, and hypertrophy of the submucosal gland, ultimately resulting in airway mucus hypersecretion [4].
The mucus, secreted by serous, mucous, and goblet cells in response to various stimuli, including inflammation [9], becomes viscous [12] and promotes bacterial colonization and biofilm growth. This hinders the ability of neutrophils and soluble antimicrobial agents to permeate dense mucus and eradicate bacteria [14,15].
In COPD, numerous inflammatory mediators are synthesized and released in response to oxidative stress, such as the pro-inflammatory transcription factor NF-κB, which is over-expressed and activated by Reactive Oxygen Species (ROS), especially in airway epithelial cells and macrophages [7]. Oxidative stress also promotes the release of signaling molecules such as phosphoinositide 3-kinase (PI3K) and p38 mitogen-activated protein kinase (MAPK). It also sustains transforming growth factor (TGF)-β signaling and the expression of matrix metalloproteinase (MMP)-9, which are implicated in small airway fibrosis and emphysema [7]. Inflammation of the respiratory tract can contribute to flare-ups and deterioration of COPD [16]. Bronchiectasis involves irreversible bronchial lumen expansion, compromising mucus clearance [17] and facilitating bacterial colonization [18]. The initial production of toxins by bacteria contributes to the activation of both microbial and danger-associated molecular patterns, subsequently heightening inflammation [18]. As a result, this inflammatory response triggers hyperplasia and metaplasia of goblet cells, eventually resulting in increased secretion of mucus in the airways [19,20,21].
NAC has undergone extensive scrutiny in numerous global clinical trials and has been used for many years in treating various respiratory conditions [13,22,23]. It is a well-documented and well-established treatment of choice, valued not only for reducing mucus viscosity and promoting discharge but also for its recognized antioxidant and anti-inflammatory properties [13,22,23].
The aim of this review is to gather data on the multifaceted properties of NAC [13], with a specific focus on its anti-inflammatory role. The analysis emphasizes findings from various studies, highlighting the impact of dosage and treatment duration on NAC’s anti-inflammatory effectiveness [24,25,26,27,28,29]. The evidence suggests that lower doses may prove effective when administered over an extended treatment period [24,25,26,27,28,29]. The goal is to present NAC, traditionally recognized as a dual-action mucolytic and anti-oxidant agent, capable of fluidifying mucus secretions and clearing the airways, as a versatile drug with a multifaceted mechanism of action, rendering it a suitable pharmacological option for a variety of chronic respiratory pathologies characterized by mucus hyperproduction, oxidative stress, and inflammation.
2. Methods
The authors performed a comprehensive non-systematic review of the existing literature on PubMed/MEDLINE and Google Scholar, until February 2024, using the following keywords: “N-acetylcysteine” AND “anti-inflammatory” OR “mucolytic” OR “antioxidant” OR “chronic respiratory disease” OR “chronic obstructive pulmonary disease” OR “idiopathic pulmonary fibrosis” OR “bronchiectasis” OR “cystic fibrosis” OR “asthma” OR “bronchitis” OR “anti-infective”.
3. NAC Exhibits Pharmacological Actions beyond Mucolytic Activity
The mechanism of action for NAC is complex and multifaceted and is not yet fully elucidated. NAC is widely utilized to decrease the viscosity and elasticity of mucus [30]. It achieves this by breaking down the disulfide bonds in mucoproteins, thereby decreasing the viscosity of respiratory secretions [22].
Apart from the replenishing of depleted GSH content, facilitated by NAC, the gradual release of NAC-derived metabolites may result in a modest increase in H2S and its oxidation byproducts including persulfides (RSSH) and polysulfides (RSSnSR) [31], known as sulfane sulfur metabolites [32]. These metabolites exhibit cytoprotective effects by aiding in disulfide reduction, acting as direct scavengers of radicals, and preventing irreversible oxidative damage of proteins [32]. Moreover, modifications involving persulfide on proteins have the potential to modify their function and signaling capabilities, thereby initiating stress-responsive cellular reactions [32].
The initial use of NAC for respiratory diseases like chronic bronchitis and COPD is primarily linked to its direct and indirect mucolytic activity, aiming to reduce the viscosity of secretions and facilitate expectoration [22]. However, pre-clinical observations indicate that its effectiveness goes beyond its mucolytic properties, encompassing anti-oxidant, anti-infective, and anti-inflammatory effects. This unique combination of properties suggests an additional and beneficial impact on patients [13,22,33,34]. The diverse actions of NAC may have implications for reducing exacerbations in individuals with chronic inflammatory respiratory conditions, such as CB/COPD. NAC’s effectiveness has been thoroughly studied in this respiratory disease, and it is employed as an adjunctive medication endorsed by several internationally recognized guidelines [35,36,37,38]. Indeed, its therapeutic value and substantial supporting evidence have been acknowledged by both the American Thoracic Society and the European Respiratory Society guidelines [39].
3.1. NAC as Anti-Oxidant
Abnormal levels of GSH in both extracellular and intracellular compartments are commonly observed in chronic pulmonary diseases, such as CF, bronchiectasis, idiopathic pulmonary fibrosis (IPF), and COPD [40,41].
During the 1980s, Moldéus and colleagues [42] pioneered the revelation that NAC demonstrated anti-oxidant effects in respiratory cells and tissues. Their findings showcased NAC’s capability to safeguard against the detrimental impacts of cigarette smoke condensates and hydrogen peroxide (H2O2) [42]. The researchers proposed that NAC’s shielding effect against ROS may be attributed to its function as a precursor to GSH and an enhancer of GSH biosynthesis [42]. Indeed, from a stringent biochemical perspective, the anti-oxidant characteristics of NAC are intricately linked to the elevation of GSH content, leading to the reduction in ROS synthesis [22]. However, NAC also possesses the capability to directly engage with oxidants such as hydroxyl radical, hydrogen peroxide, and hypochloric acid [30,34].
The pharmacological characterization of NAC’s anti-oxidant effect took place in the human ex vivo model of acute exacerbations of COPD (AECOPD) consisting of lipopolysaccharide (LPS)-stimulated human bronchi. In two different studies performed by Cazzola et al. [27] and Calzetta et al. [28], NAC led to a decrease in LPS-induced malondialdehyde, H2O2, peroxidase activity, and nitric oxide (NO) production at concentrations of 1–10 µM and 16–35 µM, respectively. Notably, there was also a normalization of GSH levels in the aforementioned two separate studies [27,28], with one study reporting increased superoxide dismutase (SOD) activity by approximately 150% compared to LPS-treated bronchi [27].
Currently, oxidative stress is recognized as a key predisposing factor in the development of COPD, with an imbalanced oxidant/anti-oxidant state particularly pronounced during AECOPD [43]. Factors such as inflammation, microbial infection, or smoke exposure can increase GSH content in bronchoalveolar lavage fluid (BALF) as a protective measure against further lung damage [40]. While GSH concentration increases in the BALF of stable COPD patients, it diminishes during AECOPD [44]. This indicates that the increase in GSH levels within the lungs is inadequate to effectively counterbalance the significant production of ROS. Maintaining adequate GSH levels is crucial to counteract excessive ROS production during AECOPD [45]. Thus, therapeutic approaches focused on increasing GSH levels could potentially be beneficial in managing the severity of AECOPD and reducing the occurrence of future episodes.
