Assessment of Blood Flow Velocity in Retinal Vasculitis Using the Retinal Function Imager—A Pilot Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Retinal Function Imager
2.3. Imaging and Image Analysis
2.4. Statistical Analysis
3. Results
| Pat. no. | Dx | HLA-A29 | Uveitis Duration since Onset (m) | Age (y) | Sex | Affected Eye(s) | Course of Uveitis | FAG Findings | Uveitis Complications | Uveitis Tx (Always Months before Imaging) | Past Surgical History (Always Months before Imaging) | Concomitant Systemic Disease | BCVA (logMAR) OD/OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BSCR | pos. | 264 | 68 | Female | OU | pers. | OU: 2;4 | OU: retinal atrophy | Fa implant 0.59 mg: | Ahmed-Valve: OD 65 m | Migraine, HTN, Hypercholesterinemia | 0.1/0.1 |
| OU: sec. glaucoma | OD: 2× (last 36 m) | CE: OD (74 m) | |||||||||||
| OU: CME in the past | OS: 3× (last 18 m) | CE: OS (126 m) | |||||||||||
| 2 | BSCR | pos. | 108 | 48 | Female | OU | pers. | OU: 0 | None | MMF 3 g/d (start: 17 m) | none | none | 0.1/0.1 |
| 3 | RVUO | neg. | 108 | 44 | Male | OU | pers. | OU: 1;4 | OD: sec. glaucoma OD: sec. cataract | Fa implant 0.59 mg: OD: (78 m) Peg-IFN alpha sc (stop 57 m) | Removal of Fa implant (steroid response): OD (67 m) | none | 0.2/0.1 |
| 4 | BSCR | pos. | 61 | 33 | Female | OD > OS | pers. | OU: 3;4 | OD > >OS: ret. atrophy OD: sec. cataract OU: CME | Dm implant: OD (16 m) TA ivi: OD (36 m) MMF: 1 g/d (start 38 m) IFX: 5 mg/kg BW iv, (stop 1 m) | CE: OD (18 m) | none | 0.7/1.0 |
| 5 | BSCR | pos. | 60 | 67 | Male | OU | pers. | OU: 0;4 | OU: sec. cataract | Fa implant 0.59 mg: OU (8 m) MMF 2g/d (stop 14 m) | CE: OU (180 m) | none | 0.6/0.1 |
| 6 | RVUO | neg. | 15 | 46 | Female | OD >> OS | pers. | OU: 1;4 | OD: sec. cataract OU: sec. glaucoma | ADA 40 mg sc/second week (start 2 m) IFX 5 mg/kg BW iv (7 times, stop 2 m) MMF 2 g/d (stop 3 m) | CE: OD (10 m) ppV(ret. detachment): OD (14 m) | Crohn’s disease, Hashimoto Thyreoiditis | 1.0/0 |
| 7 | BSCR | pos. | 8 | 48 | Female | OD > OS | pers. | OD > OS: 1 | none | none | none | Chronic Fatique Syndrom | 0.4/0 |
| 8 | RVUO | neg. | 8 | 39 | Male | OS >> OD | pers. | OU:1 OS:4 | none | none | none | none | 0/0.1 |
