Echocardiography-Derived Hemodynamic Forces Are Associated with Clinical Outcomes in Patients with Non-Ischemic Dilated Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Endpoint
2.2. Echocardiography
2.3. Deformation Imaging and Hemodynamic Forces
2.4. Statistical Analysis
3. Results
3.1. Correlation between Hemodynamic Forces and Clinical and Echocardiographic Variables
3.2. Clinical Endpoints
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dec, G.W.; Fuster, V. Idiopathic Dilated Cardiomyopathy. N. Engl. J. Med. 1994, 331, 1564–1575. [Google Scholar] [CrossRef]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef]
- Ushigome, R.; Sakata, Y.; Nochioka, K.; Miyata, S.; Miura, M.; Tadaki, S.; Yamauchi, T.; Sato, K.; Onose, T.; Tsuji, K.; et al. Improved Long-Term Prognosis of Dilated Cardiomyopathy With Implementation of Evidenced-Based Medication—Report From the CHART Studies. Circ. J. 2015, 79, 1332–1341. [Google Scholar] [CrossRef]
- Halliday, B.P.; Gulati, A.; Ali, A.; Newsome, S.; Lota, A.; Tayal, U.; Vassiliou, V.S.; Arzanauskaite, M.; Izgi, C.; Krishnathasan, K.; et al. Sex- and age-based differences in the natural history and outcome of dilated cardiomyopathy. Eur. J. Heart Fail. 2018, 20, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Buss, S.J.; Breuninger, K.; Lehrke, S.; Voss, A.; Galuschky, C.; Lossnitzer, D.; Andre, F.; Ehlermann, P.; Franke, J.; Taeger, T.; et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur. Hear. J—Cardiovasc. Imaging 2015, 16, 307–315. [Google Scholar] [CrossRef]
- Romano, S.; Judd, R.M.; Kim, R.J.; Kim, H.W.; Klem, I.; Heitner, J.F.; Shah, D.J.; Jue, J.; White, B.E.; Indorkar, R.; et al. Feature-Tracking Global Longitudinal Strain Predicts Death in a Multicenter Population of Patients With Ischemic and Nonischemic Dilated Cardiomyopathy Incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2018, 11, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Gaibazzi, N.; Bergamaschi, L.; Pizzi, C.; Tuttolomondo, D. Resting global longitudinal strain and stress echocardiography to detect coronary artery disease burden. Eur. Hear. J—Cardiovasc. Imaging 2023, 24, e86–e88. [Google Scholar] [CrossRef] [PubMed]