In this context, NAC shows efficacy in restoring the depleted pool of intracellular GSH during AECOPD [46,47] by elevating GSH levels in plasma and BALF [48]. NAC also reduces ROS secretion by alveolar macrophages (AM) [49] and exhaled H2O2 and decreases H2O2 production in the respiratory tract of COPD patients [50]. This is corroborated by De Benedetto et al., who observed a reduction in exhaled H2O2 in patients with COPD after 2 weeks of treatment with NAC at 1200 mg/day administration [51]. Intriguingly, the reduction in exhaled H2O2 in patients with COPD who received 600 mg/day NAC was observed only after 9–12 months [50], indicating the need for a longer duration of treatment for anti-oxidant effect when lower doses are employed.
Collectively, the evidence presented strongly suggests that oxidative stress plays a pivotal role in the pathogenesis of lung abnormalities observed in individuals diagnosed with COPD. Alleviating oxidative stress is anticipated to lead to diminished pulmonary damage and lower the incidence of local infections, thereby significantly slowing the progression of COPD.
3.2. NAC as an Anti-Inflammatory Drug
The anti-inflammatory attributes of NAC have been substantiated through various studies [13]. In the 1980s, multiple investigations suggested that NAC could mitigate cigarette irregularities in polymorphonuclear neutrophils (PMNs) [52], AM [49,53,54], fibroblasts [42], and epithelial cells [55,56] induced by smoke.
In particular, when given to smokers at a high dosage over an 8-week period, NAC has demonstrated efficacy in diminishing airway inflammation by reducing plasma concentrations of elastase and myeloperoxidase, lowering the content of lactoferrin, anti-chymotrypsin, and eosinophil cationic protein (ECP) in BALF, and mitigating the chemotactic activity of neutrophils [57]. Furthermore, it enhanced AM function in smokers [49].
In the 1990s, Schreck et al. observed NAC’s effectiveness in reducing the activation of nuclear factor kappa B (NF-kB) in intact Jurkat T cells [58]. Further support for the anti-inflammatory effect of NAC was provided by data demonstrating a decrease in the chemiluminescence of PMNs in BALF in vitro [59]. Additionally, NAC administered 1 h before LPS exposure in rats showed a dose-dependent reduction in lung NF-κB activation, leading to significant suppression of endotoxin-induced neutrophilic alveolitis [60].
Cytokines are pivotal in pulmonary immune defense but may lead to lung injury when produced excessively or dysregulated [60]. Given NF-κB dependent regulation of various cytokines, it emerges as a key factor in the pathobiology of pulmonary injury caused by excessive inflammation [60]. Consequently, targeting the NF-κB pathway becomes an appealing strategy for therapies aiming to limit neutrophilic lung inflammation and host-derived lung injury [60]. NAC’s anti-inflammatory activity in cells of the respiratory tract was demonstrated by Desaki et al. [61] in 2000, showing its efficacy in reducing the activation of NF-κB in human bronchial epithelial cells treated with silica.
NAC orally administered at 600 mg/day has been reported to reduce sputum ECP and Interleukin-8 (IL-8) concentrations in patients with COPD [24,25]. Additionally, at this dosage, NAC modulated the inflammatory response after ten weeks of intake in COPD patients by decreasing serum IL-8 levels [26]. This intervention also removed the inverse relationship between the pro-inflammatory cytokine IL-8 and glutathione peroxidase (GPx) and Trolox equivalent antioxidant capacity (TEAC) [26]. These findings imply that NAC affects IL-8 expression through pathways that are not dependent on GPx and plasma anti-oxidants [26]. Moreover, it eliminated the positive correlation between intercellular adhesion molecule-1 (ICAM-1) and IL-8, indicating that ICAM-1 expression is modulated by pathways not susceptible to NAC [26].
A study examining how NAC treatment affects changes induced by sepsis in conscious rats revealed a reduction in inflammatory biomarkers (IL-6, tumor necrosis factor-α (TNF-α), and IL-10) linked to sepsis [62]. In vitro, NAC reduced inflammatory cytokines, namely IL-6, IL-1β, and TNF-α, in LPS-treated macrophages under mild oxidative conditions [63]. In a hamster model of lung damage induced by SARS-CoV-2, intravenous NAC at high doses (500 mg/kg) significantly decreased levels of pro-inflammatory cytokines such as IL-1β, IL-6, IFN-γ, and TNF-α [64]. As a result of this decrease, there was reduced infiltration of macrophages at the infection site, thereby preventing lung damage [64].
In particular, elevated IL-6 levels characterize chronic inflammatory conditions of the lung and have an active role in the development of chronic respiratory conditions such as COPD and asthma, designating it as a crucial pharmacological target [65]. Reflecting airway inflammation, IL-6 concentration is heightened in sputum [66] and exhaled breath condensate of COPD patients [67]. Correlating with the number of exacerbations, IL-6 elevation may be discerned in plasma/serum during exacerbations [66,68,69]. A recent study identified a serum IL-6 concentration of 14.030 pg/mL or higher as a risk factor for more than 2 AECOPD in the subsequent year [70].
Additional evidence indicates that IL-6 could be pivotal in exacerbating COPD, particularly in cases involving co-infection with both the Gram-negative pathogen Haemophilus influenzae and rhinovirus [71].
It has been recently demonstrated that NAC exerts both anti-oxidant and concentration-dependent effects in human-isolated bronchi of patients with COPD treated with LPS [27]. The aforementioned studies conducted by Cazzola et al. [27] and Calzetta et al. [28] explored the beneficial effect of NAC in reducing airway inflammation caused by LPS. They used a wide variety of concentrations applicable in vitro, including those mimicking the levels found in plasma following daily oral doses of NAC (200 mg, 600 mg, and 1200 mg) in a validated ex vivo model of AECOPD [27,28]. NAC has demonstrated efficacy in regulating neurokinin A (NKA) release in LPS-stimulated human bronchi at concentrations ranging from 1 to 10 µM [27] and 5 to 35 µM [28] in the two different studies (Figure 1). One of the two studies showed that higher NAC concentrations (300 μM to 10 mM) were needed to reduce the production of IL-8, IL-1β, and TNF-α from LPS-stimulated human bronchi by approximately 55% [27]. In contrast, lower concentrations (1 μM) sufficed to decrease IL-6 levels by approximately 33%, likely through the inhibition of NKA release [27] (Figure 1). Accordingly, the other study showed that selectively blocking the receptor for NKA NK2 nullified the association between the reduction in NKA release and the decrease in IL-6 content [28]. This finding suggests that NAC suppresses the neurogenic inflammatory response triggered by LPS by reducing NKA production, subsequently diminishing the increase in IL-6 [28].
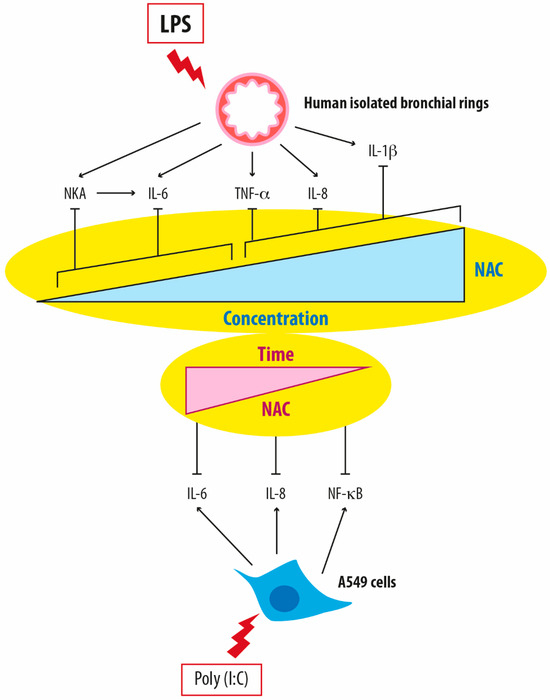
Figure 1.
