Prediction of Back Disability Using Clinical, Functional, and Biomechanical Variables in Adults with Chronic Nonspecific Low Back Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Statistical Analysis
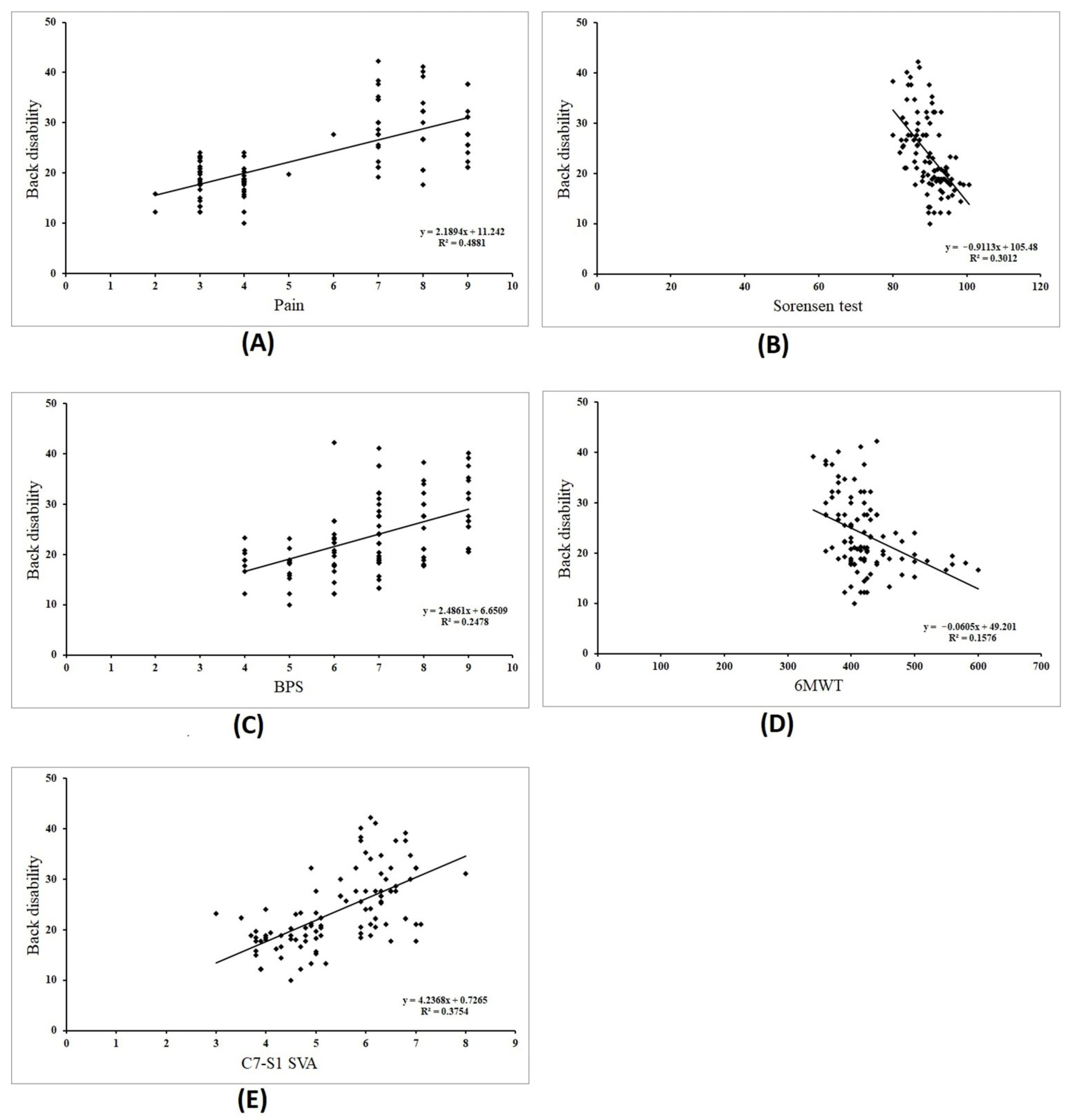
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef] [PubMed]
- Herndon, C.M.; Zoberi, K.S.; Gardner, B.J. Common questions about chronic low back pain. Am. Fam. Physician 2015, 91, 708–714. [Google Scholar] [PubMed]
- Mattiuzzi, C.; Lippi, G.; Bovo, C. Current epidemiology of low back pain. J. Hosp. Manag. Health Policy 2020, 4, 15. [Google Scholar] [CrossRef]
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.E.; Karstoft, K.I.; Lauridsen, H.H.; Manniche, C. Trajectories of disability in low back pain. Pain. Rep. 2022, 7, e985. [Google Scholar] [CrossRef] [PubMed]
- Mirzamohammadia, E.; Ghandharib, H.; Pirbornatana, M.; Mohammadia, S.; Hosseininejad, M. Assessment of disability levels in patients with low back pain based on the type of lumbar spinal disorder. J. Back. Musculoskelet. Rehabil. 2021, 34, 131–137. [Google Scholar] [CrossRef]
- Meucci, R.D.; Fassa, A.G.; Faria, N.M. Prevalence of chronic low back pain: Systematic review. Rev. Saude Publica 2015, 49, 1. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.H.; Chiel, H.J.; Trumbower, R.D.; Allen, J.L.; McKay, J.L.; Hackney, M.E.; Kesar, T.M. Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron 2015, 86, 38–54. [Google Scholar] [CrossRef]
- Murphy, D. Conservative management of cervical spine syndromes. In Dysfunction in the Cervical Spine; McGraw-Hill: New York, NY, USA, 2000; pp. 71–103. [Google Scholar]
- Doualla, M.; Aminde, J.; Aminde, L.N.; Lekpa, F.K.; Kwedi, F.M.; Yenshu, E.V.; Chichom, A.M. Factors influencing disability in patients with chronic low back pain attending a tertiary hospital in sub-Saharan Africa. BMC Musculoskelet. Disord. 2019, 20, 25. [Google Scholar] [CrossRef]
- Sun, P.; Li, K.; Yao, X.; Wu, Z.; Yang, Y. Association between functional disability with postural balance among patients with chronic low back pain. Front. Neurol. 2023, 14, 1136137. [Google Scholar] [CrossRef]
- Sirbu, E.; Onofrei, R.R.; Szasz, S.; Susan, M. Predictors of disability in patients with chronic low back pain. Arch. Med. Sci. 2023, 19, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.G.; Roios, E.; Pereira, M. Functional disability in patients with low back pain: The mediator role of suffering and beliefs about pain control in patients receiving physical and chiropractic treatment. Braz. J. Phys. Ther. 2017, 21, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Saragih, I.S.; Harahap, I.A.; Dharmajaya, R. The Relationship between Pain and Disability in Patients with Low Back Pain. Int. J. Nurs. Health Serv. 2020, 3, 147–154. [Google Scholar]