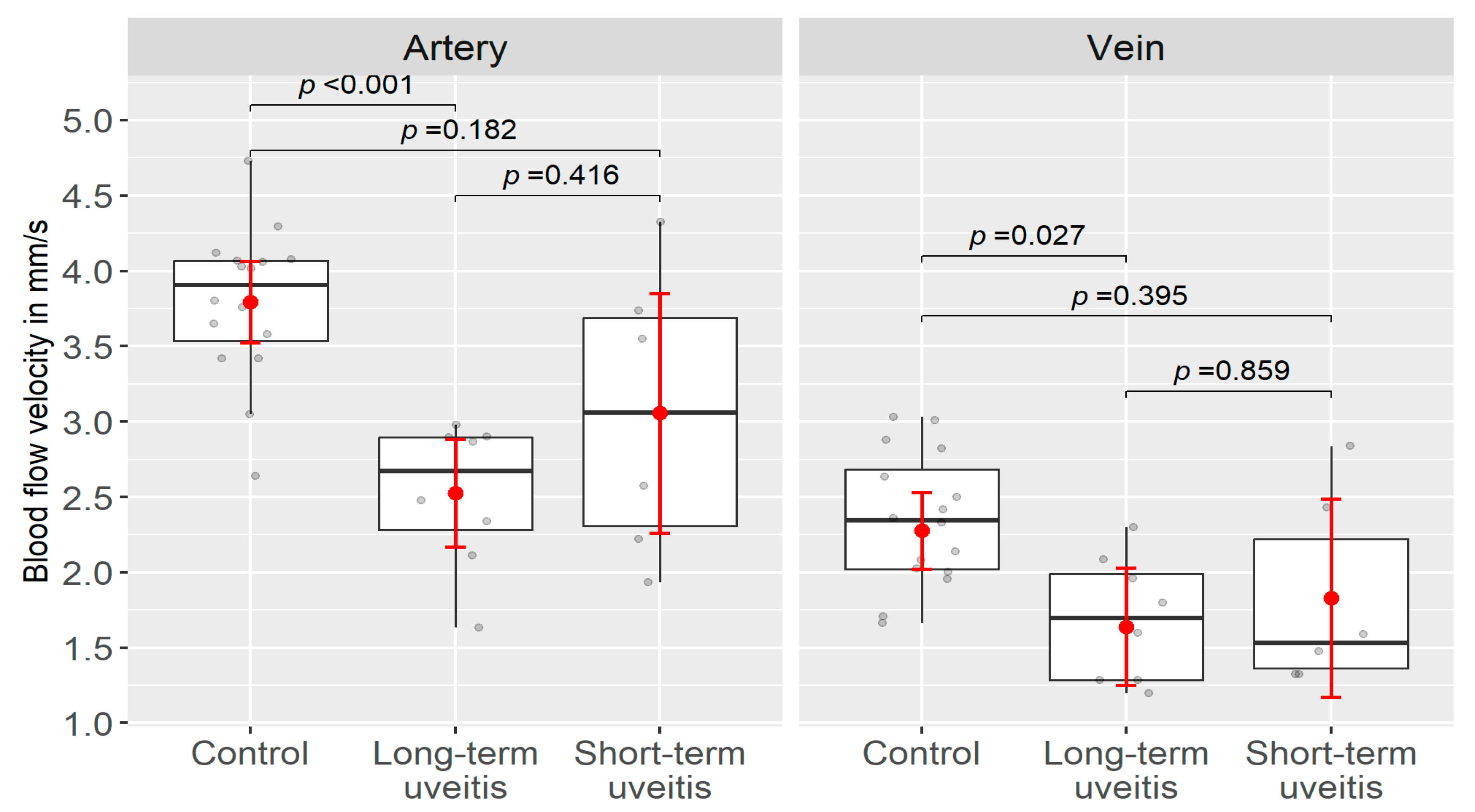
3.1. Retinal Blood Flow Velocities
3.2. Capillary Perfusion Maps/Blood Flow Velocity Maps
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hughes, E.H.; Dick, A.D. The pathology and pathogenesis of retinal vasculitis. Neuropathol. Appl. Neurobiol. 2003, 29, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ku, J.H.; Suhler, E.B.; Choi, D.; Rosenbaum, J.T. The course of retinal vasculitis. Br. J. Ophthalmol. 2014, 98, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.T.; Sibley, C.H.; Lin, P. Retinal vasculitis. Curr. Opin. Rheumatol. 2016, 28, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Standardization of Uveitis Nomenclature (SUN) Working Group. Classification Criteria for Birdshot Chorioretinitis. Am. J. Ophthalmol. 2021, 228, 65–71. [Google Scholar] [CrossRef]
- Jabs, D.A.; Nussenblatt, R.B.; Rosenbaum, J.T. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 2005, 140, 509–516. [Google Scholar] [CrossRef]
- Testi, I.; Ajamil-Rodanes, S.; Al Bloushi, A.F.; Pavesio, C. Peripheral Capillary Non-perfusion in Birdshot Retinochoroiditis: A Novel Finding on Ultra-widefield Fluorescein Angiography. Ocul. Immunol. Inflamm. 2020, 28, 1192–1195. [Google Scholar] [CrossRef]
- Pohlmann, D.; Macedo, S.; Stuebiger, N.; Joussen, A.M.; Winterhalter, S. Multimodal Imaging in Birdshot Retinochoroiditis. Ocul. Immunol. Inflamm. 2017, 25, 621–632. [Google Scholar] [CrossRef]
- Su, D.; Garg, S. The retinal function imager and clinical applications. Eye Vis. 2018, 5, 20. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, H.; Grinvald, A.; Jayadev, C.; Wang, J. A Mini Review of Clinical and Research Applications of the Retinal Function Imager. Curr. Eye Res. 2018, 43, 273–288. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Ji, K.B.; Hu, Z.; Zhang, Q.L.; Mei, H.F.; Xing, Y.Q. Retinal microvasculature features in patients with Behçet’s disease: A systematic review and meta-analysis. Sci. Rep. 2022, 14, 752. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zuo, C.; Gao, X.; Huang, D.; Lin, M. Quantitative Measurements of Vessel Density and Blood Flow Areas Primary Angle Closure Diseases: A Study of Optical Coherence Tomography Angiography. J. Clin. Med. 2022, 13, 4040. [Google Scholar] [CrossRef] [PubMed]