Differential modulation of pro-inflammatory mediators in relation to both the concentration and duration of treatment with N-acetylcysteine in in vitro models of airway inflammation. NAC: N-acetylcysteine; LPS: lipopolysaccharides; NKA: neurokinin A; IL-6: Interleukin-6; TNF-α: tumor necrosis factor-α; IL-8: Interleukin-8; IL-1β: Interleukin-1beta; NF-κB: Nuclear factor kappa B.
Logistic regression analysis revealed that NAC’s influence on NKA levels correlated positively with IL-6 levels and reduced H2O2, malondialdehyde, peroxidase activity, and NO levels in both studies [27,28]. Notably, the study by Calzetta et al. [28] revealed that even very low concentrations, mirroring plasma levels from oral administration at 200 mg/day, were effective against GSH, H2O2, peroxidase activity, and IL-6. This effect was comparable in magnitude to that obtained with higher doses, reproducing the plasma levels resulting from oral administration at 1200 mg/day and 600 mg/day [28]. These findings indicate that NAC can modulate the production of NKA, leading to a subsequent beneficial effect against the LPS-induced increase in IL-6 when administered across a spectrum of doses (Figure 1).
A detailed, well-fitted logistic regression analysis of the results obtained by Calzetta et al. [28] demonstrated that the influence of NAC on NKA production displays a bell-shaped concentration-response curve. This suggests that the inhibitory effect observed at concentrations between 5 to 35 µM is greater than at higher concentrations [28]. This distinct fitting model induced by NAC concerning NKA levels may elucidate conflicting dose-effect findings reported in in vitro and in vivo settings, particularly regarding NAC’s anti-inflammatory activity [72].
These findings imply that additional mechanisms apart from the anti-oxidant activity control the rise of IL-6 in LPS-stimulated human bronchi, particularly when NAC is administered at low concentrations equivalent to an oral dose of 200 mg/day. This suggests a genuine anti-inflammatory effect of NAC, distinct from its anti-oxidant capabilities. Given that the LPS challenge triggers neurogenic inflammation in the airways and IL-6 exacerbates NKA release, NAC at low concentrations seems to finely regulate neurogenic inflammation [73,74,75,76], a harmful condition that contributes to the cyclical relationship between oxidative stress and inflammation in human bronchi [13,27].
Notably, a recent meta-analysis by Askari et al. [77] delving into the impact of oral NAC on serum inflammatory biomarkers uncovered that only a high dosage (≥1200 mg/day) of orally administered NAC effectively reduced C-reactive protein (CRP) levels. However, a noteworthy decrease in circulating IL-6 was reported with a dosage below 1200 mg/day [77]. This suggests that while high doses may be necessary for diminishing serum IL-6, these requirements are still lower than those needed for decreasing CRP levels. In their study [77], no discernible effect on the circulating levels of VCAM-1, ICAM-1, MCP-1, IL-8, and TNF-α was reported following oral administration of NAC. This aligns with the abovementioned findings of Cazzola et al. [27], where high concentrations of NAC (ranging from 300 μM to 10 mM) were required to reduce the release of IL-1β, IL-8, and TNF-α by LPS-stimulated bronchi.
Additionally, the duration of treatment significantly influences NAC’s anti-inflammatory activity, as evidenced by Montero et al.’s study [29]. Their findings showed that A549 cells treated with doses of NAC reflecting plasma concentrations after oral doses of 600 mg and 1200 mg prevented ROS induction, decreased thiol level, correlated with intracellular GSH levels, and suppressed IL-6 and IL-8 levels while inhibiting NFκB activation [29]. The extended incubation period enabled even lower concentrations of NAC to exhibit anti-inflammatory effects, suggesting that NAC’s positive impact on IL-6 regulation may stem from both its anti-oxidant properties and other anti-inflammatory mechanisms [27,28,29].
These results provide new evidence by confirming the concentration and time-dependent effects of NAC, both in vivo and in vitro, as suggested by Sadowska et al. [72]. NAC’s effects may be attributed to both its sustained anti-oxidant activity and its impact on intracellular events, including the regulation of NF-κB activation and NKA release.
4. Clinical Effectiveness of NAC in Pulmonary Medicine
Pre-clinical observations on NAC suggest that its diverse pharmacologic characteristics, including mucolytic activity and specific anti-oxidant and anti-inflammatory effects, may have a beneficial impact on COPD (Figure 2).
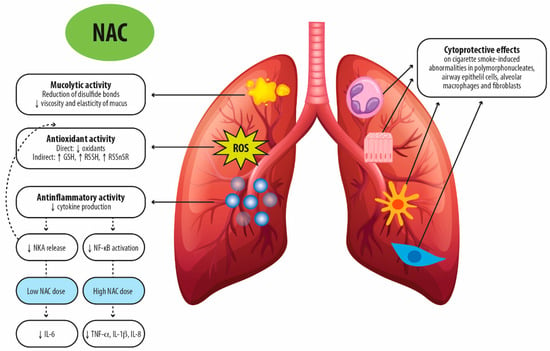
Figure 2.
Proposed molecular mechanisms for the therapeutic activity of N-acetylcysteine after oral administration in chronic respiratory diseases. NAC: N-acetylcysteine; GSH: glutathione; RSSH: persulfides; RSSnSR: polysulfides; NKA: neurokinin A; NF-κB: Nuclear factor kappa B; TNF-α: tumor necrosis factor-α; IL-1β: Interleukin-1beta; IL-8: Interleukin-8; ROS: reactive oxygen species.
A correlation between exacerbation frequency and sputum IL-8 and IL-6 levels has been established [68]. NAC, by acting on baseline levels of these cytokines, may contribute to limiting the inflammatory status and modulating exacerbation frequency. Additionally, by reducing sputum viscosity, NAC may facilitate airway clearance, influencing the course of exacerbation, as there is a link between bacterial colonization and exacerbation frequency and character [78].
The anti-infective properties and the ability to modulate the human bronchial tone of NAC can also impact the progression of COPD [13]. Patients experiencing frequent AECOPD may exhibit airway colonization by bacteria that produce biofilm [79], potentially perpetuating a harmful cycle of infection and inflammation that contributes to disease progression [80,81,82]. NAC’s antimicrobial activity has been demonstrated against various microorganisms, affecting different steps of biofilm formation [33,79]. NAC may also demonstrate strong anti-mycobacterial effects, reducing Mycobacterium tuberculosis infection and disease [83] and enhancing the effectiveness of antibiotics against bacterial infections [84,85,86,87,88]. However, it’s crucial to highlight that the plasma concentrations of NAC needed for its anti-infective properties can be much higher than what can be achieved through oral administration [13].
NAC has been shown to counteract bronchial desensitization during LPS challenges, restoring the normal contractility of airway smooth cells in an ex vivo AECOPD model [27], suggesting a potential role in protecting against exacerbations.
While certain dated studies found a small but significant positive impact of NAC on respiratory function in stable COPD patients, as measured by FEV1 [89,90] and MEF50 [90], recent research yielded disappointing results [91,92,93]. This discrepancy may stem from the limited sensitivity of these measures in detecting small airway disease and air trapping, where NAC is believed to exert its greatest effect, as evidenced by the improved FEF25–75% observed in the BRONCUS trial [91].
The randomized placebo-controlled HIACE and PANTHEON trials highlight a reduction in exacerbation rates in patients with COPD treated with 1200 mg/day of NAC for one year [88,94]. This effect appears more pronounced in stable COPD patients and mild disease cases, irrespective of the AECOPD rate [88,94]. Accordingly, a meta-analysis by Shen et al. [95] suggests that extended therapy with high doses of NAC (>600 mg/day) can decrease AECOPD.