- Harahap, I.A.; Huda, S.N.; Tanjung, D.; Siregar, C.T.; Nasution, S.Z.; Ariga, R.A. Relationship between pain intensity and disability in chronic low back pain patients. Enferm. Clín. 2021, 31, 553–555. [Google Scholar] [CrossRef]
- Demoulin, C.; Vanderthommen, M.; Duysens, C.; Crielaard, J. Spinal muscle evaluation using the Sorensen test: A critical appraisal of the literature. Jt. Bone Spine 2006, 73, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ummunah, J.O.; Ibikunle, P.O.; Ezeakunne, A.C. Relationship between isometric endurance of back extensor muscles and selected anthropometric indices among some Nigerian undergraduates. J. Back. Musculoskelet. Rehabil. 2014, 27, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Behennah, J.; Conway, R.; Fisher, J.; Osborne, N.; Steele, J. The relationship between balance performance, lumbar extension strength, trunk extension endurance, and pain in participants with chronic low back pain, and those without. Clin. Biomech. 2018, 53, 22–30. [Google Scholar] [CrossRef]
- Bozorgmehr, A.; Zahednejad, S.; Salehi, R.; Ansar, N.N.; Abbasi, S.; Mohsenifar, H. Relationships between muscular impairments, pain, and disability in patients with chronic nonspecific low back pain: A cross sectional study. J. Exerc. Rehabil. 2018, 14, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Froud, R.; Patel, S.; Rajendran, D.; Bright, P.; Bjørkli, T.; Buchbinder, R.; Eldridge, S.; Underwood, M. A Systematic Review of Outcome Measures Use, Analytical Approaches, Reporting Methods, and Publication Volume by Year in Low Back Pain Trials Published between 1980 and 2012: Respice, adspice, et prospice. PLoS ONE 2016, 11, e01645732016. [Google Scholar] [CrossRef]
- Strand, L.I.; Moe-Nilssen, R.; Ljunggren, A.E. Back Performance Scale for the Assessment of Mobility-Related Activities in People With Back Pain. Phys. Ther. 2002, 82, 1213–1223. [Google Scholar] [CrossRef]
- Hurri, H.; Vänni, T.; Muttonen, E.; Russo, F.; Iavicoli, S.; Ristolainen, L. Functional Tests Predicting Return to Work of Workers with Non-Specific Low Back Pain: Are There Any Validated and Usable Functional Tests for Occupational Health Services in Everyday Practice? A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 5188. [Google Scholar] [CrossRef] [PubMed]
- Peppin, J.F.; Marcum, S.; Kirsh, K.L. The chronic pain patient and functional assessment: Use of the 6-Minute Walk Test in a multidisciplinary pain clinic. Curr. Med. Res. Opin. 2014, 30, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Nim, C.G.; O’Sullivan, K.; O’Neill, S. Testing walking performance in patients with low back pain: Will two minutes do instead of six minutes? Disabil. Rehabil. 2023, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, T.Ş.; Akkaya, S. Is there a relationship between chronic low back pain and spinal sagittal balance? A prospective controlled study. Ankyra Med. J. 2023, 2, 95–100. [Google Scholar] [CrossRef]