- Pedrizzetti, G.; Arvidsson, P.M.; Töger, J.; Borgquist, R.; Domenichini, F.; Arheden, H.; Heiberg, E. On estimating intraventricular hemodynamic forces from endocardial dynamics: A comparative study with 4D flow MRI. J. Biomech. 2017, 60, 203–210. [Google Scholar] [CrossRef]
- Vallelonga, F.; Airale, L.; Tonti, G.; Argulian, E.; Milan, A.; Narula, J.; Pedrizzetti, G. Introduction to Hemodynamic Forces Analysis: Moving Into the New Frontier of Cardiac Deformation Analysis. J. Am. Heart Assoc. 2021, 10, e023417. [Google Scholar] [CrossRef]
- Airale, L.; Vallelonga, F.; Forni, T.; Leone, D.; Magnino, C.; Avenatti, E.; Iannaccone, A.; Astarita, A.; Mingrone, G.; Cesareo, M.; et al. A Novel Approach to Left Ventricular Filling Pressure Assessment: The Role of Hemodynamic Forces Analysis. Front. Cardiovasc. Med. 2021, 8, 704909. [Google Scholar] [CrossRef]
- Dal Ferro, M.; De Paris, V.; Collia, D.; Stolfo, D.; Caiffa, T.; Barbati, G.; Korcova, R.; Pinamonti, B.; Zovatto, L.; Zecchin, M.; et al. Left Ventricular Response to Cardiac Resynchronization Therapy: Insights From Hemodynamic Forces Computed by Speckle Tracking. Front. Cardiovasc. Med. 2019, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Lapinskas, T.; Pedrizzetti, G.; Stoiber, L.; Düngen, H.D.; Edelmann, F.; Pieske, B.; Kelle, S. The Intraventricular Hemodynamic Forces Estimated Using Routine CMR Cine Images: A New Marker of the Failing Heart. JACC Cardiovasc. Imaging. 2019, 12, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Pedrizzetti, G. On the computation of hemodynamic forces in the heart chambers. J. Biomech. 2019, 95, 109323. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Bolger, A.F.; Ebbers, T.; Carlhall, C.J. Left ventricular hemodynamic forces are altered in patients with dilated cardiomyopathy. J. Cardiovasc. Magn. Reson. 2015, 17, P282. