US-Guided Interventional Procedures for Total Hip Arthroplasty
Abstract
1. Introduction
2. Why Total Hip Arthroplasty Fails
3. US-Guided Procedures around the Hip
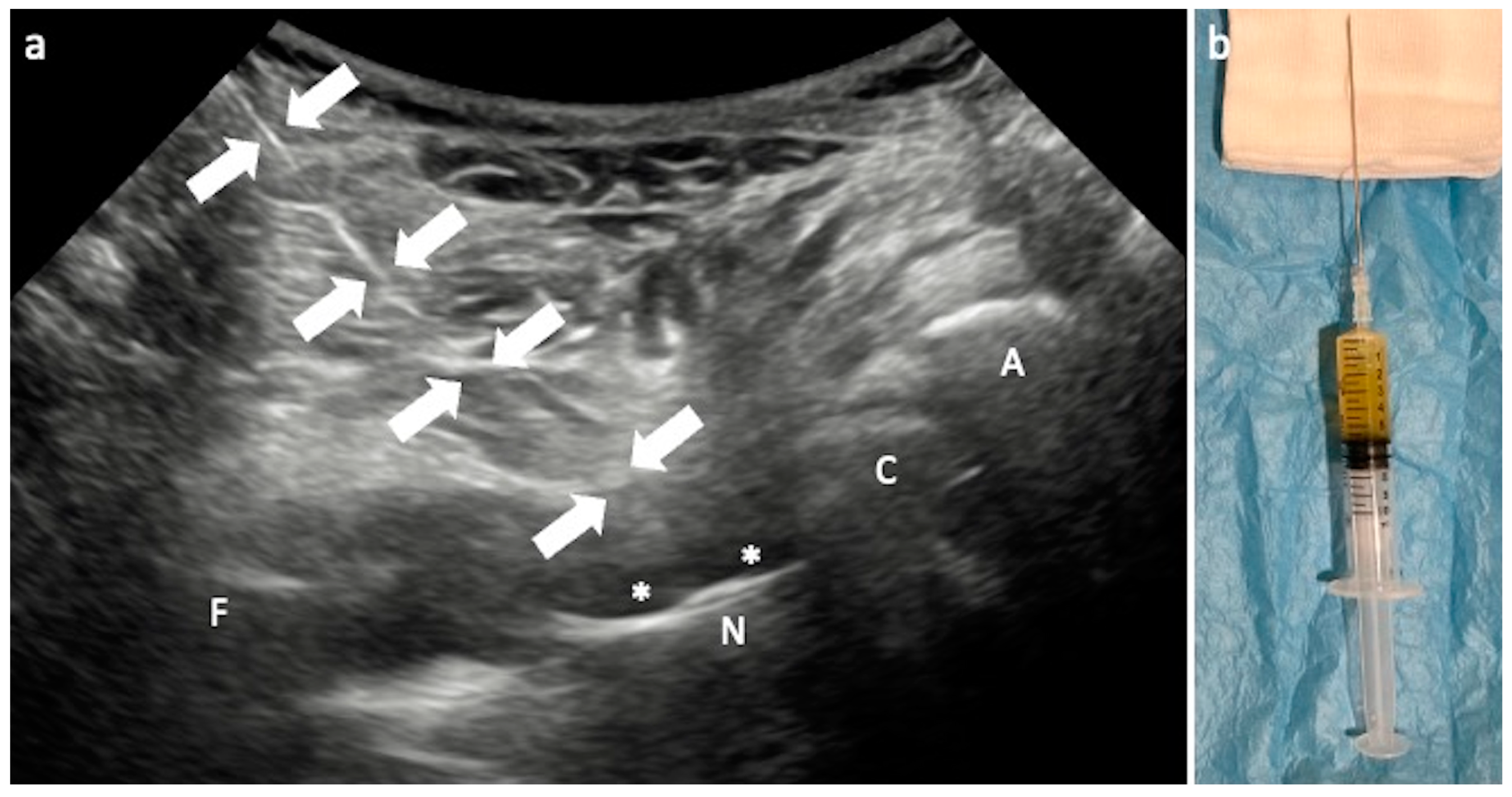
3.1. Arthrocentesis and Periprosthetic Biopsy
Technical Considerations
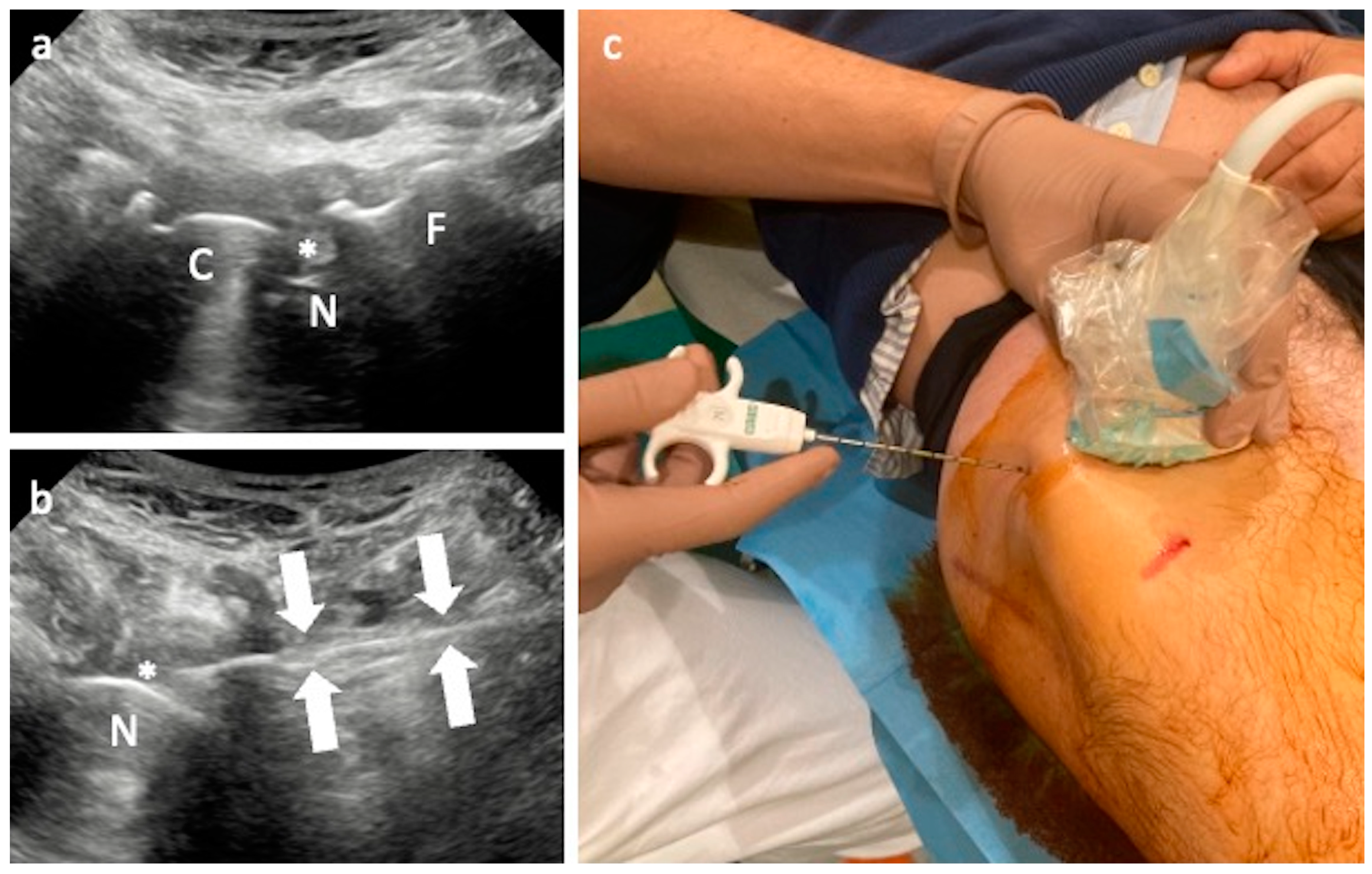
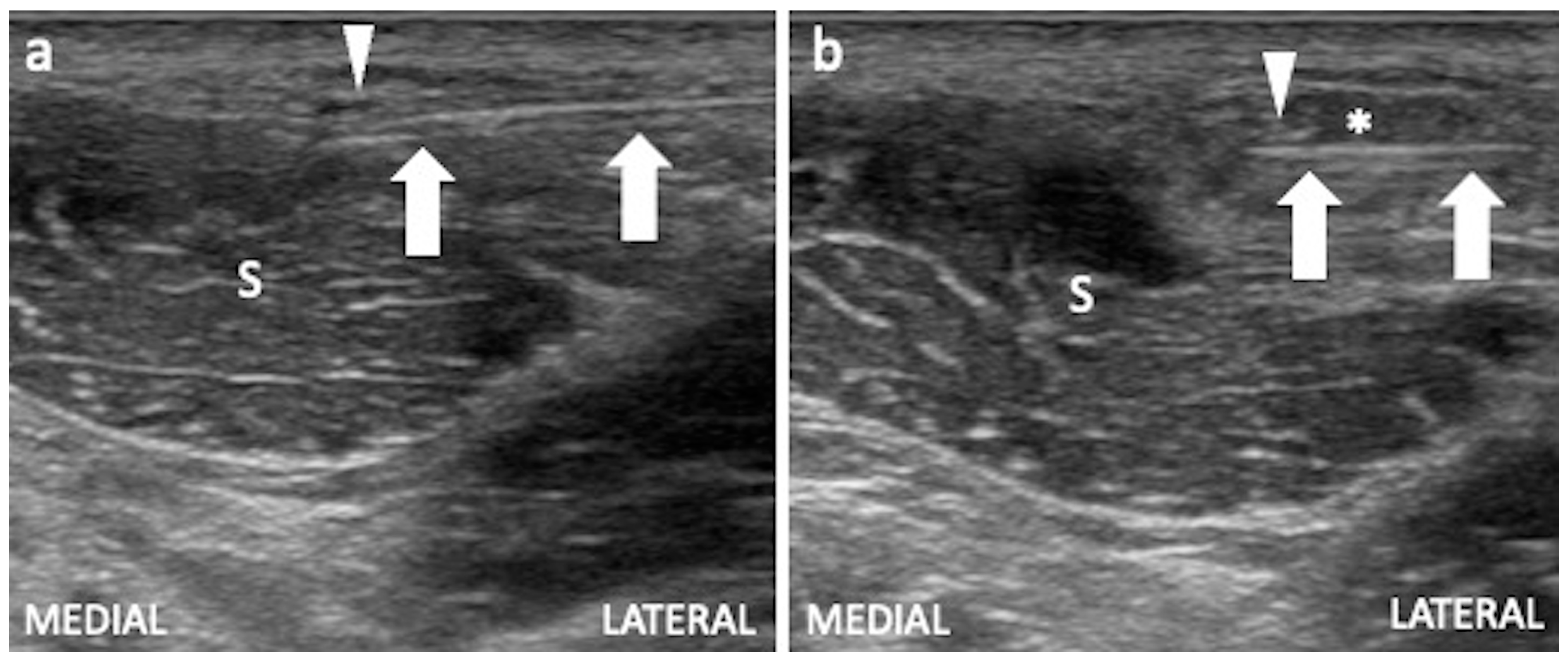
3.2. Iliopsoas Tendinopathy
Technical Considerations
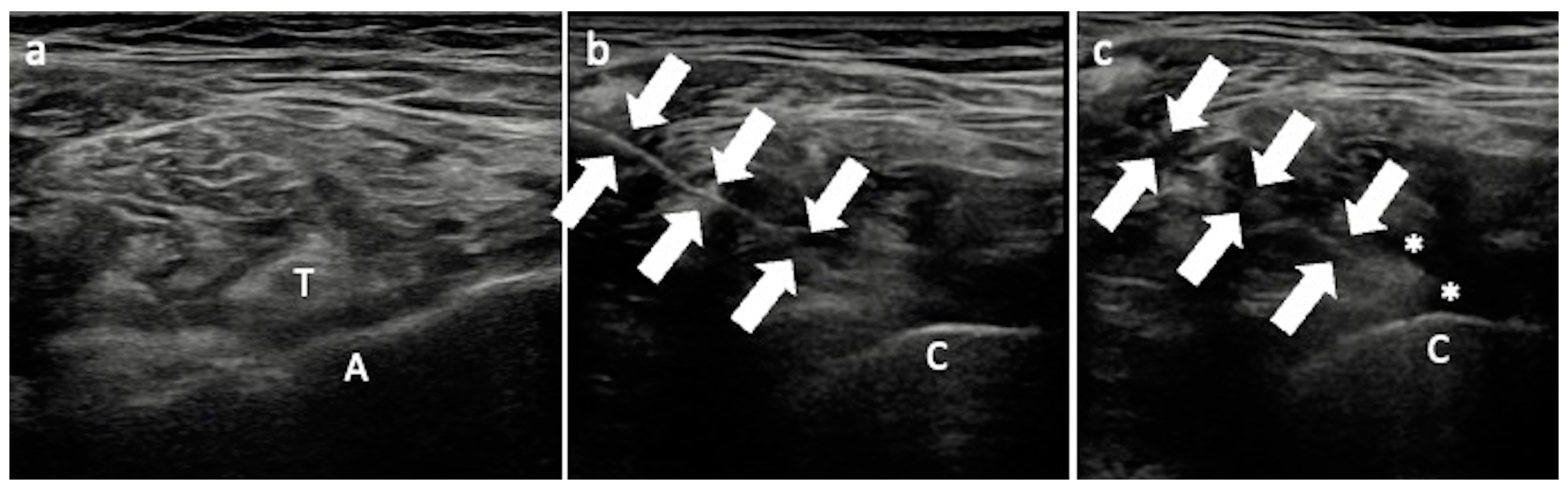
3.3. Greater Trochanteric Pain Syndrome
Technical Considerations
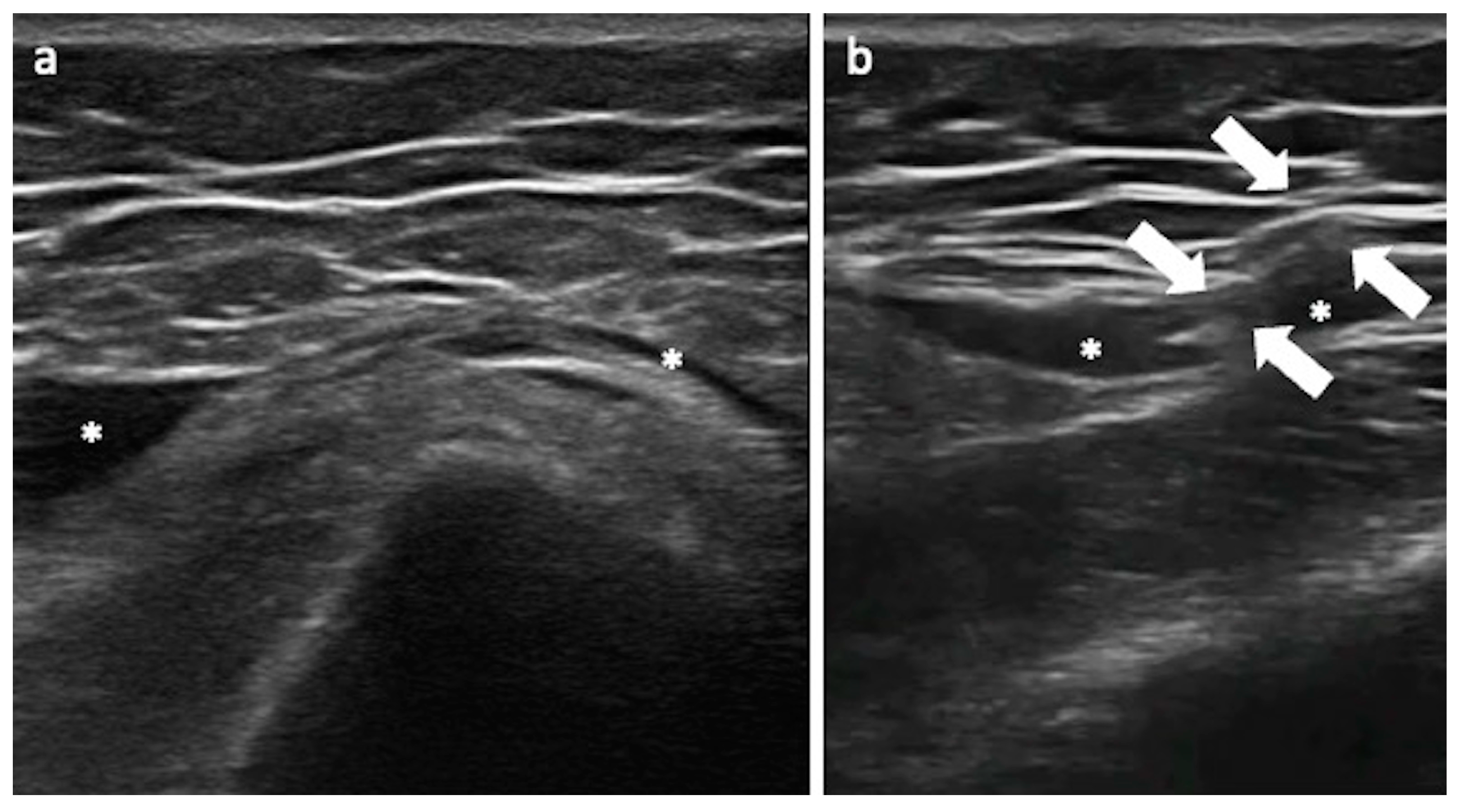
3.4. Nerve Injuries
3.4.1. Sciatic Nerve and Technical Considerations
3.4.2. Lateral Femoral Cutaneous Nerve and Technical Considerations
4. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lane, N.E. Clinical Practice. Osteoarthritis of the Hip. N. Engl. J. Med. 2007, 357, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.M.; Arostegui, I.; Escobar, A.; Azkarate, J.; Goenaga, J.I.; Lafuente, I. Prevalence of Knee and Hip Osteoarthritis and the Appropriateness of Joint Replacement in an Older Population. Arch. Intern. Med. 2008, 168, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Gademan, M.G.J.; Hofstede, S.N.; Vliet Vlieland, T.P.M.; Nelissen, R.G.H.H.; Marang-van de Mheen, P.J. Indication Criteria for Total Hip or Knee Arthroplasty in Osteoarthritis: A State-of-the-Science Overview. BMC Musculoskelet. Disord. 2016, 17, 463. [Google Scholar] [CrossRef] [PubMed]
- Gomez, P.F.; Morcuende, J.A. Early Attempts at Hip Arthroplasty—1700s to 1950s. Iowa Orthop. J. 2005, 25, 25–29. [Google Scholar] [PubMed]
- Fabi, D.; Levine, B.; Paprosky, W.; Della Valle, C.; Sporer, S.; Klein, G.; Levine, H.; Hartzband, M. Metal-on-Metal Total Hip Arthroplasty: Causes and High Incidence of Early Failure. Orthopedics 2012, 35, e1009–e1016. [Google Scholar] [CrossRef] [PubMed]
- Kenney, C.; Dick, S.; Lea, J.; Liu, J.; Ebraheim, N.A. A Systematic Review of the Causes of Failure of Revision Total Hip Arthroplasty. J. Orthop. 2019, 16, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Gitto, S.; Messina, C.; Serpi, F.; Salvatore, C.; Castiglioni, I.; Zagra, L.; De Vecchi, E.; Sconfienza, L.M. MRI-Based Artificial Intelligence to Predict Infection Following Total Hip Arthroplasty Failure. Radiol. Med. 2023, 128, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Erivan, R.; Villatte, G.; Ollivier, M.; Paprosky, W.G. Painful Hip Arthroplasty: What Should We Find? Diagnostic Approach and Results. J. Arthroplast. 2019, 34, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Chianca, V.; Orlandi, D.; Messina, C.; Albano, D.; Corazza, A.; Rapisarda, S.; Pozzi, G.; Cazzato, R.L.; Mauri, G.; Silvestri, E.; et al. Interventional Therapeutic Procedures to Treat Degenerative and Inflammatory Musculoskeletal Conditions: State of the Art. Radiol. Med. 2019, 124, 1112–1120. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Albano, D.; Allen, G.; Bazzocchi, A.; Bignotti, B.; Chianca, V.; Facal de Castro, F.; Drakonaki, E.E.; Gallardo, E.; Gielen, J.; et al. Clinical Indications for Musculoskeletal Ultrasound Updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) Consensus. Eur. Radiol. 2018, 28, 5338–5351. [Google Scholar] [CrossRef]