Another meta-analysis conducted by Cazzola et al. [96] reports that oral administration of NAC for 4 months or more effectively reduces the frequency of AECOPD. However, higher doses (≥1200 mg/day) were needed for this effect, while NAC at the dosage of ≤600 mg/day prevented exacerbation of chronic bronchitis [96]. In another meta-analysis, the same authors also highlight that high doses of NAC (1200 mg/day), employed as additional therapy in COPD patients, prevent AECOPD [97].
In contrast, a systematic review by Fowdar et al. [92] indicates that both low-dose NAC (≤600 mg/day) and high-dose NAC (>600 mg/day) can safely reduce the percentage of patients experiencing one exacerbation or more after at least 6 months of treatment. This suggests that administering NAC over the long term can decrease the likelihood of COPD exacerbations, irrespective of dosage. In chronic bronchitis, NAC 600 mg/day over a duration of 3–6 months prevented acute exacerbations and improved symptoms [98,99].
A recent meta-analysis including twenty studies and a total of 4044 patients evaluated oral NAC efficacy either in patients with CB/pre-COPD (patients with chronic bronchitis and no diagnosis of COPD) or with confirmed COPD: in both groups, patients treated with NAC exhibited a noteworthy decrease in the occurrence of exacerbations [100]. Furthermore, CB/pre-COPD patients treated with NAC showed a significant likelihood of experiencing symptom improvement and/or improved QoL compared to those receiving a placebo [100].
NAC is linked to a favorable safety profile, with a beneficial impact observed in both patients with stable COPD and individuals at risk of AECOPD, regardless of inhaled corticosteroid use [88,92,96,97,101].
Some years ago, mucolytic agents, like NAC, were recognized as the most cost-effective treatment option for managing severe COPD patients prone to frequent exacerbations [102].
Based on the currently available findings, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy incorporates a section titled “Mucolytic (mucokinetics, mucoregulators) and anti-oxidant agents (NAC, carbocysteine)” [35]. The recommendations explicitly state that “in COPD patients not receiving inhaled corticosteroids, regular treatment with mucolytics such as carbocysteine and N-acetylcysteine may reduce exacerbations and modestly improve health status” [35]. Accordingly, the European Respiratory Society/American Thoracic Society guideline emphasizes that mucolytic therapy diminishes the probability of hospitalization in COPD patients [39]. Moreover, when administered in elevated doses, it has the potential to decrease exacerbations [39].
Extensive evidence from randomized clinical trials and thorough meta-analyses supports the efficacy of long-term NAC administration in decreasing the risk of AECOPD [88,94,96,97] and establishes a dose-related protective effect [96,97]. Additionally, pre-clinical in vitro studies indicate that chronic administration may require lower doses for efficacy [97]. However, there remains a crucial need for larger and more robust randomized controlled trials to optimize dosage and duration of treatment. Such trials can contribute to instilling greater confidence and facilitate the widespread adoption of NAC as a safe and effective therapy for COPD management.
Concerning lung diseases other than COPD, thiol drugs have been proposed to have anti-fibrotic activity in IPF, attributed to their antioxidant and anti-inflammatory effects [103,104,105]. This condition involves the generation of oxygen radicals and a reduction in GSH levels [106]. Despite these potential benefits, clinical trials for IPF have produced inconsistent results [103,106,107,108]. Using oral NAC, either by itself or in conjunction with pirfenidone or nintedanib, might be an effective treatment option for certain IPF patients [109], particularly those with the rs3750920 (TOLLIP) TT genotype [110]. Data on the overall efficacy and safety of NAC in a pharmacogenomic context, tailored to patients most likely to benefit, are eagerly awaited [108].
In addition, NAC is also utilized for treating non-CF bronchiectasis, where it could help decrease the frequency of exacerbations [111]. A recent randomized placebo-controlled pilot study evaluated the impact of NAC on sputum neutrophil elastase levels, which serve as a surrogate marker for exacerbations, in adult patients with bronchiectasis [112]. They observed a nearly 50% reduction in sputum neutrophil elastase and improvements in lung function and QoL-related domains, without increased adverse events compared to placebo [112].
A multicenter, double-blind, randomized, placebo-controlled trial is presently in progress to investigate the sustained effectiveness of oral NAC on exacerbation rates and QoL in bronchiectasis [113]. The study aims to specifically focus on the antioxidant, anti-inflammatory, and antibacterial properties of NAC in bronchiectasis [113].
NAC is also used in CF, which is characterized by frequent respiratory infections, PMN-mediated inflammation, and increased oxidative stress [114]. However, evidence of NAC effectiveness in CF is still poor, despite some encouraging results [115,116,117], and further studies on larger samples are needed [114].
Interestingly, NAC as adjunctive therapy has been associated with reduced rates of progression to respiratory failure, with some studies reporting shorter hospital stays and others indicating decreased mortality in patients hospitalized for COVID-19 pneumonia [118].
5. Conclusions
In conclusion, although adjusting the anti-oxidant status may indirectly trigger anti-inflammatory effects, recent findings in vitro [28] highlight a distinctive anti-inflammatory activity of NAC at low concentrations, equivalent to an oral dosage as low as 200 mg/day. This bolsters the evidence suggesting that NAC may disrupt the harmful cycle between oxidative stress and inflammation, a detrimental condition prevalent in the airways of patients undergoing acute exacerbations of chronic respiratory conditions [13]. The prospect of utilizing NAC in COPD treatment appears promising, especially with prolonged administration (beyond 6 months). While the proposed mechanism, potentially involving NF-κB inhibition and the inhibition of NKA release, holds promise for influencing the production of diverse mediators and regulating inflammation more profoundly, further investigation is required for a comprehensive understanding. Clinical trial results suggest that prolonged NAC administration can significantly enhance respiratory symptoms and decrease the frequency of exacerbations in COPD and CB/pre-COPD patients.
Author Contributions
All Authors contributed to the definition and contextualization of contents, critically edited the manuscript, and approved it for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
Editorial assistance and medical writing were provided by Valeria Benedusi and Aashni Shah (Polistudium srl, Milan, Italy). This assistance was supported by Zambon S.p.A.
Conflicts of Interest
P.S. has received lectures fees at national and international meetings and consultancy fees from Boehringer Ingelgheim, Chiesi Farmaceutici, Astra Zeneca, Berlin-Chemie, Edmondpharma, Guidotti, Neopharmed, Novartis, Valeas, GlaxoSmithKline, Alfasigma, Zambon, Dompè and Sanofi; research grants from Air Liquide, Almirall, Boehringer Ingelgheim, Chiesi Farmaceutici, Pfizer, Edmondpharma, GSK, and Astra Zeneca. J.C.S., F.D., G.L. and M.S. have no conflicts of interest or competing interests. D.R. has received fees for lectures from Astra Zeneca, Berlin Chemie, Glaxo Smith Kline, Menarini, Roche; honoraria for consulting and participation in advisory boards from Astra Zeneca.
References
- Sheffner, A.L. The reduction in vitro in viscosity of mucoprotein solutions by a new mucolytic agent, N-acetyl-L-cysteine. Ann. N. Y. Acad. Sci. 1963, 106, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Prescott, L.F.; Park, J.; Ballantyne, A.; Adriaenssens, P.; Proudfoot, A.T. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet 1977, 2, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/medicines/publications/essentialmedicines/en/ (accessed on 6 May 2024).