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Martiniello, A.R.; Bianchi, V.; D’Onofrio, A.; Caso, P.; Tonti, G. Changes in electrical activation modify the orientation of left ventricular flow momentum: Novel observations using echocardiographic particle image velocimetry. Eur. Heart J. Cardiovasc. Imaging. 2016, 17, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Vos, J.L.; Raafs, A.G.; Henkens, M.T.H.M.; Van Deursen, C.J.; Pedrizzetti, G.; Rodwell, L.; Heymans, S.R.B.; Nijveldt, R. CMR derived left ventricular intraventricular pressure gradients identify different patterns associated with prognosis in patients with dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2023, 43, ehac544-265. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Bahler, R.C. Assessment of Prognosis in Idiopathic Dilated Cardiomyopathy. Chest 2002, 121, 1016–1019. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Domenichini, F. Nature optimizes the swirling flow in the human left ventricle. Phys. Rev. Lett. 2005, 95, 108101. [Google Scholar] [CrossRef] [PubMed]
- Pedrizzetti, G.; La Canna, G.; Alfieri, O.; Tonti, G. The vortex—An early predictor of cardiovascular outcome? Nat. Rev. Cardiol. 2014, 11, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Faganello, G.; Collia, D.; Furlotti, S.; Pagura, L.; Zaccari, M.; Pedrizzetti, G.; Di Lenarda, A. A new integrated approach to cardiac mechanics: Reference values for normal left ventricle. Int. J. Cardiovasc. Imaging 2020, 36, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Capuano, F.; Cocchia, R.; Ranieri, B.; Contaldi, C.; Lacava, G.; Capone, V.; Chianese, S.; Rega, S.; Annunziata, R.; et al. Reference ranges of left ventricular hemodynamic forces in healthy adults: A speckle-tracking echocardiographic study. J. Clin. Med. 2021, 10, 5937. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Narula, J. Reclassifying Heart Failure: Predominantly Subendocardial, Subepicardial, and Transmural. Heart Fail. Clin. 2008, 4, 379–382. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Lapinskas, T.; Tonti, G.; Stoiber, L.; Zaliunas, R.; Gebker, R.; Pieske, B.; Kelle, S. The Relationship Between EF and Strain Permits a More Accurate Assessment of LV Systolic Function. JACC Cardiovasc. Imaging 2019, 12, 1893–1895. [Google Scholar] [CrossRef] [PubMed]