- Albano, D.; Gitto, S.; Serpi, F.; Aliprandi, A.; Maria Sconfienza, L.; Messina, C. Ultrasound-Guided Musculoskeletal Interventional Procedures Around the Hip: A Practical Guide. J. Ultrason. 2023, 23, 15–22. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Adriaensen, M.; Albano, D.; Alcala-Galiano, A.; Allen, G.; Aparisi Gómez, M.P.; Aringhieri, G.; Bazzocchi, A.; Beggs, I.; Chianca, V.; et al. Clinical Indications for Image-Guided Interventional Procedures in the Musculoskeletal System: A Delphi-Based Consensus Paper from the European Society of Musculoskeletal Radiology (ESSR)-Part VI, Foot and Ankle. Eur. Radiol. 2022, 32, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Gundtoft, P.H.; Overgaard, S.; Schønheyder, H.C.; Møller, J.K.; Kjærsgaard-Andersen, P.; Pedersen, A.B. The “True” Incidence of Surgically Treated Deep Prosthetic Joint Infection after 32,896 Primary Total Hip Arthroplasties: A Prospective Cohort Study. Acta Orthop. 2015, 86, 326–334. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic Joint Infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Messina, C.; Zagra, L.; Andreata, M.; De Vecchi, E.; Gitto, S.; Sconfienza, L.M. Failed Total Hip Arthroplasty: Diagnostic Performance of Conventional MRI Features and Locoregional Lymphadenopathy to Identify Infected Implants. J. Magn. Reson. Imaging JMRI 2021, 53, 201–210. [Google Scholar] [CrossRef]
- Blanc, P.; Bonnet, E.; Giordano, G.; Monteil, J.; Salabert, A.-S.; Payoux, P. The Use of Labelled Leucocyte Scintigraphy to Evaluate Chronic Periprosthetic Joint Infections: A Retrospective Multicentre Study on 168 Patients. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2019, 38, 1625–1631. [Google Scholar] [CrossRef]
- Catelas, I.; Lehoux, E.A.; Ning, Z.; Figeys, D.; Baskey, S.J.; Beaulé, P.E. Differential Proteomic Analysis of Synovial Fluid from Hip Arthroplasty Patients with a Pseudotumor vs. Periprosthetic Osteolysis. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2018, 36, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, S.; Wang, Z.; Yan, X.; Luo, H. Systematic Review and Meta-Analysis of Single-Stage vs Two-Stage Revision for Periprosthetic Joint Infection: A Call for a Prospective Randomized Trial. BMC Musculoskelet. Disord. 2024, 25, 153. [Google Scholar] [CrossRef]
- Silvestri, E.; Barile, A.; Albano, D.; Messina, C.; Orlandi, D.; Corazza, A.; Zugaro, L.; Masciocchi, C.; Sconfienza, L.M. Interventional Therapeutic Procedures in the Musculoskeletal System: An Italian Survey by the Italian College of Musculoskeletal Radiology. Radiol. Med. 2018, 123, 314–321. [Google Scholar] [CrossRef]
- Lachiewicz, P.F.; Rogers, G.D.; Thomason, H.C. Aspiration of the Hip Joint before Revision Total Hip Arthroplasty. Clinical and Laboratory Factors Influencing Attainment of a Positive Culture. J. Bone Jt. Surg. Am. 1996, 78, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lu, Q.; Chai, W.; Hao, L.-B.; Lu, S.-B.; Chen, J.-Y. Saline Solution Lavage and Reaspiration for Culture with a Blood Culture System Is a Feasible Method for Diagnosing Periprosthetic Joint Infection in Patients with Insufficient Synovial Fluid. J. Bone Jt. Surg. Am. 2019, 101, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Partridge, D.G.; Winnard, C.; Townsend, R.; Cooper, R.; Stockley, I. Joint Aspiration, Including Culture of Reaspirated Saline after a “Dry Tap”, Is Sensitive and Specific for the Diagnosis of Hip and Knee Prosthetic Joint Infection. Bone Jt. J. 2018, 100-B, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Ting, N.T.; Della Valle, C.J. Diagnosis of Periprosthetic Joint Infection—An Algorithm-Based Approach. J. Arthroplast. 