- Harrison, D.E.; Colloca, C.J.; Harrison, D.D.; Janik, T.J.; Haas, J.W.; Keller, T.S. Anterior thoracic posture increases thoracolumbar disc loading. Eur. Spine J. 2005, 14, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Chaléat-Valayer, E.; Mac-Thiong, J.M.; Paquet, J.; Berthonnaud, E.; Siani, F.; Roussouly, P. Sagittal spino-pelvic alignment in chronic low back pain. Eur. Spine J. 2011, 20, 634–640. [Google Scholar] [CrossRef]
- Naserkhaki, S.; Jaremko, J.L.; El-Rich, M. Effects of inter-individual lumbar spine geometry variation on load-sharing: Geometrically personalized Finite Element study. J. Biomech. 2016, 49, 2909–2917. [Google Scholar] [CrossRef]
- Katzman, W.; Parimi, N.; Gladin, A.; Fan, B.; Wong, S.; Mergenthaler, J.; Lane, N.E. Reliability of sagittal vertical axis measurement and association with measures of age-related hyperkyphosis. J. Phys. Ther. Sci. 2018, 30, 1417–1423. [Google Scholar] [CrossRef]
- Verburg, A.; Dulmen, S.; Kiers, H.; Sanden, M.; Wees, P. Development of a standard set of outcome measures for non-specific low back pain in Dutch primary care physiotherapy practices: A Delphi study. Eur. Spine J. 2019, 28, 1550–1564. [Google Scholar] [CrossRef]
- Silva, F.G.; Costa, L.O.; Hancock, M.J.; Palomo, G.A.; Costa, L.C.; Silva, T. No prognostic model for people with recent-onset low back pain has yet been demonstrated to be suitable for use in clinical practice: A systematic review. J. Physiother. 2022, 68, 99–109. [Google Scholar] [CrossRef]
- Mukasa, D.; Sung, J. A prediction model of low back pain risk: A population-based cohort study in Korea. Korean J. Pain. 2020, 33, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Petrozzi, M.J.; Rubinstein, S.M.; Ferreira, P.H.; Leaver, A.; Mackey, M.G. Predictors of low back disability in chiropractic and physical therapy settings. Chiropr. Man. Ther. 2020, 28, 41. [Google Scholar] [CrossRef]
- Petersen, T.; Laslett, M.; Juhl, C. Clinical classification in low back pain: Best-evidence diagnostic rules based on systematic reviews. BMC Musculoskelet. Disord. 2017, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Kayihan, G. Relationship between daily physical activity level and low back pain in young, female desk-job workers. Int. J. Occup. Med. Environ. Health 2014, 27, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Fairbank, J.C.; Pynsent, P.B. The oswestry disability index. Spine 2000, 22, 2940–2952. [Google Scholar] [CrossRef] [PubMed]
- Vianin, M. Psychometric properties and clinical usefulness of the Oswestry Disability Index. J. Chiropr. Med. 2008, 7, 161–163. [Google Scholar] [CrossRef]