- Rogers, D.F.; Barnes, P.J. Treatment of airway mucus hypersecretion. Ann. Med. 2006, 38, 116–125. [Google Scholar] [CrossRef]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.S.; Lanzetti, M.; Nesi, R.T.; Nagato, A.C.; Silva, C.P.; Kennedy-Feitosa, E.; Melo, A.C.; Cattani-Cavalieri, I.; Porto, L.C.; Valenca, S.S. Oxidative Stress and Inflammation in Acute and Chronic Lung Injuries. Antioxidants 2023, 12, 548. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef]
- Hauber, H.P.; Foley, S.C.; Hamid, Q. Mucin overproduction in chronic inflammatory lung disease. Can. Respir. J. 2006, 13, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Curran, D.R.; Cohn, L. Advances in mucous cell metaplasia: A plug for mucus as a therapeutic focus in chronic airway disease. Am. J. Respir. Cell Mol. Biol. 2010, 42, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.A.; Chen, A.C.; Radicioni, G.; Lourie, R.; Martin, M.; Broomfield, A.; Sheng, Y.H.; Hasnain, S.Z.; Radford-Smith, G.; Simms, L.A.; et al. Airway Mucus Hyperconcentration in Non–Cystic Fibrosis Bronchiectasis. Am. J. Respir. Crit. Care Med. 2020, 201, 661–670. [Google Scholar] [CrossRef]
- Tilley, A.E.; Walters, M.S.; Shaykhiev, R.; Crystal, R.G. Cilia Dysfunction in Lung Disease. Annu. Rev. Physiol. 2015, 77, 379–406. [Google Scholar] [CrossRef]
- MacNee, W. Pathology, pathogenesis, and pathophysiology. BMJ 2006, 332, 1202–1204. [Google Scholar] [CrossRef]
- Calzetta, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. Multifaceted activity of N-acetyl-l-cysteine in chronic obstructive pulmonary disease. Expert. Rev. Respir. Med. 2018, 12, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Randell, S.H.; Boucher, R.C.; University of North Carolina Virtual Lung Group. Effective mucus clearance is essential for respiratory health. Am. J. Respir. Cell Mol. Biol. 2006, 35, 20–28. [Google Scholar] [CrossRef]
- Matsui, H.; Verghese, M.W.; Kesimer, M.; Schwab, U.E.; Randell, S.H.; Sheehan, J.K.; Grubb, B.R.; Boucher, R.C. Reduced three-dimensional motility in dehydrated airway mucus prevents neutrophil capture and killing bacteria on airway epithelial surfaces. J. Immunol. 2005, 175, 1090–1099. [Google Scholar] [CrossRef]
- Papi, A.; Luppi, F.; Franco, F.; Fabbri, L.M. Pathophysiology of exacerbations of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2006, 3, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Basavaraj, A.; Choate, R.; Addrizzo-Harris, D.; Aksamit, T.R.; Barker, A.; Daley, C.L.; Daniels, M.L.; Eden, E.; DiMango, A.; Fennelly, K.; et al. Airway clearance techniques in bronchiectasis: Analysis from the United States Bronchiectasis and Non-TB Mycobacteria Research Registry. Chest 2020, 158, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Borekci, S.; Halis, A.N.; Aygun, G.; Musellim, B. Bacterial colonization and associated factors in patients with bronchiectasis. Ann. Thorac. Med. 2016, 11, 55–59. [Google Scholar] [CrossRef]
- Korfhagen, T.R.; Kitzmiller, J.; Chen, G.; Sridharan, A.; Haitchi, H.M.; Hegde, R.S.; Divanovic, S.; Karp, C.L.; Whitsett, J.A. SAM-pointed domain ETS factor mediates epithelial cell-intrinsic innate immune signaling during airway mucous metaplasia. Proc. Natl. Acad. Sci. USA 2012, 109, 16630–16635. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, M.J.; Byers, D.E.; Alexander-Brett, J.; Wang, X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat. Rev. Immunol. 2014, 14, 686–698. [Google Scholar] [CrossRef]
- Tyner, J.W.; Kim, E.Y.; Ide, K.; Pelletier, M.R.; Roswit, W.T.; Morton, J.D.; Battaile, J.T.; Patel, A.C.; Patterson, G.A.; Castro, M.; et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J. Clin. Investig. 2006, 116, 309–321. [Google Scholar] [CrossRef]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; de Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, A.M.; Verbraecken, J.; Darquennes, K.; De Backer, W.A. Role of N-acetylcysteine in the management of COPD. Int. J. Chron. Obstruct Pulmon Dis. 2006, 1, 425–434. [Google Scholar] [CrossRef] [PubMed]
- De Backer, W.; Van Overveld, F. Sputum ECP levels in COPD patients decrease after treatment with N-acetylcysteine (NAC). Eur. Respir. J. 1997, 12, 225s. [Google Scholar]
- Van Overveld, F.J.; Demkow, U.; Górecka, D.; De Backer, W.A.; Zielinski, J. New developments in the treatment of COPD: Comparing the effects of inhaled corticosteroids and N-acetylcysteine. J. Physiol. Pharmacol. 2005, 56 (Suppl. S4), 135–142. [Google Scholar]
- Sadowska, A.M.; Van Overveld, F.J.; Gorecka, D.; Zdral, A.; Filewska, M.; Demkow, U.A.; Luyten, C.; Saenen, E.; Zielinski, J.; De Backer, W.A. The interrelationship between markers of inflammation and oxidative stress in chronic obstructive pulmonary disease: Modulation by inhaled steroids and antioxidant. Respir. Med. 2005, 99, 241–249. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Facciolo, F.; Rogliani, P.; Matera, M.G. Pharmacological investigation on the anti-oxidant and anti-inflammatory activity of N-acetylcysteine in an ex vivo model of COPD exacerbation. Respir. Res. 2017, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Rogliani, P.; Facciolo, F.; Rinaldi, B.; Cazzola, M.; Matera, M.G. N-Acetylcysteine protects human bronchi by modulating the release of neurokinin A in an ex vivo model of COPD exacerbation. Biomed. Pharmacother. 2018, 103, 1–8. [Google Scholar] [CrossRef]
- Montero, P.; Roger, I.; Estornut, C.; Milara, J.; Cortijo, J. Influence of dose and exposition time in the effectiveness of N-Acetyl-l-cysteine treatment in A549 human epithelial cells. Heliyon 2023, 9, e15613. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. H2S: A Novel Gasotransmitter that Signals by Sulfhydration. Trends Biochem. Sci. 2015, 40, 687–700. [Google Scholar] [CrossRef]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef]
- Tieu, S.; Charchoglyan, A.; Paulsen, L.; Wagter-Lesperance, L.C.; Shandilya, U.K.; Bridle, B.W.; Mallard, B.A.; Karrow, N.A. N-Acetylcysteine and Its Immunomodulatory Properties in Humans and Domesticated Animals. Antioxidants 2023, 12, 1867. [Google Scholar] [CrossRef]
- Santus, P.; Corsico, A.; Solidoro, P.; Braido, F.; Di Marco, F.; Scichilone, N. Oxidative stress and respiratory system: Pharmacological and clinical reappraisal of N-acetylcysteine. COPD J. Chronic Obstr. Pulm. Dis. 2014, 11, 705–717. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease—GOLD. 2024 GOLD Report. Available online: https://goldcopd.org/2024-gold-report/ (accessed on 22 January 2024).