2017, 32, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Porrino, J.; Wang, A.; Moats, A.; Mulcahy, H.; Kani, K. Prosthetic Joint Infections: Diagnosis, Management, and Complications of the Two-Stage Replacement Arthroplasty. Skeletal Radiol. 2020, 49, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.Y.; Crawford, A.M.; Kobes, P.H.; Allen, H.; Leake, R.L.; Hanrahan, C.J.; Mills, M.K. Septic Arthritis: An Evidence-Based Review of Diagnosis and Image-Guided Aspiration. AJR Am. J. Roentgenol. 2020, 215, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Sconfienza, L.M.; Albano, D.; Messina, C.; D’Apolito, R.; De Vecchi, E.; Zagra, L. Ultrasound-Guided Periprosthetic Biopsy in Failed Total Hip Arthroplasty: A Novel Approach to Test Infection in Patients With Dry Joints. J. Arthroplast. 2021, 36, 2962–2967. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.L.; Morrison, J.J. Hip Arthroplasty Pseudotumors: Pathogenesis, Imaging, and Clinical Decision Making. J. Clin. Imaging Sci. 2016, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Berend, K.R.; Morris, M.J.; Adams, J.B.; Lombardi, A.V. Metal-on-Metal Hip Arthroplasty: Going, Going, Gone…—Affirms. J. Bone Jt. Surg. Br. 2012, 94, 75–77. [Google Scholar] [CrossRef]
- Hauptfleisch, J.; Pandit, H.; Grammatopoulos, G.; Gill, H.S.; Murray, D.W.; Ostlere, S. A MRI Classification of Periprosthetic Soft Tissue Masses (Pseudotumours) Associated with Metal-on-Metal Resurfacing Hip Arthroplasty. Skeletal Radiol. 2012, 41, 149–155. [Google Scholar] [CrossRef]
- Muraoka, K.; Naito, M.; Nakamura, Y.; Hagio, T.; Takano, K. Usefulness of Ultrasonography for Detection of Pseudotumors after Metal-on-Metal Total Hip Arthroplasty. J. Arthroplast. 2015, 30, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Nishii, T.; Sakai, T.; Takao, M.; Yoshikawa, H.; Sugano, N. Is Ultrasound Screening Reliable for Adverse Local Tissue Reaction after Hip Arthroplasty? J. Arthroplast. 2014, 29, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- Tarasevicius, S.; Loiba, V.; Stucinskas, J.; Robertsson, O.; Wingstrand, H. Size of Cup Affects the Anterior Capsular Distance in Total Hip Arthroplasty, as Measured with Ultrasound. BMC Musculoskelet. Disord. 2014, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.J.; Lee, S.; Marks, P.; Schneider, M.E. Ultrasound Screening for Adverse Local Tissue Reaction after Hip Arthroplasty. Ultrasound Med. Biol. 2017, 43, 1549–1556. [Google Scholar] [CrossRef]
- Visuri, T.; Pulkkinen, P.; Paavolainen, P. Malignant Tumors at the Site of Total Hip Prosthesis. Analytic Review of 46 Cases. J. Arthroplast. 2006, 21, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Chung, H.W.; Oh, T.S.; Lee, J.-S. Practical Guidelines for Ultrasound-Guided Core Needle Biopsy of Soft-Tissue Lesions: Transformation from Beginner to Specialist. Korean J. Radiol. 2017, 18, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Long, S.S.; Surrey, D.; Nazarian, L.N. Common Sonographic Findings in the Painful Hip after Hip Arthroplasty. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2012, 31, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Hardwick-Morris, M.; Twiggs, J.; Miles, B.; Al-Dirini, R.M.A.; Taylor, M.; Balakumar, J.; Walter, W.L. Iliopsoas Tendonitis after Total Hip Arthroplasty: An Improved Detection Method with Applications to Preoperative Planning. Bone Jt. Open 2023, 4, 3–12. [Google Scholar] [CrossRef]