- Ostelo, R.W.; Deyo, R.A.; Stratford, P.; Waddell, G.; Croft, P.; Von Korff, M.; Bouter, L.M.; de Vet, H.C. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimal important change. Spine 2008, 33, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Reneman, M.F.; Posthumus, J.B.; Stewart, R.E. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int. J. Rehabil. Res. 2008, 31, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. 11), S240–S252. [Google Scholar]
- Ghroubi, S.; Jribi, S.; Jdidi, J.; Yahia, A.; Elleuch, W.; Chaaben, M.; Dammak, J.; Elleuch, M.H. Study of the validity and reproducibility of the Biering- Sorensen test in chronic low back pain. Ann. Phys. Rehabil. Med. 2015, 58 (Suppl. 1), e93–e97. [Google Scholar] [CrossRef]
- Shaw, J.; Jacobs, J.V.; Van Dillen, L.R.; Beneck, G.J.; Smith, J.A. Understanding the Biering-Sørensen test: Contributors to extensor endurance in young adults with and without a history of low back pain. J. Electromyogr. Kinesiol. 2024, 74, 102854. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, L.; Strand, L.I.; Lygren, H. Reliability and validity of the back performance scale: Observing activity limitation in patients with back pain. Spine 2004, 29, 903–907. [Google Scholar] [CrossRef]
- Benz, T.; Lehmann, S.; Elfering, A.; Sandor, P.S.; Angst, F. Comprehensiveness and validity of a multidimensional assessment in patients with chronic low back pain: A prospective cohort study. BMC Musculoskelet. Disord. 2021, 22, 291. [Google Scholar] [CrossRef]
- Fell, B.L.; Hanekom, S.; Heine, M. Six-minute walk test protocol variations in low-resource settings—A scoping review. S. Afr. J. Physiother. 2021, 77, 1549. [Google Scholar] [CrossRef]
- Aota, Y.; Saito, T.; Uesugi, M.; Kato, S.; Kuniya, H.; Koh, R. Optimal arm position for evaluation of spinal sagittal balance. J. Spinal Disord. Tech. 2011, 24, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Lafage, V.; Schwab, F.; Vira, S.; Hart, R.; Burton, D.; Smith, J.S.; Boachie-Adjei, O.; Shelokov, A.; Hostin, R.; Shaffrey, C.I.; et al. Does vertebral level of pedicle subtraction osteotomy correlate with degree of spinopelvic parameter correction? J. Neurosurg. Spine 2011, 14, 184–191. [Google Scholar] [CrossRef]
- Savarese, L.G.; Menezes-Reis, R.; Bonugli, G.P.; Herrero, C.F.P.D.S.; Defino, H.L.A.; Nogueira-Barbosa, M.H. Spinopelvic sagittal balance: What does the radiologist need to know? Radiol. Bras. 2020, 53, 175–184. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.; Fan, Y.; Zhou, Z.; Gu, G.; Wang, C.; Feng, C.; Chen, J.; He, S.; Ni, H. Sagittal alignment of the cervical spine: Radiographic analysis of 111 asymptomatic adolescents, a retrospective observational study. BMC Musculoskelet. Disord. 2022, 23, 840. [Google Scholar] [CrossRef] [PubMed]
- Vanti, C.; Conti, C.; Faresin, F.; Ferrari, S.; Piccarreta, R. The Relationship Between Clinical Instability and Endurance Tests, Pain, and Disability in Nonspecific Low Back Pain. J. Manip. Physiol. Ther. 2016, 39, 359–368. [Google Scholar] [CrossRef]