- Pedrizzetti, G.; Tanacli, R.; Lapinskas, T.; Zovatto, L.; Pieske, B.; Tonti, G.; Kelle, S. Integration between volumetric change and strain for describing the global mechanical function of the left ventricle. Med. Eng. Phys. 2019, 74, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Raafs, A.G.; Boscutti, A.; Henkens, M.T.H.M.; van den Broek, W.W.A.; Verdonschot, J.A.J.; Weerts, J.; Stolfo, D.; Nuzzi, V.; Manca, P.; Hazebroek, M.R.; et al. Global Longitudinal Strain is Incremental to Left Ventricular Ejection Fraction for the Prediction of Outcome in Optimally Treated Dilated Cardiomyopathy Patients. J. Am. Heart Assoc. 2022, 11, e024505. [Google Scholar] [CrossRef]
- Fabiani, I.; Pugliese, N.R.; Pedrizzetti, G.; Tonti, G.; Castiglione, V.; Chubuchny, V.; Taddei, C.; Gimelli, A.; Del Punta, L.; Balletti, A.; et al. Haemodynamic forces predicting remodelling and outcome in patients with heart failure treated with sacubitril/valsartan. ESC Hear. Fail. 2023, 10, 2927–2938. [Google Scholar] [CrossRef]


| Total Population (n. 97) | No MACE (n. 78) | MACE (n. 19) | p Value | |
|---|---|---|---|---|
| Clinical variables | ||||
| Age (years) | 62 ± 14 | 59.4 ± 14.0 | 73.8 ± 9.4 | <0.001 |
| Male sex, n (%) | 67 (69%) | 59 (76%) | 8 (42%) | 0.071 |
| Weight (kg) | 75 ± 15 | 76 ± 15 | 71 ± 16 | 0.176 |
| Height (cm) | 168 ± 10 | 169 ± 10 | 161 ± 9 | 0.002 |
| BSA (m2) | 1.84 ± 0.21 | 1.86 ± 0.20 | 1.74 ± 0.21 | 0.032 |
| BMI (kg/m2) | 26.5 ± 4.5 | 26.5 ± 4.5 | 27.0 ± 5.0 | 0.728 |
| Obesity, n (%) | 19 (20%) | 16 (21%) | 3 (16%) | 0.975 |
| Hypertension, n (%) | 50 (52%) | 36 (46%) | 14 (74%) | 0.031 |
| Dyslipidemia, n (%) | 42 (43%) | 31 (40%) | 11 (58%) | 0.152 |
| Diabetes, n (%) | 28 (29%) | 20 (26%) | 8 (42%) | 0.041 |
| COPD, n (%) | 9 (9%) | 7 (9%) | 2 (11%) | 0.834 |
| CKD, n (%) | 5 (5%) | 3 (4%) | 2 (11%) | 0.260 |
| Peripheral artery disease, n (%) | 5 (5%) | 3 (4%) | 2 (11%) | 0.260 |
| Previous or active cancer, n (%) | 13 (13%) | 10 (13%) | 4 (21%) | 0.465 |