- Odri, G.A.; Padiolleau, G.B.; Gouin, F.T. Oversized Cups as a Major Risk Factor of Postoperative Pain after Total Hip Arthroplasty. J. Arthroplast. 2014, 29, 753–756. [Google Scholar] [CrossRef]
- Dora, C.; Houweling, M.; Koch, P.; Sierra, R.J. Iliopsoas Impingement after Total Hip Replacement: The Results of Non-Operative Management, Tenotomy or Acetabular Revision. J. Bone Jt. Surg. Br. 2007, 89, 1031–1035. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Chen, K.; Xiao, F.; Shen, C.; Peng, J.; Chen, X. Iliopsoas Tendonitis Following Total Hip Replacement in Highly Dysplastic Hips: A Retrospective Study. J. Orthop. Surg. 2019, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Piechota, M.; Maczuch, J.; Skupiński, J.; Kukawska-Sysio, K.; Wawrzynek, W. Internal Snapping Hip Syndrome in Dynamic Ultrasonography. J. Ultrason. 2016, 16, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Jasani, V.; Richards, P.; Wynn-Jones, C. Pain Related to the Psoas Muscle after Total Hip Replacement. J. Bone Jt. Surg. Br. 2002, 84, 991–993. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Tai, C.C.; Richards, S.; Skyrme, A.D.; Walter, W.L.; Walter, W.K. Iliopsoas Tendonitis a Complication after Total Hip Arthroplasty. J. Arthroplast. 2007, 22, 166–170. [Google Scholar] [CrossRef]
- Shapira, J.; Chen, S.L.; Wojnowski, N.M.; Lall, A.C.; Rosinsky, P.J.; Maldonado, D.R.; Domb, B.G. Outcomes of Nonoperative Management, Iliopsoas Tenotomy, and Revision Arthroplasty for Iliopsoas Impingement after Total Hip Arthroplasty: A Systematic Review. J. Arthroplast. 2019, 34, 2184–2191. [Google Scholar] [CrossRef]
- Adler, R.S.; Buly, R.; Ambrose, R.; Sculco, T. Diagnostic and Therapeutic Use of Sonography-Guided Iliopsoas Peritendinous Injections. AJR Am. J. Roentgenol. 2005, 185, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.A.; Lachiewicz, P.F. Groin Pain after Replacement of the Hip: Aetiology, Evaluation and Treatment. J. Bone Jt. Surg. Br. 2012, 94, 145–151. [Google Scholar] [CrossRef]
- Odak, S.; Ivory, J. Management of Abductor Mechanism Deficiency Following Total Hip Replacement. Bone Jt. J. 2013, 95-B, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Pfirrmann, C.W.A.; Notzli, H.P.; Dora, C.; Hodler, J.; Zanetti, M. Abductor Tendons and Muscles Assessed at MR Imaging after Total Hip Arthroplasty in Asymptomatic and Symptomatic Patients. Radiology 2005, 235, 969–976. [Google Scholar] [CrossRef]
- Albano, D.; Pansa, S.; Messina, C.; Gitto, S.; Serpi, F.; Fusco, S.; Midiri, F.; Zagra, L.; Sconfienza, L.M. MRI of Total Hip Arthroplasty: Technical Aspects and Imaging Findings. Insights Imaging 2024, 15, 152. [Google Scholar] [CrossRef]
- Marín-Pena, O.; Papavasiliou, A.V.; Olivero, M.; Galanis, N.; Tey-Pons, M.; Khanduja, V. Non-Surgical Treatment as the First Step to Manage Peritrochanteric Space Disorders. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2021, 29, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Sconfienza, L.M.; Adriaensen, M.; Alcala-Galiano, A.; Allen, G.; Aparisi Gómez, M.P.; Aringhieri, G.; Bazzocchi, A.; Beggs, I.; Chianca, V.; Corazza, A.; et al. Clinical Indications for Image-Guided Interventional Procedures in the Musculoskeletal System: A Delphi-Based Consensus Paper from the European Society of Musculoskeletal Radiology (ESSR)-Part IV, Hip. Eur. Radiol. 2022, 32, 551–560. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.R.; Lee, K.S.; Blankenbaker, D.G.; del Rio, A.M.; Keene, J.S. Ultrasound-Guided Corticosteroid Injections for Treatment of Greater Trochanteric Pain Syndrome: Greater Trochanter Bursa versus Subgluteus Medius Bursa. AJR Am. J. Roentgenol. 2013, 201, W313–W317. [Google Scholar] [CrossRef]