- Abdelraouf, O.R.; Abdel-Aziem, A.A. The relationship between core endurance and back dysfunction in collegiate male athletes with and without nonspecific low back pain. Int. J. Sports Phys. Ther. 2016, 11, 337–344. [Google Scholar]
- Danneels, L.A.; Vanderstraeten, G.G.; Cambier, D.C.; Witvrouw, E.E.; De Cuyper, H.J. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur. Spine J. 2000, 9, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W. Core stability exercise in chronic low back pain. Orthop. Clin. N. Am. 2003, 34, 245–254. [Google Scholar] [CrossRef]
- Wallwork, T.L.; Stanton, W.R.; Freke, M.; Hides, J.A. The effect of chronic low back pain on size and contraction of the lumbar multifidus muscle. Man. Ther. 2009, 14, 496–500. [Google Scholar] [CrossRef]
- Jewell, D.V.; Moore, J.D.; Goldstein, M.S. Delivering the physical therapy value proposition: A call to action. Phys. Ther. 2013, 93, 104–114. [Google Scholar] [CrossRef]
- Elabd, A.M.; Elabd, O.M. Relationships between forward head posture and lumbopelvic sagittal alignment in older adults with chronic low back pain. J. Bodyw. Mov. Ther. 2021, 28, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Haas, J.W.; Moustafa, I.M.; Betz, J.W.; Oakley, P.A. Can the Mismatch of Measured Pelvic Morphology vs. Lumbar Lordosis Predict Chronic Low Back Pain Patients? J. Clin. Med. 2024, 13, 2178. [Google Scholar] [CrossRef]
- Elabd, A.; Elerian, A.; Ahmed, S.; Elhafez, H.; Geneidy, A.; Ashour, A.; Elabd, O. Effect of Forward Head Posture Correction Added to Lumber Stabilization Exercises on Lumbopelvic Organization in Mechanical Low Back Pain Patients: A Randomized Clinical Trial. Fizjoter. Pol. 2020, 20, 132–140. [Google Scholar]
- Elabd, A.M.; Rasslan, S.B.; Elhafez, H.M.; Elabd, O.M.; Behiry, M.A.; Elerian, A.I. Efficacy of Integrating Cervical Posture Correction With Lumbar Stabilization Exercises for Mechanical Low Back Pain: A Randomized Blinded Clinical Trial. J. Appl. Biomech. 2021, 37, 43–51. [Google Scholar] [CrossRef]
- Borghuis, J.; Hof, A.L.; Lemmink, K.A. The importance of sensory-motor control in providing core stability: Implications for measurement and training. Sports Med. 2008, 38, 893–916. [Google Scholar] [CrossRef]
- Bezgin, S.; Arslan, S.A.; Sertel, M.; Vergili, O.; Kocaman, A.; Oral, M. The relationship between balance, trunk muscular endurance, and functional level in individuals with chronic low back pain. Ann. Med. Res. 2020, 27, 582–587. [Google Scholar] [CrossRef]
- Lin, C.C.; McAuley, J.H.; Macedo, L.; Barnett, D.C.; Smeets, R.J.; Verbunt, J.A. Relationship between physical activity and disability in low back pain: A systematic review and meta-analysis. Pain 2011, 152, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Elabd, A.; Elabd, O. Effect of aerobic exercises on patients with chronic mechanical low back pain: A randomized controlled clinical trial. J. Bodyw. Mov. Ther. 2024, 37, 379–385. [Google Scholar] [CrossRef] [PubMed]