- Miravitlles, M.; Calle, M.; Molina, J.; Almagro, P.; Gómez, J.T.; Trigueros, J.A.; Cosío, B.G.; Casanova, C.; López-Camposb, J.L.; Riescob, J.A.; et al. Spanish COPD Guidelines (GesEPOC) 2021: Updated Pharmacological treatment of stable COPD. Arch. Bronconeumol. 2022, 58, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Criner, G.J.; Bourbeau, J.; Diekemper, R.L.; Ouellette, D.R.; Goodridge, D.; Hernandez, P.; Curren, K.; Balter, M.S.; Bhutani, M.; Camp, P.G.; et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest 2015, 147, 894–942. [Google Scholar] [CrossRef] [PubMed]
- Bourbeau, J.; Bhutani, M.; Hernandez, P.; Marciniuk, D.D.; Aaron, S.D.; Balter, M.; Beauchesne, M.F.; D’Urzo, A.; Goldstein, R.; Kaplan, A.; et al. Full article: CTS position statement: Pharmacotherapy in patients with COPD—An update. Can. J. Respir. Crit. Care Sleep Med. 2017, 1, 222. [Google Scholar]
- Wedzicha, J.A.; Calverley, P.M.; Albert, R.K.; Anzueto, A.; Criner, G.J.; Hurst, J.R.; Miravitlles, M.; Papi, A.; Rabe, K.F.; Rigau, D.; et al. Prevention of COPD exacerbations: A European Respiratory Society/American Thoracic Society guideline. Eur. Respir. J. 2017, 50, 1602265. [Google Scholar] [CrossRef]
- Gould, N.S.; Day, B.J. Targeting maladaptive glutathione responses in lung disease. Biochem. Pharmacol. 2011, 81, 187. [Google Scholar] [CrossRef] [PubMed]
- Olveira, G.; Olveira, C.; Dorado, A.; García-Fuentes, E.; Rubio, E.; Tinahones, F.; Soriguer, F.; Murri, M. Cellular and plasma oxidative stress biomarkers are raised in adults with bronchiectasis. Clin. Nutr. 2013, 32, 112–117. [Google Scholar] [CrossRef]
- Moldéus, P.; Cotgreave, I.A.; Berggren, M. Lung protection by a thiol-containing antioxidant: N-acetylcysteine. Respiration 1986, 50 (Suppl. S1), 31–42. [Google Scholar] [CrossRef]
- Kirkham, P.A.; Barnes, P.J. Oxidative stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Drost, E.M.; Skwarski, K.M.; Sauleda, J.; Soler, N.; Roca, J.; Agusti, A.; MacNee, W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 2005, 60, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Matera, M.G.; Calzetta, L.; Cazzola, M. Oxidation pathway and exacerbations in COPD: The role of NAC. Expert Rev. Respir. Med. 2016, 10, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, M.M.; Marsden, M.; Selby, C.; Morrison, D.; MacNee, W. Effect of N-acetyl cysteine on the concentrations of thiols in plasma, bronchoalveolar lavage fluid, and lung tissue. Thorax 1994, 49, 670–675. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W.; Bridgeman, M.M.; Marsden, M.; Drost, E.; Lannan, S.; Selby, C.; Donaldson, K. The effects of N-acetylcysteine and glutathione on smoke-induced changes in lung phagocytes and epithelial cells. Am. J. Med. 1991, 91, 60S–66S. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, M.M.; Marsden, M.; MacNee, W.; Flenley, D.C.; Ryle, A.P. Cysteine and glutathione concentrations in plasma and bronchoalveolar lavage fluid after treatment with N-acetylcysteine. Thorax 1991, 46, 39–42. [Google Scholar] [CrossRef]
- Linden, M.; Wieslander, E.; Eklund, A.; Larsson, K.; Brattsand, R. Effects of oral N-acetylcysteine on cell content and macrophage function in bronchoalveolar lavage from healthy smokers. Eur. Respir. J. 1988, 1, 645–650. [Google Scholar] [CrossRef]
- Kasielski, M.; Nowak, D. Long-term administration of N-acetylcysteine decreases hydrogen peroxide exhalation in subjects with chronic obstructive pulmonary disease. Respir. Med. 2001, 95, 448–456. [Google Scholar] [CrossRef]
- De Benedetto, F.; Aceto, A.; Dragani, B.; Spacone, A.; Formisano, S.; Pela, R.; Donner, C.F.; Sanguinetti, C.M. Long-term oral n-acetylcysteine reduces exhaled hydrogen peroxide in stable COPD. Pulm. Pharmacol. Ther. 2005, 18, 41–47. [Google Scholar] [CrossRef]
- Bridges, R.B. Protective action of thiols on neutrophil function. Eur. J. Respir. Dis. Suppl. 1985, 139, 40–48. [Google Scholar]
- Voisin, C.; Aerts, C.; Wallaert, B. Prevention of in vitro oxidant-mediated alveolar macrophage injury by cellular glutathione and precursors. Bull. Eur. Physiopathol. Respir. 1987, 23, 309–313. [Google Scholar] [PubMed]
- Bergstrand, H.; Björnson, A.; Eklund, A.; Hernbrand, R.; Larsson, K.; Linden, M.; Nilsson, A. Stimuli-induced superoxide radical generation in vitro by human alveolar macrophages from smokers: Modulation by N-acetylcysteine treatment in vivo. J. Free Radic. Biol. Med. 1986, 2, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Moldéus, P.; Berggren, M.; Grafström, R. N-acetylcysteine protection against the toxicity of cigarette smoke and cigarette smoke condensates in various tissues and cells in vitro. Eur. J. Respir. Dis. Suppl. 1985, 139, 123–129. [Google Scholar]
- Cotgreave, I.A.; Moldéus, P. Lung protection by thiol-containing antioxidants. Bull. Eur. Physiopathol. Respir. 1987, 23, 275–277. [Google Scholar] [PubMed]
- Eklund, A.; Eriksson, O.; Hakansson, L.; Larsson, K.; Ohlsson, K.; Venge, P.; Bergstrand, H.; Bjornson, A.; Brattsand, R.; Glennow, C. Oral N-acetylcysteine reduces selected humoral markers of inflammatory cell activity in BAL fluid from healthy smokers: Correlation to effects on cellular variables. Eur. Respir. J. 1988, 1, 832–838. [Google Scholar] [CrossRef]
- Schreck, R.; Meier, B.; Männel, D.N.; Dröge, W.; Baeuerle, P.A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J. Exp. Med. 1992, 175, 1181–1194. [Google Scholar] [CrossRef]
- Jankowska, R.; Passowicz-Muszyńska, E.; Medrala, W.; Banaś, T.; Marcinkowska, A. The influence of n-acetylcysteine on chemiluminescence of granulocytes in peripheral blood of patients with chronic bronchitis. Pneumonol. Alergol. Pol. 1993, 61, 586–591. [Google Scholar] [PubMed]
- Blackwell, T.S.; Blackwell, T.R.; Holden, E.P.; Christman, B.W.; Christman, J.W. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J. Immunol. 1996, 157, 1630–1637. [Google Scholar] [CrossRef]
- Desaki, M.; Takizawa, H.; Kasama, T.; Kobayashi, K.; Morita, Y.; Yamamoto, K. Nuclear factor-kappa b activation in silica-induced interleukin 8 production by human bronchial epithelial cells. Cytokine 2000, 12, 1257–1260. [Google Scholar] [CrossRef]
- Hsu, B.G.; Lee, R.P.; Yang, F.L.; Harn, H.J.; Chen, H.I. Post-treatment with N-acetylcysteine ameliorates endotoxin shock-induced organ damage in conscious rats. Life Sci. 2006, 79, 2010–2016. [Google Scholar] [CrossRef]
- Palacio, J.R.; Markert, U.R.; Martínez, P. Anti-inflammatory properties of N-acetylcysteine on lipopolysaccharide-activated macrophages. Inflamm. Res. 2011, 60, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Behera, P.; Parida, D.; Mohapatra, A.P.; Das, S.K.; Kumari, S.; Avula, K.; Mohapatra, A.; Syed, G.H.; Senapati, S. Therapeutic role of N-acetyl cysteine (NAC) for the treatment and/or management of SARS-CoV-2-induced lung damage in hamster model. Eur. J. Pharmacol. 2023, 938, 175392. [Google Scholar] [CrossRef]