- Albano, D.; Messina, C.; Gitto, S.; Serpi, F.; Sconfienza, L.M. Imaging-Guided Musculoskeletal Interventions in the Lower Limb. Radiol. Clin. North Am. 2023, 61, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.A.; Yablon, C.M.; Henning, P.T.; Kazmers, I.S.; Urquhart, A.; Hallstrom, B.; Bedi, A.; Parameswaran, A. Greater Trochanteric Pain Syndrome: Percutaneous Tendon Fenestration Versus Platelet-Rich Plasma Injection for Treatment of Gluteal Tendinosis. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2016, 35, 2413–2420. [Google Scholar] [CrossRef]
- Ali, M.; Oderuth, E.; Atchia, I.; Malviya, A. The Use of Platelet-Rich Plasma in the Treatment of Greater Trochanteric Pain Syndrome: A Systematic Literature Review. J. Hip Preserv. Surg. 2018, 5, 209–219. [Google Scholar] [CrossRef]
- Fitzpatrick, J.; Bulsara, M.K.; O’Donnell, J.; McCrory, P.R.; Zheng, M.H. The Effectiveness of Platelet-Rich Plasma Injections in Gluteal Tendinopathy: A Randomized, Double-Blind Controlled Trial Comparing a Single Platelet-Rich Plasma Injection With a Single Corticosteroid Injection. Am. J. Sports Med. 2018, 46, 933–939. [Google Scholar] [CrossRef]
- Begkas, D.; Chatzopoulos, S.-T.; Touzopoulos, P.; Balanika, A.; Pastroudis, A. Ultrasound-Guided Platelet-Rich Plasma Application Versus Corticosteroid Injections for the Treatment of Greater Trochanteric Pain Syndrome: A Prospective Controlled Randomized Comparative Clinical Study. Cureus 2020, 12, e6583. [Google Scholar] [CrossRef]
- Weber, E.R.; Daube, J.R.; Coventry, M.B. Peripheral Neuropathies Associated with Total Hip Arthroplasty. J. Bone Jt. Surg. Am. 1976, 58, 66–69. [Google Scholar] [CrossRef]
- Johanson, N.A.; Pellicci, P.M.; Tsairis, P.; Salvati, E.A. Nerve Injury in Total Hip Arthroplasty. Clin. Orthop. 1983, 179, 214–222. [Google Scholar] [CrossRef]
- Stone, R.G.; Weeks, L.E.; Hajdu, M.; Stinchfield, F.E. Evaluation of Sciatic Nerve Compromise during Total Hip Arthroplasty. Clin. Orthop. 1985, 201, 26–31. [Google Scholar] [CrossRef]
- Thejeel, B.; Lin, J.; Queler, S.; Nimura, C.; Lin, Y.; Valle, A.G.D.; Sneag, D.B. Magnetic Resonance Imaging of Femoral Nerve Injury in the Setting of Anterior Approach Total Hip Arthroplasty. Clin. Imaging 2024, 108, 110112. [Google Scholar] [CrossRef] [PubMed]
- Martinoli, C.; Miguel-Perez, M.; Padua, L.; Gandolfo, N.; Zicca, A.; Tagliafico, A. Imaging of Neuropathies about the Hip. Eur. J. Radiol. 2013, 82, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Aringhieri, G.; Messina, C.; De Flaviis, L.; Sconfienza, L.M. High-Frequency and Ultra-High Frequency Ultrasound: Musculoskeletal Imaging up to 70 MHz. Semin. Musculoskelet. Radiol. 2020, 24, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Schmalzried, T.P.; Amstutz, H.C.; Dorey, F.J. Nerve Palsy Associated with Total Hip Replacement. Risk Factors and Prognosis. J. Bone Jt. Surg. Am. 1991, 73, 1074–1080. [Google Scholar] [CrossRef]
- Hasija, R.; Kelly, J.J.; Shah, N.V.; Newman, J.M.; Chan, J.J.; Robinson, J.; Maheshwari, A.V. Nerve Injuries Associated with Total Hip Arthroplasty. J. Clin. Orthop. Trauma 2018, 9, 81–86. [Google Scholar] [CrossRef]