- Dhirachaikulpanich, D.; Chanthongdee, K.; Zheng, Y.; Beare, N.A.V. A systematic review of OCT and OCT angiography in retinal vasculitis. J. Ophthal Inflamm. Infect. 2023, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Werkmeister, R.M.; Dragostinoff, N.; Palkovits, S.; Told, R.; Boltz, A.; Leitgeb, R.A.; Gröschl, M.; Garhöfer, G.; Schmetterer, L. Measurement of absolute blood flow velocity and blood flow in the human retina by dual-beam bidirectional Doppler fourier-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2012, 12, 6062–6071. [Google Scholar] [CrossRef]
- Dai, C.; Liu, X.; Zhang, H.F.; Puliafito, C.A.; Jiao, S. Absolute retinal blood flow measurement with a dual-beam Doppler optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2013, 9, 7998–8003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, O.; Liu, G.; Liang, L.; Gao, S.S.; Pechauer, A.D.; Jia, Y. En face Doppler total retinal blood flow measurement with 70 kHz spectral optical coherence tomography. J. Biomed. Opt. 2015, 20, 066004. [Google Scholar] [CrossRef]
- Tani, T.; Song, Y.S.; Yoshioka, T.; Omae, T.; Ishibazawa, A.; Akiba, M.; Yoshida, A. Repeatability and Reproducibility of Retinal Blood Flow Measurement Using a Doppler Optical Coherence Tomography Flowmeter in Healthy Subjects. Invest. Ophthalmol. Vis. Sci. 2017, 58, 2891–2898. [Google Scholar] [CrossRef] [PubMed]
- Pechauer, A.D.; Hwang, T.S.; Hagag, A.M.; Liu, L.; Tan, O.; Zhang, X.; Parker, M.; Huang, D.; Wilson, D.J.; Jia, Y. Assessing total retinal blood flow in diabetic retinopathy using multiplane en face Doppler optical coherence tomography. Br. J. Ophthalmol. 2018, 102, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Cano, J.; Farzad, S.; Khansari, M.M.; Tan, O.; Huang, D.; Lim, J.I.; Shahidi, M. Relating retinal blood flow and vessel morphology in sickle cell retinopathy. Eye 2020, 34, 886–891. [Google Scholar] [CrossRef]
- Oner, A.; Akal, A.; Erdogan, N.; Dogan, H.; Oner, M. Color Doppler imaging of ocular hemodynamic changes in Behcet disease and uveitis patients with different etiologies. Curr. Eye Res. 2006, 31, 519–523. [Google Scholar] [CrossRef]