- Rincon, M.; Irvin, C.G. Role of IL-6 in Asthma and Other Inflammatory Pulmonary Diseases. Int. J. Biol. Sci. 2012, 8, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Grubek-Jaworska, H.; Paplińska, M.; Hermanowicz-Salamon, J.; Białek-Gosk, K.; Dąbrowska, M.; Grabczak, E.; Domagała-Kulawik, J.; Stępień, J.; Chazan, R. IL-6 and IL-13 in induced sputum of COPD and asthma patients: Correlation with respiratory tests. Respiration 2012, 84, 101–107. [Google Scholar] [CrossRef]
- Bucchioni, E.; Kharitonov, S.A.; Allegra, L.; Barnes, P.J. High levels of interleukin-6 in the exhaled breath condensate of patients with COPD. Respir. Med. 2003, 97, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.; Seemungal, T.A.; Sapsford, R.J.; Wedzicha, J.A. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 2000, 55, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Hussein, F.G.M.; Mohammed, R.S.; Khattab, R.A.; Al-Sharawy, L.A. Serum interleukin-6 in chronic obstructive pulmonary disease patients and its relation to severity and acute exacerbation. Egypt. J. Bronchol. 2022, 16, 10. [Google Scholar] [CrossRef]
- Huang, H.; Huang, X.; Zeng, K.; Deng, F.; Lin, C.; Huang, W. Interleukin-6 is a Strong Predictor of the Frequency of COPD Exacerbation within 1 Year. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 2945–2951. [Google Scholar] [CrossRef]
- Wilkinson, T.M.A.; Hurst, J.R.; Perera, W.R.; Wilks, M.; Donaldson, G.C.; Wedzicha, J.A. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest 2006, 129, 317–324. [Google Scholar] [CrossRef]
- Sadowska, A.M.; Manuel-Y-Keenoy, B.; De Backer, W.A. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: Discordant in vitro and in vivo dose-effects: A review. Pulm. Pharmacol. Ther. 2007, 20, 9–22. [Google Scholar] [CrossRef]
- Calzetta, L.; Luongo, L.; Cazzola, M.; Page, C.; Rogliani, P.; Facciolo, F.; Maione, S.; Capuano, A.; Rinaldi, B.; Matera, M.G. Contribution of sensory nerves to LPS-induced hyperresponsiveness of human isolated bronchi. Life Sci. 2015, 131, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Soggiu, A.; Roncada, P.; Bonizzi, L.; Pistocchini, E.; Urbani, A.; Rinaldi, B.; Matera, M.G. Propofol protects against opioid-induced hyperresponsiveness of airway smooth muscle in a horse model of target-controlled infusion anaesthesia. Eur. J. Pharmacol. 2015, 765, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Page, C.P.; Rinaldi, B.; Capuano, A.; Matera, M.G. Protein prenylation contributes to the effects of LPS on EFS-induced responses in human isolated bronchi. Am. J. Respir. Cell Mol. Biol. 2011, 45, 704–710. [Google Scholar] [CrossRef] [PubMed]
- De Laurentiis, A.; Candolfi, M.; Pisera, D.; Seilicovich, A. Effects of lipopolysaccharide on neurokinin A content and release in the hypothalamic-pituitary axis. Regul. Pept. 2003, 111, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Faryabi, R.; Mozaffari, H.; Darooghegi Mofrad, M. The effects of N-Acetylcysteine on serum level of inflammatory biomarkers in adults. Findings from a systematic review and meta-analysis of randomized clinical trials. Cytokine 2020, 135, 155239. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.S.; Seemungal, T.A.; Wilks, M.; Lloyd-Owen, S.J.; Donaldson, G.C.; Wedzicha, J.A. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002, 57, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Page, C.; Rossolini, G.M.; Pallecchi, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. The effect of N-acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Respir. Med. 2016, 117, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Hassett, D.J.; Borchers, M.T.; Panos, R.J. Chronic obstructive pulmonary disease (COPD): Evaluation from clinical, immunological and bacterial pathogenesis perspectives. J. Microbiol. 2014, 52, 211–226. [Google Scholar] [CrossRef]
- Eldika, N.; Sethi, S. Role of nontypeable Haemophilus influenzae in exacerbations and progression of chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2006, 12, 118–124. [Google Scholar] [CrossRef]
- Martínez-Solano, L.; Macia, M.D.; Fajardo, A.; Oliver, A.; Martinez, J.L. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin. Infect. Dis. 2008, 47, 1526–1533. [Google Scholar] [CrossRef]
- Amaral, E.P.; Conceição, E.L.; Costa, D.L.; Rocha, M.S.; Marinho, J.M.; Cordeiro-Santos, M.; D’Império-Lima, M.R.; Barbosa, T.; Sher, A.; Andrade, B.B. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol. 2016, 16, 251. [Google Scholar] [CrossRef]
- Lea, J.; Conlin, A.E.; Sekirov, I.; Restelli, V.; Ayakar, K.G.; Turnbull, L.; Doyle, P.; Noble, M.; Rennie, R.; Schreiber, W.E.; et al. In vitro efficacy of N-acetylcysteine on bacteria associated with chronic suppurative otitis media. J. Otolaryngol.-Head Neck Surg. 2014, 43, 20. [Google Scholar] [CrossRef]
- del Prado, G.; Ruiz, V.; Naves, P.; Rodríguez-Cerrato, V.; Soriano, F.; del Carmen Ponte, M. Biofilm formation by Streptococcus pneumoniae strains and effects of human serum albumin, ibuprofen, N-acetyl-l-cysteine, amoxicillin, erythromycin, and levofloxacin. Diagn. Microbiol. Infect. Dis. 2010, 67, 311–318. [Google Scholar] [CrossRef]
- Aslam, S.; Darouiche, R.O. Role of antibiofilm-antimicrobial agents in controlling device-related infections. Int. J. Artif. Organs 2011, 34, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Riise, G.C.; Qvarfordt, I.; Larsson, S.; Eliasson, V.; Andersson, B.A. Inhibitory effect of N-acetylcysteine on adherence of Streptococcus pneumoniae and Haemophilus influenzae to human oropharyngeal epithelial cells in vitro. Respiration 2000, 67, 552–558. [Google Scholar] [CrossRef]
- Zheng, J.P.; Wen, F.Q.; Bai, C.X.; Wan, H.Y.; Kang, J.; Chen, P.; Yao, W.Z.; Ma, L.J.; Li, X.; Raiteri, L.; et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): A randomised, double-blind placebo-controlled trial. Lancet Respir. Med. 2014, 2, 187–194. [Google Scholar] [CrossRef]
- Aylward, M.; Maddock, J.; Dewland, P. Clinical evaluation of acetylcysteine in the treatment of patients with chronic obstructive bronchitis: A balanced double-blind trial with placebo control. Eur. J. Respir. Dis. Suppl. 1980, 111, 81–89. [Google Scholar] [PubMed]
- Pela, R.; Calcagni, A.M.; Subiaco, S.; Isidori, P.; Tubaldi, A.; Sanguinetti, C.M. N-acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPD. Respiration 1999, 66, 495–500. [Google Scholar] [CrossRef]
- Decramer, M.; Rutten-van Mölken, M.; Dekhuijzen, P.R.; Troosters, T.; van Herwaarden, C.; Pellegrino, R.; van Schayck, C.O.; Olivieri, D.; Del Donno, M.; De Backer, W.; et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet 2005, 365, 1552–1560. [Google Scholar] [CrossRef]