- Rodríguez-Piñero, M.; Vidal Vargas, V.; Jiménez Sarmiento, A.S. Long-Term Efficacy of Ultrasound-Guided Injection of IncobotulinumtoxinA in Piriformis Syndrome. Pain Med. 2018, 19, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Patton, R.S.; Runner, R.P.; Lyons, R.J.; Bradbury, T.L. Clinical Outcomes of Patients With Lateral Femoral Cutaneous Nerve Injury After Direct Anterior Total Hip Arthroplasty. J. Arthroplast. 2018, 33, 2919–2926.e1. [Google Scholar] [CrossRef] [PubMed]
- Goulding, K.; Beaulé, P.E.; Kim, P.R.; Fazekas, A. Incidence of Lateral Femoral Cutaneous Nerve Neuropraxia after Anterior Approach Hip Arthroplasty. Clin. Orthop. 2010, 468, 2397–2404. [Google Scholar] [CrossRef]
- Homma, Y.; Baba, T.; Sano, K.; Ochi, H.; Matsumoto, M.; Kobayashi, H.; Yuasa, T.; Maruyama, Y.; Kaneko, K. Lateral Femoral Cutaneous Nerve Injury with the Direct Anterior Approach for Total Hip Arthroplasty. Int. Orthop. 2016, 40, 1587–1593. [Google Scholar] [CrossRef]
- Ozaki, Y.; Homma, Y.; Baba, T.; Sano, K.; Desroches, A.; Kaneko, K. Spontaneous Healing of Lateral Femoral Cutaneous Nerve Injury and Improved Quality of Life after Total Hip Arthroplasty via a Direct Anterior Approach. J. Orthop. Surg. Hong Kong 2017, 25, 2309499016684750. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Baba, T.; Homma, Y.; Tanabe, H.; Ochi, H.; Bannno, S.; Watari, T.; Kaneko, K. Preoperative Ultrasound to Identify Distribution of the Lateral Femoral Cutaneous Nerve in Total Hip Arthroplasty Using the Direct Anterior Approach. SICOT-J 2018, 4, 42. [Google Scholar] [CrossRef]
- Wijntjes, J.; Borchert, A.; van Alfen, N. Nerve Ultrasound in Traumatic and Iatrogenic Peripheral Nerve Injury. Diagn. Basel Switz. 2020, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Sconfienza, L.M.; Adriaensen, M.; Albano, D.; Alcala-Galiano, A.; Allen, G.; Aparisi Gómez, M.P.; Aringhieri, G.; Bazzocchi, A.; Beggs, I.; Chianca, V.; et al. Clinical Indications for Image-Guided Interventional Procedures in the Musculoskeletal System: A Delphi-Based Consensus Paper from the European Society of Musculoskeletal Radiology (ESSR)-Part VII, Nerves of the Lower Limb. Eur. Radiol. 2022, 32, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Matičič, U.B.; Omejec, G.; Salapura, V.; Snoj, Ž. Ultrasound-Guided Injections in Pelvic Entrapment Neuropathies. J. Ultrason. 2021, 21, e139–e146. [Google Scholar] [CrossRef] [PubMed]
- Mwikirize, C.; Nosher, J.L.; Hacihaliloglu, I. Convolution Neural Networks for Real-Time Needle Detection and Localization in 2D Ultrasound. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Brock, M.; Roghmann, F.; Sonntag, C.; Sommerer, F.; Tian, Z.; Löppenberg, B.; Palisaar, R.J.; Noldus, J.; Hanske, J.; von Bodman, C. Fusion of Magnetic Resonance Imaging and Real-Time Elastography to Visualize Prostate Cancer: A Prospective Analysis Using Whole Mount Sections after Radical Prostatectomy. Ultraschall Med. Stuttg. Ger. 2015, 36, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Calandri, M.; Ruggeri, V.; Carucci, P.; Mirabella, S.; Veltri, A.; Fonio, P.; Gazzera, C. Thermal Ablation with Fusion Imaging Guidance of Hepatocellular Carcinoma without Conspicuity on Conventional or Contrast-Enhanced US: Surrounding Anatomical Landmarks Matter. Radiol. Med. 2019, 124, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mi, S.; Guo, R.; Ma, X.; Han, M. Application of ultrasound fusion imaging technique for unilateral percutaneous vertebroplasty in treatment of osteoporotic thoracolumbar compression fracture. J. Xray. Sci. Technol. 2020, 28, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Klauser, A.S.; De Zordo, T.; Feuchtner, G.M.; Djedovic, G.; Weiler, R.B.; Faschingbauer, R.; Schirmer, M.; Moriggl, B. Fusion of Real-Time US with CT Images to Guide Sacroiliac Joint Injection In Vitro and In Vivo. Radiology 2010, 256, 547–553. [Google Scholar] [CrossRef]