| Previous heart failure hosp., n (%) | 13 (13%) | 11 (14%) | 2 (11%) | 0.682 |
| Charlson Comorbidity Index | 3 (1–4) | 2 (1–3) | 5 (3–6) | <0.001 |
| Serum creatinine (mg/dL) | 0.88 (0.75–1.05) | 0.86 (0.74–1.05) | 0.94 (0.82–1.12) | 0.075 |
| Hemoglobin (g/dL) | 13.7 ± 1.9 | 14.0 ± 1.8 | 12.5 ± 1.6 | 0.002 |
| Heart rate (bpm) | 72 ± 16 | 73 ± 16 | 71 ± 14 | 0.612 |
| QRS duration (ms) | 117 ± 37 | 114 ± 36 | 129 ± 37 | 0.115 |
| QRS ≥ 120 ms, n (%) | 49 (51%) | 35 (45%) | 14 (74%) | 0.024 |
| Systolic BP (mmHg) | 128 ± 17 | 128 ± 17 | 131 ± 18 | 0.514 |
| Diastolic BP (mmHg) | 75 ± 12 | 75 ± 12 | 74 ± 12 | 0.637 |
| Follow-up time (years) | 4.2(3.1–5.1) | 4.2 (3.3–5.3) | 4.1 (2.9–5.4) | 0.620 |
| Medications, use of | ||||
| Beta blockers, n (%) | 52 (54%) | 38 (49%) | 14 (74%) | 0.050 |
| ACE-Inhibitors/ARBs, n (%) | 68 (70%) | 52 (67%) | 16 (84%) | 0.134 |
| MRAs, n (%) | 28 (29%) | 19 (24%) | 9 (47%) | 0.047 |
| Loop diuretics, n (%) | 29 (30%) | 19 (24%) | 10 (53%) | 0.016 |
| Ivabradine, n (%) | 13 (13%) | 10 (13%) | 3 (16%) | 0.715 |
| Echocardiography | ||||
| Interventricular septum (mm) | 11.0 ± 1.9 | 10.9 ± 1.7 | 11.5 ± 2.5 | 0.356 |
| Inferior-lateral wall (mm) | 10.2 ± 1.9 | 10.0 ± 1.7 | 10.7 ± 2.3 | 0.270 |
| RWT | 0.37 ± 0.09 | 0.35 ± 0.08 | 0.41 ± 0.1 | 0.042 |
| LV EDVi (mL/m2) | 69 (60–82 | 68 (60–84) | 70 (52–78) | 0.676 |
| LVMi (g/m2) | 133 ± 32 | 134 ± 34 | 133 ± 35 | 0.870 |
| Sphericity index | 0.58 (0.53–0.64) | 0.58 (0.54–0.64) | 0.58 (0.53–0.67) | 0.927 |
| LVEF (%) | 39.2 ± 8.6 | 39.1 ± 8.3 | 39.3 ± 10.2 | 0.948 |
| LVEF < 40%, n (%) | 47 (49%) | 38 (49%) | 9 (47%) | 0.916 |
| GLS (%) | −13.6 ± 3.6 | −13.7 ± 3.6 | −13.0 ± 3.9 | 0.514 |
| GCS (%) | −17.1 ± 4.6 | −16.9 ± 4.4 | −17.9 ± 5.5 | 0.722 |
| TAPSE (mm) | 20 (18–23) | 20 (18–22) | 22 (20–25) | 0.037 |
| RV FWS (%) | −22.1 ± 4.4 | −21.7 ± 4.6 | −23.7 ± 3.7 | 0.094 |
| LAVi (mL/m2) | 30 (24–40) | 29 (22–38) | 34 (27–41] | 0.269 |
| LA reservoir strain (%) | 21.2 ± 8.2 | 21.7 ± 8.2 | 19.0 ± 8.4 | 0.206 |
| E wave (m/s) | 0.63 (90.52–0.79) | 0.61 (0.51–0.74) | 0.67 (0.56–0.88) | 0.137 |