- Dimitrova, G.; Kato, S. Color Doppler imaging of retinal diseases. Surv. Ophthalmol. 2010, 55, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Jonescu-Cuypers, C.P.; Harris, A.; Bartz-Schmidt, K.U.; Kagemann, L.; Boros, A.S.; Heimann, U.E.; Lenz, B.H.; Hilgers, R.D.; Krieglstein, G.K. Reproducibility of circadian retinal and optic nerve head blood flow measurements by Heidelberg retina flowmetry. Br. J. Ophthalmol. 2004, 88, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Konno, S.; Feke, G.T.; Yoshida, A.; Fujio, N.; Goger, D.G.; Buzney, S.M. Retinal blood flow changes in type I diabetes. A long-term follow-up study. Invest. Ophthalmol. Vis. Sci. 1996, 37, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Feke, G.T.; Mori, F.; Nagaoka, T.; Fujio, N.; Ogasawara, H.; Konno, S.; Mcmeel, J.W. Reproducibility and clinical application of a newly developed stabilized retinal laser Doppler instrument. Am. J. Ophthalmol. 2003, 135, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.P., Jr.; Garcia, P.T.; Rosen, R.B. Retinal blood flow in the normal human eye using the canon laser blood flowmeter. Ophthalmic Res. 2002, 34, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.A.; Elsner, A.E.; Sapoznik, K.A.; Warner, R.L.; Gast, T.J. Adaptive optics imaging of the human retina. Prog. Retin. Eye Res. 2019, 68, 1–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Castro, A.; Huang, G.; Sawides, L.; Luo, T.; Burns, S.A. Rapid high resolution imaging with a dual-channel scanning technique. Opt. Lett. 2016, 41, 1881–1884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nelson, D.A.; Krupsky, S.; Pollack, A.; Aloni, E.; Belkin, M.; Vanzetta, I.; Rosner, M.; Grinvald, A. Special report: Noninvasive multi-parameter functional optical imaging of the eye. Ophthalmic Surg. Lasers Imaging 2005, 36, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Izhaky, D.; Nelson, D.A.; Burgansky-Eliash, Z.; Grinvald, A. Functional imaging using the retinal function imager: Direct imaging of blood velocity, achieving fluorescein angiography-like images without any contrast agent, qualitative oximetry, and functional metabolic signals. Jpn. J. Ophthalmol. 2009, 53, 345–351. [Google Scholar] [CrossRef]