- Albano, D.; Messina, C.; Gitto, S.; Fusco, S.; Sconfienza, L.M.; Bellelli, A. US/CT Fusion Imaging and Virtual Navigation to Guide Lumbar Intradiscal Oxygen-Ozone Therapy: A Pilot Study. J. Ultrasound 2024, 27, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; Kadom, N.; Ghobadi, C.; Strauss, B.; Al Dandan, O.; Aggarwal, A.; Anzai, Y.; Griffith, B.; Lazarow, F.; Straus, C.M.; et al. Virtual and Augmented Reality: Potential Applications in Radiology. Acta Radiol. Stockh. Swed. 2020, 61, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Pratt, P.; Ives, M.; Lawton, G.; Simmons, J.; Radev, N.; Spyropoulou, L.; Amiras, D. Through the HoloLensTM Looking Glass: Augmented Reality for Extremity Reconstruction Surgery Using 3D Vascular Models with Perforating Vessels. Eur. Radiol. Exp. 2018, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Racadio, J.M.; Nachabe, R.; Homan, R.; Schierling, R.; Racadio, J.M.; Babić, D. Augmented Reality on a C-Arm System: A Preclinical Assessment for Percutaneous Needle Localization. Radiology 2016, 281, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.S.; Lee, J.H.; Park, Y.S.; Cho, J.; Koh, J.C. Virtual Reality Simulator’s Effectiveness on the Spine Procedure Education for Trainee: A Randomized Controlled Trial. Korean J. Anesthesiol. 2023, 76, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Pierzchajlo, N.; Stevenson, T.C.; Huynh, H.; Nguyen, J.; Boatright, S.; Arya, P.; Chakravarti, S.; Mehrki, Y.; Brown, N.J.; Gendreau, J.; et al. Augmented Reality in Minimally Invasive Spinal Surgery: A Narrative Review of Available Technology. World Neurosurg. 2023, 176, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Messina, C.; Gitto, S.; Chianca, V.; Sconfienza, L.M. Bone Biopsies Guided by Augmented Reality: A Pilot Study. Eur. Radiol. Exp. 2023, 7, 40. [Google Scholar] [CrossRef]
- Ugwoke, C.K.; Albano, D.; Umek, N.; Dumić-Čule, I.; Snoj, Ž. Application of Virtual Reality Systems in Bone Trauma Procedures. Med. Kaunas Lith. 2023, 59, 562. [Google Scholar] [CrossRef]
- Solbiati, M.; Passera, K.M.; Rotilio, A.; Oliva, F.; Marre, I.; Goldberg, S.N.; Ierace, T.; Solbiati, L. Augmented Reality for Interventional Oncology: Proof-of-Concept Study of a Novel High-End Guidance System Platform. Eur. Radiol. Exp. 2018, 2, 18. [Google Scholar] [CrossRef]
- Solbiati, M.; Ierace, T.; Muglia, R.; Pedicini, V.; Iezzi, R.; Passera, K.M.; Rotilio, A.C.; Goldberg, S.N.; Solbiati, L.A. Thermal Ablation of Liver Tumors Guided by Augmented Reality: An Initial Clinical Experience. Cancers 2022, 14, 1312. [Google Scholar] [CrossRef]
- Mihcin, S.; Melzer, A. Principles of Focused Ultrasound. Minim. Invasive Ther. Allied Technol. 2018, 27, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Masciocchi, C.; Zugaro, L.; Arrigoni, F.; Gravina, G.L.; Mariani, S.; La Marra, A.; Zoccali, C.; Flamini, S.; Barile, A. Radiofrequency Ablation versus Magnetic Resonance Guided Focused Ultrasound Surgery for Minimally Invasive Treatment of Osteoid Osteoma: A Propensity Score Matching Study. Eur. Radiol. 