| E/A ratio | 0.75 (0.64–1.09) | 0.75 (0.64–1.09) | 0.74 (0.58–1.05) | 0.587 |
| E/e’ ratio * | 11.3 (7.5–16.1) | 10.2 (7.3–14.2) | 16.4 (11.6–20.3) | 0.011 |
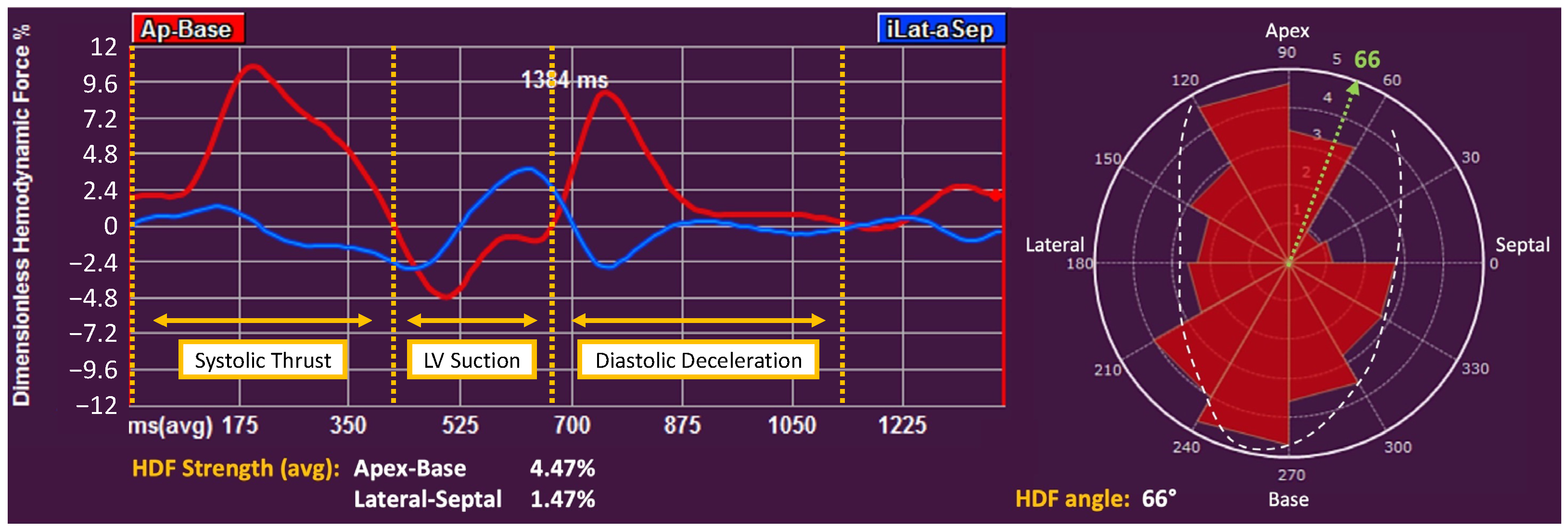
| Hemodynamic forces (HDFs) | ||||
| HDFs apex-base (%) | 5.6 (4.4–6.7) | 5.7 (4.5–6.8) | 5.0 (4.4–6.3) | 0.375 |
| HDFs lateral-septal (%) | 1.6 (1.3–2.0) | 1.7 (1.3–2.0) | 1.4 (1.0–1.9) | 0.015 |
| HDFs-angle (°) | 68 (64–71) | 68 (63–71) | 71 (67–75) | 0.005 |
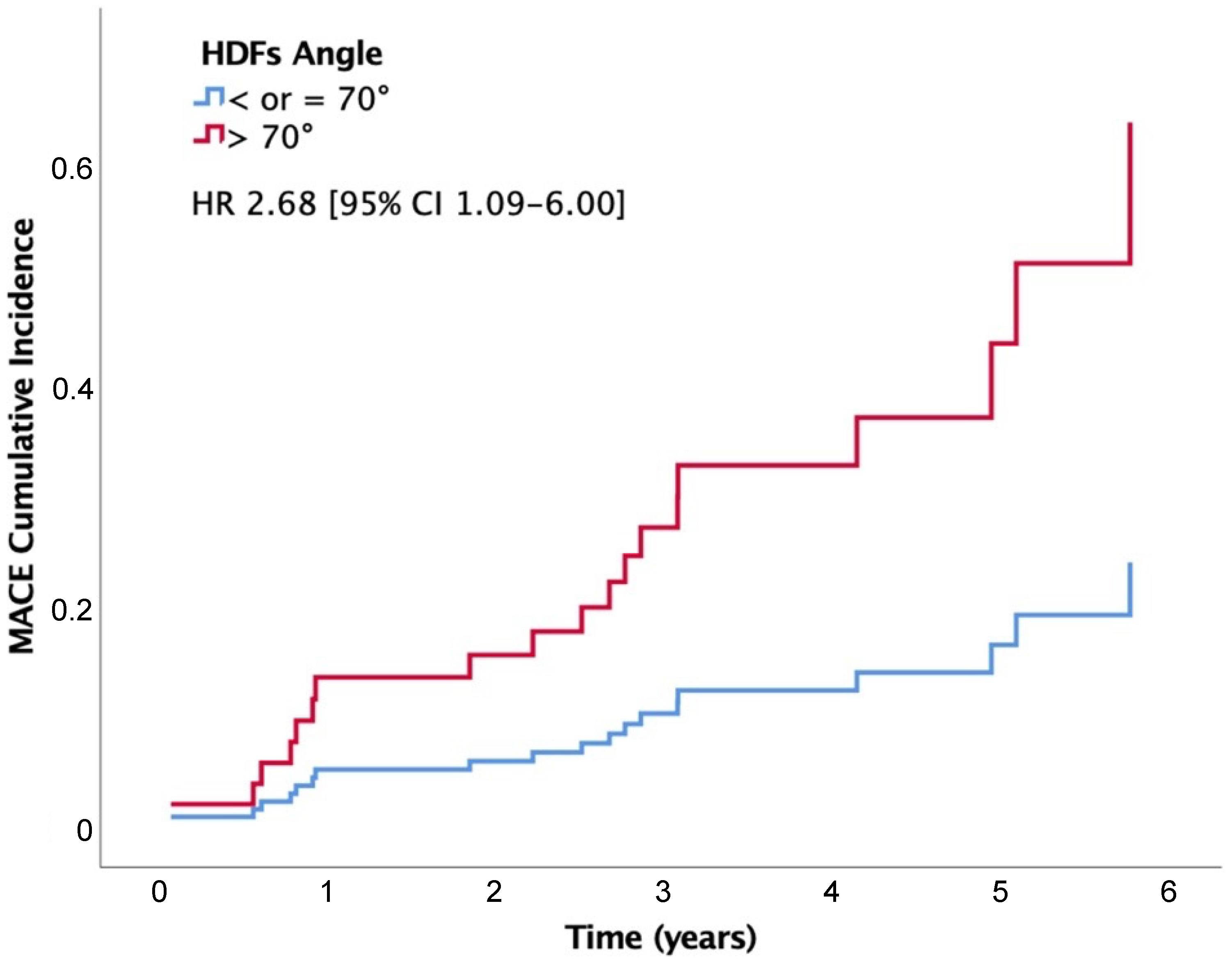
| HDFs-angle > 70°, n (%) | 30 (31%) | 20 (26%) | 10 (53%) | 0.022 |
| Systolic Thrust (%) | 6.0 (4.5–7.6) | 6.2 (4.6–7.6) | 5.3 (4.5–7.0) | 0.140 |
| LV Suction (%) | −2.7 (−3.7;−2.0) | −2.6 (−3.7;−1.9) | −2.8 (−4.2;−2.1) | 0.519 |
| Diastolic Deceleration (%) | 2.6 (1.8–3.9) | 2.7 (1.8–3.9) | 2.0 (1.8–4.0) | 0.581 |
| LV Suction Reversal, n (%) | 52 (54%) | 42 (54%) | 10 (53%) | 0.924 |
| Systolic Thrust angle (°) | 74 (70–78) | 74 (69–78) | 76 (72–78) | 0.016 |
| LV Suction angle (°) | 59 (53–66) | 58 (53–65) | 63 (58–73) | 0.029 |
| Hazard Ratio [95% CI] | p Value | |
|---|---|---|
| Clinical variables | ||
| Age (per year) | 1.09 (1.04–1.15) | <0.001 |
| Male sex | 0.40 (0.16–0.99) | 0.049 |
| Weight (per kg) | 0.98 (0.94–1.01) | 0.175 |
| Height (per cm) | 0.93 (0.88–0.97) | 0.002 |
| BSA (per m2) | 0.07 (0.01–0.69) | 0.023 |
| BMI (per kg/m2) | 1.03 (0.93–1.14) | 0.598 |
| Obesity | 1.06 (0.35–3.20) | 0.919 |
| Hypertension | 3.12 (1.12–8.68) | 0.030 |
| Dyslipidemia | 1.96 (0.79–4.89) | 0.147 |
| Diabetes | 2.65 (1.07–6.55) | 0.035 |
| COPD | 1.37 (0.31–5.98) | 0.675 |
| CKD | 1.96 (0.45–8.52) | 0.368 |
| Peripheral artery disease | 2.39 (0.55–10.37) | 0.246 |
| Previous or active cancer | 1.39 (0.80–2.43) | 0.245 |
| Previous heart failure hosp. | 0.86 (0.20–3.71) | 0.836 |
| Charlson Comorbidity Index (per unit) | 1.71 (1.34–2.18) | <0.001 |
| Serum creatinine (per mg/dL) | 1.20 (0.89–1.62) | 0.240 |
| Hemoglobin (per g/dL) | 0.65 (0.49–0.85) | 0.002 |
| Heart rate (per bpm) | 0.99 (0.97–1.03) | 0.838 |
| QRS duration (per ms) | 1.01 (0.99–1.02) | 0.200 |