- Nelson, D.A.; Burgansky-Eliash, Z.; Barash, H.; Loewenstein, A.; Barak, A.; Bartov, E.; Rock, T.; Grinvald, A. High-resolution wide-field imaging of perfused capillaries without the use of contrast agent. Clin. Ophthalmol. 2011, 5, 1095–1106. [Google Scholar] [CrossRef]
- Chhablani, J.; Bartsch, D.U.; Cheng, L.; Gomez, L.; Alshareef, R.A.; Rezeq, S.S.; Garg, S.J.; Burgansky-Eliash, Z.; Freeman, W.R. Segmental reproducibility of retinal blood flow velocity measurements using retinal function imager. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Ganekal, S. Retinal functional imager (RFI): Non-invasive functional imaging of the retina. Nepal. J. Ophthalmol. 2013, 5, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; DeBuc, D.C.; Rundek, T.; Lam, B.L.; Wright, C.B.; Shen, M.; Tao, A.; Wang, J. Automated segmentation and fractal analysis of high-resolution non-invasive capillary perfusion maps of the human retina. Microvasc. Res. 2013, 89, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Somfai, G.M.; Campagnoli, T.R.; Smiddy, W.E.; DeBuc, D.C. Interactive retinal blood flow analysis of the macular region. Microvasc. Res. 2016, 104, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jayadev, C.; Jain, N.; Mohan, A.; Yadav, N.K. Clinical applications of the retinal functional imager: A brief review. Indian. J. Ophthalmol. 2019, 67, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Campagnoli, T.R.; Somfai, G.M.; Tian, J.; DeBuc, D.C.; Smiddy, W.E. Exploratory study of non-invasive, high-resolution functional macular imaging in subjects with diabetic retinopathy. Int. J. Ophthalmol. 2021, 14, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Christian, K.; Barash, H.; Izhaky, D.; Burgansky-Eliash, Z.; Nelson, D.A.; Barak, A.; Lowenstein, A.; Grinvald, A. High reproducibility of retinal blood flow velocity measurements using the retinal function imager. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1054. [Google Scholar]