| Variables | Mean (SD) | Minimum | Maximum |
|---|---|---|---|
| Age (year) | 33.0 (6.0) | 22.0 | 40.0 |
| Weight (kg) | 79.4 (10.4) | 57.0 | 95.0 |
| Height (cm) | 171.3 (7.3) | 160.0 | 185.0 |
| BMI (kg/m2) | 27.0 (2.0) | 22.3 | 29.4 |
| ODI (%) | 23.7 (7.4) | 10 | 42.2 |
| Variables | Mean (SD) (n = 100) | r | p-Value 1 | r (p-Value 2) |
|---|---|---|---|---|
| Pain (cm) | 5.67 ± 2.36 | 0.699 | 0.0001 * | 0.723 (0.0001 *) |
| Sorensen (seconds) | 89.79 ± 4.45 | −0.549 | 0.0001 * | |
| BPS (points) | 6.84 ± 1.48 | 0.501 | 0.0001 * | |
| 6MWT (m) | 422.24 ± 48.56 | −0.397 | 0.0001 * | |
| C7-S1 SVA (cm) | 5.41 ± 1.07 | 0.613 | 0.0001 * |
| Dependent Variables (Y) | Independent Variable (x) | Bivariate Regression Model | 95% CI | p-Value |
|---|---|---|---|---|
| Back disability | Pain | Y = 11.24 + 2.189x | 1.74–2.63 | 0.0001 * |
| Sorensen | Y = 105.48 − 0.911x | −1.19–−0.63 | 0.0001 * | |
| BPS | Y = 6.65 + 2.486x | 1.61–3.35 | 0.0001 * | |
| 6MWT | Y = 49.20 − 0.060x | −0.08–−0.03 | 0.0001 * | |
| C7-S1 SVA | Y = 0.72 + 4.23x | 3.14–5.33 | 0.0001 * |
| Dependent Variables (Y) | Independent Variable (x) | Multivariate Regression Model | 95% CI | p-Value 1 | p-Value 2 |
|---|---|---|---|---|---|
| Back disability | Constant | 46.84 | 14.52–79.16 | 0.005 * | 0.0001 * |
| Pain (x1) | 1.540 | 0.61–2.46 | 0.001 * | ||
| Sorensen (x2) | −0.339 | −0.64–−0.04 | 0.028 * | ||
| BPS (x3) | −0.050 | −1.04–0.94 | 0.921 | ||
| 6MWT (x4) | −0.010 | −0.03–0.02 | 0.439 | ||
| C7-S1 SVA (x5) | 0.541 | −1.21–2.29 | 0.542 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elabd, O.M.; Oakley, P.A.; Elabd, A.M. Prediction of Back Disability Using Clinical, Functional, and Biomechanical Variables in Adults with Chronic Nonspecific Low Back Pain. J. Clin. Med. 2024, 13, 3980. https://doi.org/10.3390/jcm13133980
Elabd OM, Oakley PA, Elabd AM. Prediction of Back Disability Using Clinical, Functional, and Biomechanical Variables in Adults with Chronic Nonspecific Low Back Pain. Journal of Clinical Medicine. 2024; 13(13):3980. https://doi.org/10.3390/jcm13133980
Chicago/Turabian StyleElabd, Omar M., Paul A. Oakley, and Aliaa M. Elabd. 2024. "Prediction of Back Disability Using Clinical, Functional, and Biomechanical Variables in Adults with Chronic Nonspecific Low Back Pain" Journal of Clinical Medicine 13, no. 13: 3980. https://doi.org/10.3390/jcm13133980
APA StyleElabd, O. M., Oakley, P. A., & Elabd, A. M. (2024). Prediction of Back Disability Using Clinical, Functional, and Biomechanical Variables in Adults with Chronic Nonspecific Low Back Pain. Journal of Clinical Medicine, 13(13), 3980. https://doi.org/10.3390/jcm13133980