| QRS ≥ 120 ms | 2.70 (0.97–7.57) | 0.058 |
| Systolic BP (per mmHg) | 1.01 (0.99–1.04) | 0.384 |
| Diastolic BP (per mmHg) | 0.99 (0.96–1.04) | 0.924 |
| Medications, use of | ||
| Beta blockers | 1.76 (0.95–3.27) | 0.073 |
| ACE-Inhibitors/ARBs | 1.42 (0.78–2.59) | 0.257 |
| MRAs | 1.44 (0.90–2.28) | 0.127 |
| Loop diuretics | 2.75 (1.12–6.77) | 0.028 |
| Ivabradine | 1.15 (0.34–3.96) | 0.823 |
| Echocardiography | ||
| Interventricular septum (per mm) | 1.15 (0.92–1.45) | 0.223 |
| Inferior-lateral wall (per mm) | 1.14 (0.92–1.42) | 0.247 |
| RWT (per unit) | 1.05 (1.01–1.09) | 0.019 |
| LV EDVi (per mL/m2) | 1.00 (0.98–1.02) | 0.948 |
| LVMi (per g/m2) | 1.00 (0.98–1.01) | 0.739 |
| Sphericity index (per unit) | 1.03 (0.96–1.09) | 0.442 |
| LVEF (per %) | 1.00 (0.95–1.06) | 0.954 |
| LVEF < 40% | 1.14 (0.46–2.82) | 0.785 |
| GLS (per %) | 1.01 (0.89–1.15) | 0.909 |
| GCS (per %) | 0.97 (0.87–1.08) | 0.589 |
| TAPSE (per mm) | 1.17 (1.02–1.34) | 0.025 |
| RV FWS (per %) | 0.92 (0.81–1.04) | 0.200 |
| LAVi (per mL/m2) | 1.01(0.97–1.05) | 0.621 |
| LA reservoir strain (per %) | 0.95 (0.89–1.01) | 0.097 |
| E wave (per m/s) | 2.36 (0.50–11.19) | 0.281 |
| E/A ratio (per unit) | 1.04 (0.44–2.49) | 0.926 |
| E/e’ ratio (per unit) * | 1.11 (1.02–1.20) | 0.012 |
| Hemodynamic forces | ||
| HDFs Apex-Base (per %) | 0.86 (0.66–1.13) | 0.290 |
| HDFs Lateral-Septal (per %) | 0.27 (0.09–0.80) | 0.019 |
| HDFs-angle (per °) | 1.16 (1.04–1.30) | 0.007 |
| HDFs-angle > 70° | 2.68 (1.09–6.60) | 0.032 |
| Systolic Thrust (per %) | 0.88 (0.73–1.07) | 0.190 |
| LV Suction (per %) | 0.88 (0.62–1.26) | 0.489 |
| Diastolic Deceleration (per %) | 0.96 (0.70–1.31) | 0.774 |
| LV Suction Reversal | 1.08 (0.44–2.67) | 0.869 |
| Systolic Thrust angle (per °) | 1.09 (0.99–1.20) | 0.075 |
| LV Suction angle (per °) | 1.05 (1.00–1.11) | 0.041 |
| Hazard Ratio [95% CI] | p Value | |
|---|---|---|
| Age (per year) | 1.09 (1.03–1.14) | 0.001 |
| HDFs-angle (per °) | 1.11 (1.00–1.23) | 0.042 |
| p = 0.030 for change in −2Log Likelihood | ||