- Burgansky-Eliash, Z.; Nelson, D.A.; Bar-Tal, O.P.; Lowenstein, A.; Grinvald, A.; Barak, A. Reduced retinal blood flow velocity in diabetic retinopathy. Retina 2010, 30, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Landa, G.; Amde, W.; Haileselassie, Y.; Rosen, R.B. Cilioretinal arteries in diabetic eyes are associated with increased retinal blood flow velocity and occurrence of diabetic macular edema. Retina 2011, 31, 304–311. [Google Scholar] [CrossRef]
- Burgansky-Eliash, Z.; Barak, A.; Barash, H.; Nelson, D.A.; Pupko, O.; Lowenstein, A.; Grinvald, A.; Rubinstein, A. Increased retinal blood flow velocity in patients with early diabetes mellitus. Retina 2012, 32, 112–119. [Google Scholar] [CrossRef]
- Stuebiger, N.; Smiddy, W.; Wang, J.; Jiang, H.; DeBuc, D.C. Assesment of Conjunctival Microangiopathy in a Patient with Diabetes Mellitus Using the Retinal Function Imager. J. Clin. Exp. Ophthalmol. 2015, 6, 400. [Google Scholar] [CrossRef] [PubMed]
- Burgansky-Eliash, Z.; Bartov, E.; Barak, A.; Grinvald, A.; Gaton, D. Blood-flow velocity in glaucoma patients measured with the retinal function imager. Curr. Eye Res. 2016, 41, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Enz, T.J.; Bittner, M.; Tribble, J.R.; Williams, P.A.; Thiel, M.A.; Schmid, M.K.; Bachmann, L.M.; Bochmann, F. Comparative Assessment of Retinal Blood Flow Velocity Changes Following Brimonidine and Brinzolamide Administration Using Retinal Function Imaging. Transl. Vis. Sci. Technol. 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.D.; Jiang, H.; Wang, J. Characterization of retinal microvasculature in acute non-arteritic anterior ischemic optic neuropathy using the retinal functional imager: A prospective case series. Eye Vis. 2019, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Barak, A.; Burgansky-Eliash, Z.; Barash, H.; Nelson, D.A.; Grinvald, A.; Loewenstein, A. The effect of intravitreal bevacizumab (Avastin) injection on retinal blood flow velocity in patients with choroidal neovascularization. Eur. J. Ophthalmol. 