2016, 26, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Düx, D.M.; Baal, J.D.; Bitton, R.; Chen, J.; Brunsing, R.L.; Sheth, V.R.; Rosenberg, J.; Kim, K.; Ozhinsky, E.; Avedian, R.; et al. MR-Guided Focused Ultrasound Therapy of Extra-Abdominal Desmoid Tumors: A Multicenter Retrospective Study of 105 Patients. Eur. Radiol. 2024, 34, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, A.-S.; Iannessi, A.; Natale, R.; Beaumont, H.; Patriti, S.; Xiong-Ying, J.; Baudin, G.; Thyss, A. Focused Ultrasound for the Treatment of Bone Metastases: Effectiveness and Feasibility. J. Ther. Ultrasound 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Baal, J.D.; Chen, W.C.; Baal, U.; Wagle, S.; Baal, J.H.; Link, T.M.; Bucknor, M.D. Efficacy and Safety of Magnetic Resonance-Guided Focused Ultrasound for the Treatment of Painful Bone Metastases: A Systematic Review and Meta-Analysis. Skeletal Radiol. 2021, 50, 2459–2469. [Google Scholar] [CrossRef]
- Masciocchi, C.; Conchiglia, A.; Gregori, L.M.; Arrigoni, F.; Zugaro, L.; Barile, A. Critical Role of HIFU in Musculoskeletal Interventions. Radiol. Med. 2014, 119, 470–475. [Google Scholar] [CrossRef]
- Leporace, M.; Lancellotta, V.; Baccolini, V.; Calabria, F.; Castrovillari, F.; Filippiadis, D.K.; Tagliaferri, L.; Iezzi, R. Magnetic resonance-guided focused ultrasound versus percutaneous thermal ablation in local control of bone oligometastases: A systematic review and meta-analysis. Radiol. Med. 2024, 129, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Ciatawi, K.; Dusak, I.W.S.; Wiratnaya, I.G.E. High-intensity focused ultrasound-a needleless management for osteoid osteoma: A systematic review. Musculoskelet. Surg. 2024, 108, 21–30. [Google Scholar] [CrossRef] [PubMed]
- De Maio, A.; Alfieri, G.; Mattone, M.; Ghanouni, P.; Napoli, A. High-Intensity Focused Ultrasound Surgery for Tumor Ablation: A Review of Current Applications. Radiol. Imaging. Cancer. 2024, 6, e230074. [Google Scholar] [CrossRef]
- McGill, K.C.; Baal, J.D.; Bucknor, M.D. Update on Musculoskeletal Applications of Magnetic Resonance-Guided Focused Ultrasound. Skeletal Radiol. 2024. [Google Scholar] [CrossRef]
- Bucknor, M.D.; Baal, J.D.; McGill, K.C.; Infosino, A.; Link, T.M. Musculoskeletal Applications of Magnetic Resonance-Guided Focused Ultrasound. Semin. Musculoskelet. Radiol. 2021, 25, 725–734. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albano, D.; Cintioli, R.; Messina, C.; Serpi, F.; Gitto, S.; Mascitti, L.; Vignati, G.; Glielmo, P.; Vitali, P.; Zagra, L.; et al. US-Guided Interventional Procedures for Total Hip Arthroplasty. J. Clin. Med. 2024, 13, 3976. https://doi.org/10.3390/jcm13133976
Albano D, Cintioli R, Messina C, Serpi F, Gitto S, Mascitti L, Vignati G, Glielmo P, Vitali P, Zagra L, et al. US-Guided Interventional Procedures for Total Hip Arthroplasty. Journal of Clinical Medicine. 2024; 13(13):3976. https://doi.org/10.3390/jcm13133976
Chicago/Turabian StyleAlbano, Domenico, Roberto Cintioli, Carmelo Messina, Francesca Serpi, Salvatore Gitto, Laura Mascitti, Giacomo Vignati, Pierluigi Glielmo, Paolo Vitali, Luigi Zagra, and et al. 2024. "US-Guided Interventional Procedures for Total Hip Arthroplasty" Journal of Clinical Medicine 13, no. 13: 3976. https://doi.org/10.3390/jcm13133976
APA StyleAlbano, D., Cintioli, R., Messina, C., Serpi, F., Gitto, S., Mascitti, L., Vignati, G., Glielmo, P., Vitali, P., Zagra, L., Snoj, Ž., & Sconfienza, L. M. (2024). US-Guided Interventional Procedures for Total Hip Arthroplasty. Journal of Clinical Medicine, 13(13), 3976. https://doi.org/10.3390/jcm13133976