- Fowdar, K.; Chen, H.; He, Z.; Zhang, J.; Zhong, X.; Zhang, J.; Li, M.; Bai, J. The effect of N-acetylcysteine on exacerbations of chronic obstructive pulmonary disease: A meta-analysis and systematic review. Heart Lung 2017, 46, 120–128. [Google Scholar] [CrossRef]
- Schermer, T.; Chavannes, N.; Dekhuijzen, R.; Wouters, E.; Muris, J.; Akkermans, R.; van Schayck, O.; van Weel, C. Fluticasone and N-acetylcysteine in primary care patients with COPD or chronic bronchitis. Respir. Med. 2009, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Tse, H.N.; Raiteri, L.; Wong, K.Y.; Yee, K.S.; Ng, L.Y.; Wai, K.Y.; Loo, C.K.; Chan, M.H. High-dose N-acetylcysteine in stable COPD: The 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest 2013, 144, 106–118. [Google Scholar] [CrossRef]
- Shen, Y.; Cai, W.; Lei, S.; Zhang, Z. Effect of high/low dose N-acetylcysteine on chronic obstructive pulmonary disease: A systematic review and meta-analysis. COPD J. Chronic Obstr. Pulm. Dis. 2014, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Page, C.; Jardim, J.; Chuchalin, A.G.; Rogliani, P.; Matera, M.G. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: A meta-analysis. Eur. Respir. Rev. 2015, 24, 451–461. [Google Scholar] [CrossRef]
- Cazzola, M.; Rogliani, P.; Calzetta, L.; Hanania, N.A.; Matera, M.G. Impact of Mucolytic Agents on COPD Exacerbations: A Pair-wise and Network Meta-analysis. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Stey, C.; Steurer, J.; Bachmann, S.; Medici, T.C.; Tramèr, M.R. The effect of oral N-acetylcysteine in chronic bronchitis: A quantitative systematic review. Eur. Respir. J. 2000, 16, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, E.M.; Berthet, P.; Ruffmann, R.; Leuenberger, P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: A meta-analysis of published double-blind, placebo-controlled clinical trials. Clin. Ther. 2000, 22, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Alfano, F.; Bigoni, T.; Mancini, L.; Mawass, A.; Baraldi, F.; Aljama, C.; Contoli, M.; Miravitlles, M. N-acetylcysteine Treatment in Chronic Obstructive Pulmonary Disease (COPD) and Chronic Bronchitis/Pre-COPD: Distinct Meta-analyses. Arch. Bronconeumol. 2024, 60, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Poole, P.; Chong, J.; Cates, C.J. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015, 7, CD001287. [Google Scholar] [CrossRef]
- Poole, P.J.; Black, P.N. Oral mucolytic drugs for exacerbations of chronic obstructive pulmonary disease: Systematic review. BMJ 2001, 322, 1271–1274. [Google Scholar] [CrossRef]
- Mokra, D.; Mokry, J.; Barosova, R.; Hanusrichterova, J. Advances in the Use of N-Acetylcysteine in Chronic Respiratory Diseases. Antioxidants 2023, 12, 1713. [Google Scholar] [CrossRef] [PubMed]
- Maghsadi, Z.; Azadmehr, A.; Moghadamnia, A.A.; Feizi, F.; Hamidi, N. N-Acetylcysteine attenuated pulmonary fibrosis induced by bleomycin via immunomodulation responses. Res. Pharm. Sci. 2023, 18, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Zhang, J.; Wang, Z.; Wu, Q.; Zhou, X. Efficacy and safety of N-acetylcysteine therapy for idiopathic pulmonary fibrosis: An updated systematic review and meta-analysis. Exp. Ther. Med. 2019, 18, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Idiopathic Pulmonary Fibrosis Clinical Research Network; Martinez, F.J.; de Andrade, J.A.; Anstrom, K.J.; King, T.E.; Raghu, G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Calzetta, L.; Cavalli, F.; Matera, M.G.; Cazzola, M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Pulm. Pharmacol. Ther. 2016, 40, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Podolanczuk, A.J.; Noth, I.; Raghu, G. Idiopathic pulmonary fibrosis: Prime time for a precision-based approach to treatment with N-acetylcysteine. Eur. Respir. J. 2021, 57, 2003551. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Z.; Kotuk, M.; Iraz, M.; Kuku, I.; Ulu, R.; Armutcu, F.; Ozen, S. Attenuation of bleomycin-induced lung fibrosis by oral sulfhydryl containing antioxidants in rats: Erdosteine and N-acetylcysteine. Pulm. Pharmacol. Ther. 2005, 18, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Oldham, J.M.; Ma, S.F.; Martinez, F.J.; Anstrom, K.J.; Raghu, G.; Schwartz, D.A.; Valenzi, E.; Witt, L.; Lee, C.; Vij, R.; et al. TOLLIP, MUC5B, and the Response to N-Acetylcysteine among Individuals with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2015, 192, 1475–1482. [Google Scholar] [CrossRef]
- Qi, Q.; Ailiyaer, Y.; Liu, R.; Zhang, Y.; Li, C.; Liu, M.; Wang, X.; Jing, L.; Li, Y. Effect of N-acetylcysteine on exacerbations of bronchiectasis (BENE): A randomized controlled trial. Respir. Res. 2019, 20, 73. [Google Scholar] [CrossRef]
- Jayaram, L.; King, P.T.; Hunt, J.; Lim, M.; Park, C.; Hu, E.; Dousha, L.; Ha, P.; Bartlett, J.B.; Southcott, A.M.; et al. Evaluation of high dose N-Acetylcysteine on airway inflammation and quality of life outcomes in adults with bronchiectasis: A randomised placebo-controlled pilot study. Pulm. Pharmacol. Ther. 2024, 84, 102283. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, Y.; Zi, K.; Shen, Y.; Wang, T.; Qin, J.; Chen, L.; Chen, M.; Liu, L.; Li, W.; et al. The effect of N-acetylcysteine in patients with non-cystic fibrosis bronchiectasis (NINCFB): Study protocol for a multicentre, double-blind, randomised, placebo-controlled trial. BMC Pulm. Med. 2022, 22, 401. [Google Scholar] [CrossRef] [PubMed]
- Guerini, M.; Condrò, G.; Friuli, V.; Maggi, L.; Perugini, P. N-acetylcysteine (NAC) and Its Role in Clinical Practice Management of Cystic Fibrosis (CF): A Review. Pharmaceuticals 2022, 15, 217. [Google Scholar] [CrossRef] [PubMed]
- Skov, M.; Pressler, T.; Lykkesfeldt, J.; Poulsen, H.E.; Jensen, P.Ø.; Johansen, H.K.; Qvist, T.; Kræmer, D.; Høiby, N.; Ciofu, O. The effect of short-term, high-dose oral N-acetylcysteine treatment on oxidative stress markers in cystic fibrosis patients with chronic P. aeruginosa infection—A pilot study. J. Cyst. Fibros. 2015, 14, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.; Lymp, J.; Thompson, V.; Dunn, C.; Davies, Z.; Chatfield, B.; Nichols, D.; Clancy, J.; Vender, R.; Egan, M.E.; et al. Long-term treatment with oral N-acetylcysteine: Affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. J. Cyst. Fibros. 2015, 14, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Tirouvanziam, R.; Conrad, C.K.; Bottiglieri, T.; Herzenberg, L.A.; Moss, R.B.; Herzenberg, L.A. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4628–4633. [Google Scholar] [CrossRef]
- Santus, P.; Danzo, F.; Zuffi, A.; Pini, S.; Saad, M.; Visconti, A.; Radovanovic, D. Oxidative stress and viral Infections: Rationale, experiences, and perspectives on N-acetylcysteine. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8582–8590. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).