2012, 22, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Burgansky-Eliash, Z.; Barash, H.; Nelson, D.; Grinvald, A.; Sorkin, A.; Lowenstein, A.; Barak, A. Retinal blood flow velocity in patients with age-related macular degeneration. Curr. Eye Res. 2014, 39, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Bohni, S.C.; Howell, J.P.; Bittner, M.; Faes, L.; Bachmann, L.M.; Thiel, M.A.; Schmid, M.K. Blood flow velocity measured using the Retinal Function Imager predicts successful ranibizumab treatment in neovascular age related macular degeneration: Early prospective cohort study. Eye 2015, 29, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Allaf, A.M.; Wang, J.; Simms, A.G.; Jiang, H. Age-related alterations in retinal capillary function. Microvasc. Res. 2023, 148, 104508. [Google Scholar] [CrossRef] [PubMed]
- Beutelspacher, S.V.; Serbecic, N.; Barash, H.; Burgansky-Eliash, Z.; Grinvald, A.; Jonas, J.B. Central serous chorioretinopathy shows reduced retinal flow circulation in retinal function imaging (RFI). Acta Ophthalmol. 2011, 89, e479–e482. [Google Scholar] [CrossRef]
- Landa, G.; Rosen, R.B. A new vascular pattern for idiopathic juxtafoveal telangiectasia revealed by the retinal function imager. Ophthalmic Surg. Lasers Imaging 2010, 41, 413–417. [Google Scholar] [CrossRef]
- Feng, X.; Kedhar, S.; Bhoomibunchoo, C. Retinal blood flow velocity in patients with active uveitis using the retinal function imager. Chin. Med. J. 2013, 126, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, aka Least-Squares Means; R package version 1.10.1. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 19 May 2024).
- R Core Team. 2021. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 19 May 2024).
- Beutelspacher, S.V.; Serbecic, N.; Barash, H.; Burgansky-Eliash, Z.; Grinvald, A.; Krastel, H.; Jonas, J.B. Retinal blood flow velocity measured by retinal function imaging in retinitis pigmentosa. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1855–1858. [Google Scholar] [CrossRef] [PubMed]
- Jittpoonkuson, T.; Garcia, P.; Landa, G.; Rosen, R. Retinal blood-flow velocity and oximetry status monitoring in a central retinal vein occlusion patient. Retin. Physician 2009, 6, 52–54. [Google Scholar]
- Jiang, H.; Delgado, S.; Tan, J.; Liu, C.; Rammohan, K.W.; DeBuc, D.C.; Lam, B.L.; Feuer, W.J.; Wang, J. Impaired retinal microcirculation in multiple sclerosis. Mult. Scler. 2016, 22, 1812–1820. [Google Scholar] [CrossRef]
- Stuebiger, N.; Ruprecht, K.; Pleyer, U. Intraocular inflammation in multiple sclerosis. Ophthalmologe 2018, 115, 531–542. [Google Scholar] [CrossRef]
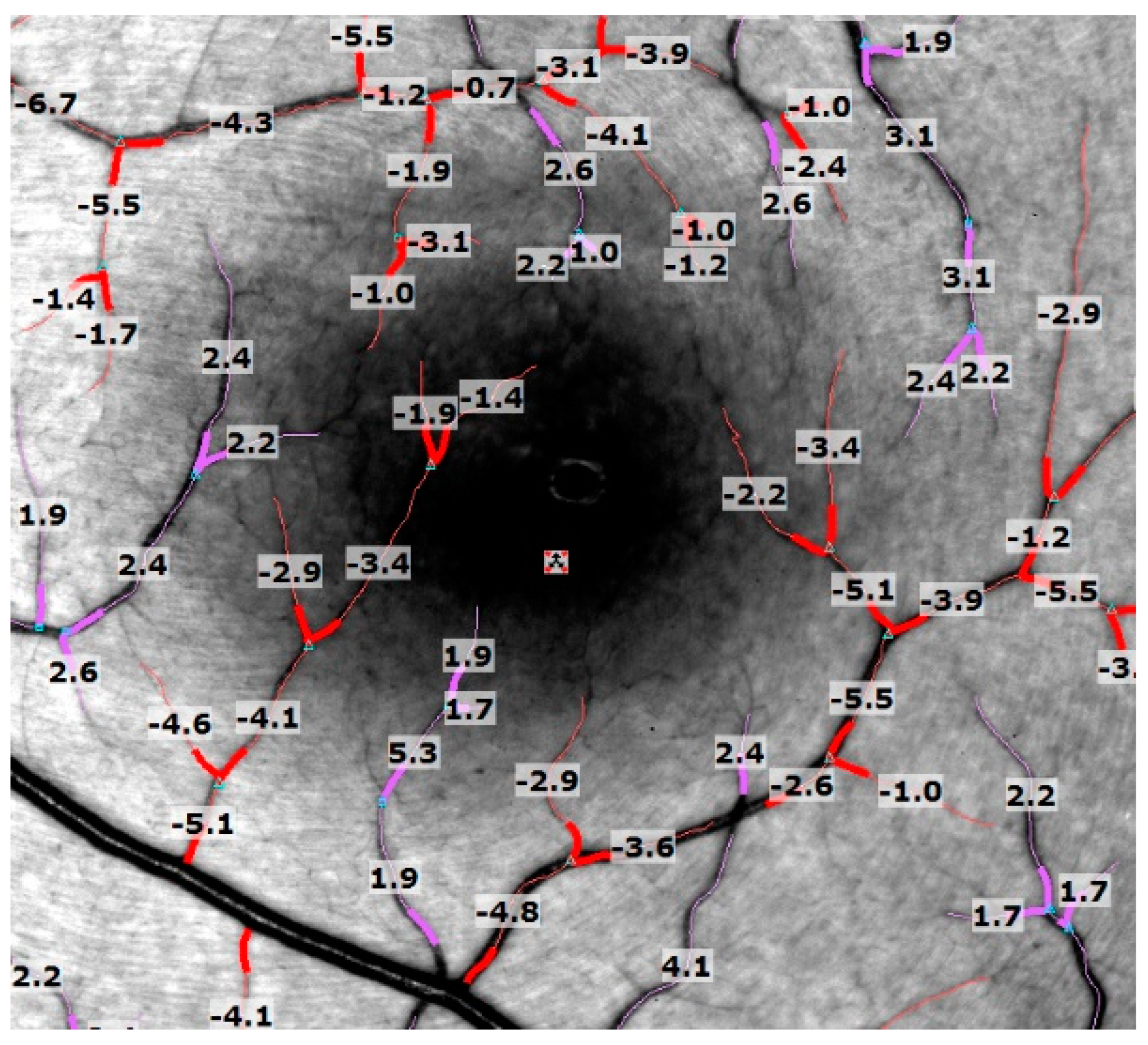
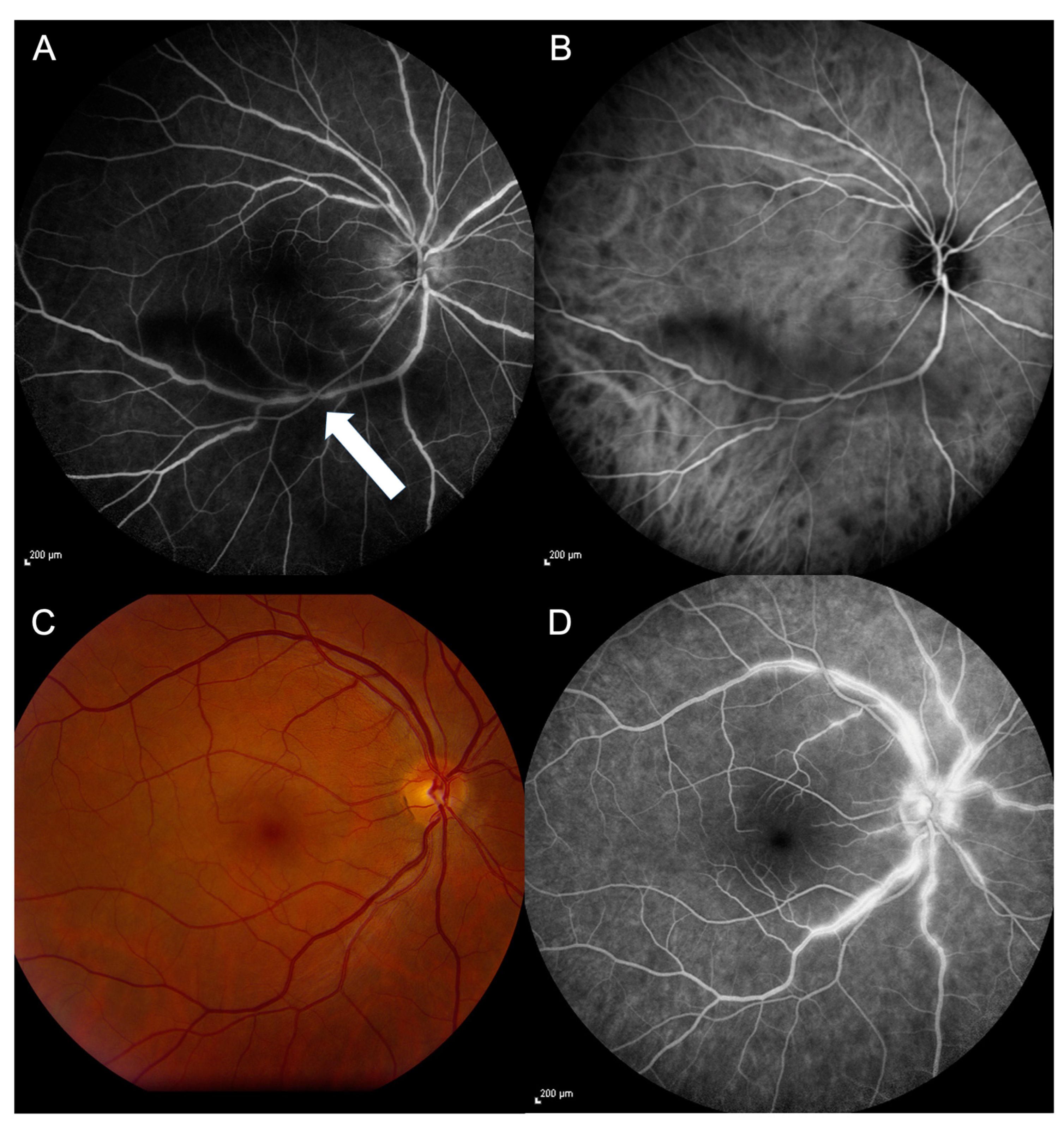

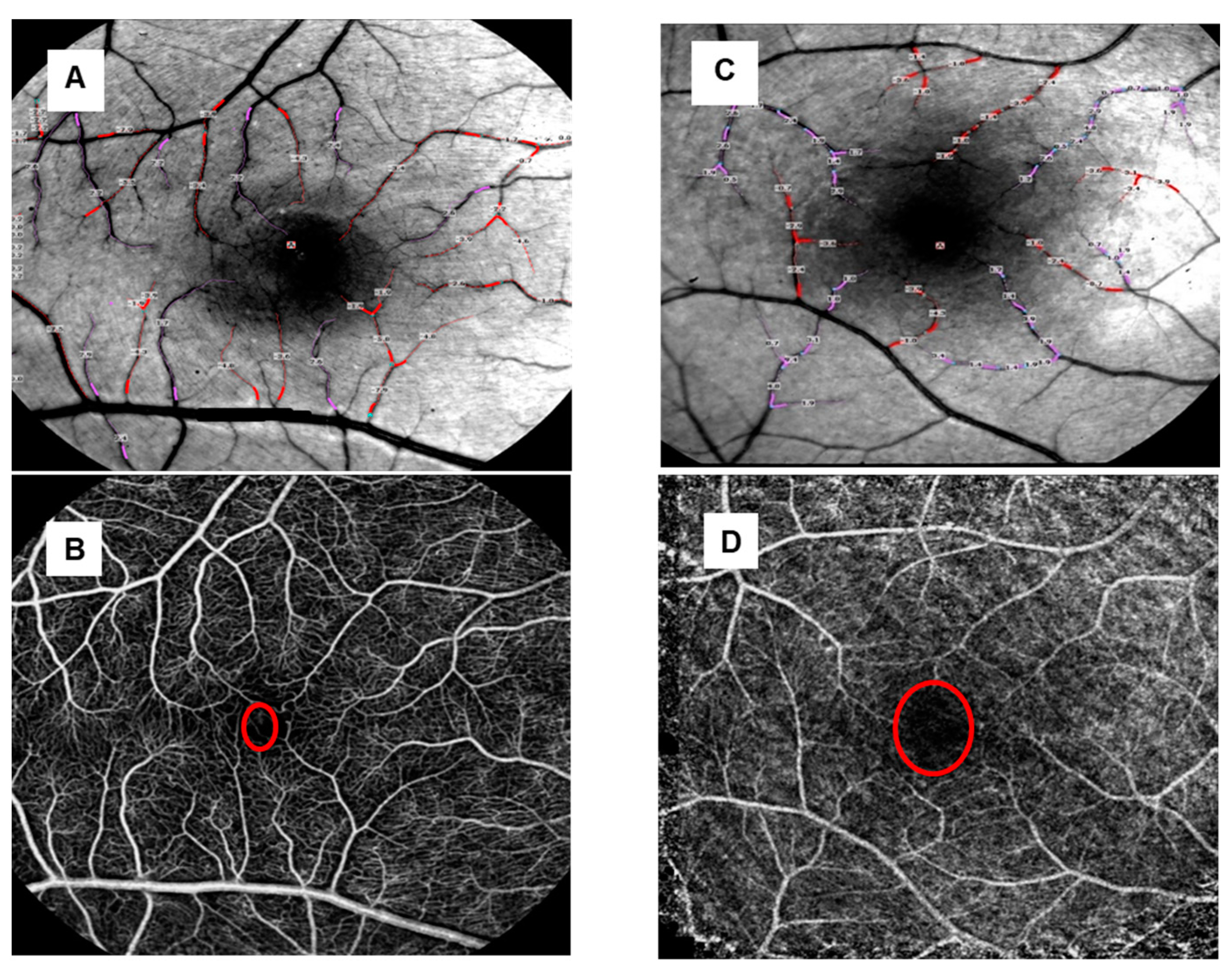
| Group | Arterioles BFV (mm/s) (Mean ± SD) | p-Value | Venules BFV (mm/s) (Mean ± SD) | p-Value | BCVA logMAR (Median ± SD) |
|---|---|---|---|---|---|
| Patient group | 2.75 ± 0.74 | p < 0.001 * | 1.75 ± 0.51 | p = 0.016 * | 0.1 ± 0.36 |
| Control group | 3.79 ± 0.50 | 2.35 ± 0.44 | 0 ± 0.01 | ||
| Long-term uveitis group | 2.53 ± 0.48 | p = 0.416 * | 1.69 ± 0.41 | p = 0.859 * | 0.1 ± 0.37 |
| Short-term uveitis group | 3.06 ± 0.95 | 1.83 ± 0.64 | 0.05 ± 0.15 | ||
| Long-term uveitis group | 2.53 ± 0.48 | p < 0.001 * | 1.69 ± 0.41 | p < 0.027 * | 0.1 ± 0.37 |
| Short-term uveitis group | 3.06 ± 0.95 | p = 0.182 * | 1.83 ± 0.64 | p = 0.395 * | 0.05 ± 0.15 |
| Control group | 3.79 ± 0.50 | 2.34 ± 0.43 | 0 ± 0.01 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuebiger, N.; Lee, W.-H.; Birtel, J.; Druchkiv, V.; Davis, J.L.; DeBuc, D.C. Assessment of Blood Flow Velocity in Retinal Vasculitis Using the Retinal Function Imager—A Pilot Study. J. Clin. Med. 2024, 13, 3975. https://doi.org/10.3390/jcm13133975
Stuebiger N, Lee W-H, Birtel J, Druchkiv V, Davis JL, DeBuc DC. Assessment of Blood Flow Velocity in Retinal Vasculitis Using the Retinal Function Imager—A Pilot Study. Journal of Clinical Medicine. 2024; 13(13):3975. https://doi.org/10.3390/jcm13133975
Chicago/Turabian StyleStuebiger, Nicole, Wen-Hsiang Lee, Johannes Birtel, Vasyl Druchkiv, Janet L. Davis, and Delia Cabrera DeBuc. 2024. "Assessment of Blood Flow Velocity in Retinal Vasculitis Using the Retinal Function Imager—A Pilot Study" Journal of Clinical Medicine 13, no. 13: 3975. https://doi.org/10.3390/jcm13133975
APA StyleStuebiger, N., Lee, W.-H., Birtel, J., Druchkiv, V., Davis, J. L., & DeBuc, D. C. (2024). Assessment of Blood Flow Velocity in Retinal Vasculitis Using the Retinal Function Imager—A Pilot Study. Journal of Clinical Medicine, 13(13), 3975. https://doi.org/10.3390/jcm13133975