| Charlson Comorbidity Index (per unit) | 1.63 (1.29–2.06) | <0.001 |
| HDFs-angle (per °) | 1.13 (1.02–1.26) | 0.021 |
| p = 0.013 for change in −2Log Likelihood | ||
| Hemoglobin (per g/dL) | 0.64 (0.49–0.84) | 0.001 |
| HDFs-angle (per °) | 1.18 (1.06–1.33) | 0.004 |
| p = 0.001 for change in −2Log Likelihood | ||
| LVEF (per %) | 0.96 (0.90–1.02) | 0.188 |
| HDFs-angle (per °) | 1.20 (1.06–1.36) | 0.003 |
| p = 0.001 for change in −2Log Likelihood | ||
| LV EDVi (per mL/m2) | 1.01 (0.99–1.03) | 0.325 |
| HDFs-angle (per °) | 1.19 (1.05–1.34) | 0.005 |
| p = 0.002 for change in −2Log Likelihood | ||
| GLS (per %) | 1.12 (0.96–1.29) | 0.142 |
| HDFs-angle (per °) | 1.20 (1.07–1.35) | 0.002 |
| p = 0.001 for change in −2Log Likelihood | ||
| TAPSE (per mm) | 1.19 (1.03–1.37) | 0.019 |
| HDFs-angle (per °) | 1.17 (1.05–1.30) | 0.006 |
| p = 0.003 for change in −2Log Likelihood | ||
| LA reservoir strain (per %) | 0.94 (0.88–0.99) | 0.046 |
| HDFs-angle (per °) | 1.17 (1.05–1.30) | 0.003 |
| p = 0.002 for change in −2Log Likelihood | ||
| E/e′ ratio (per unit) * | 1.12 (1.02–1.23) | 0.017 |
| HDFs-angle (per °) | 1.18 (1.02–1.37) | 0.028 |
| p = 0.013 for change in −2Log Likelihood | ||
| HDFs-Angle ≤ 70° (n. 67) | HDFs-Angle > 70° (n. 30) | p Value | |
|---|---|---|---|
| Clinical variables | |||
| Age (years) | 60.9 ± 14.4 | 65.1 ± 14.3 | 0.195 |
| Male sex, n (%) | 50 (75%) | 17 (57%) | 0.077 |
| Weight (kg) | 77 ± 16 | 70 ± 13 | 0.040 |
| Height (cm) | 169 ± 9 | 166 ± 12 | 0.288 |
| BSA (m2) | 1.86 ± 0.21 | 1.78 ± 0.21 | 0.066 |
| BMI (kg/m2) | 27.0 ± 4.8 | 25.6 ± 4.0 | 0.139 |
| Obesity, n (%) | 17 (25%) | 4 (13%) | 0.286 |
| Hypertension, n (%) | 33 (49%) | 17 (57%) | 0.500 |
| Dyslipidemia, n (%) | 28 (42%) | 14 (47%) | 0.654 |
| Diabetes, n (%) | 20 (30%) | 8 (27%) | 0.749 |
| COPD, n (%) | 7 (10%) | 2 (7%) | 0.716 |
| CKD, n (%) | 4 (6%) | 1 (3%) | 0.567 |
| Peripheral artery disease, n (%) | 3 (5%) | 2 (7%) | 0.643 |
| Previous or active cancer, n (%) | 9 (13%) | 5 (17%) | 0.664 |
| Previous heart failure hosp., n (%) | 11 (14%) | 2 (11%) | 0.682 |
| Charlson Comorbidity Index | 3 (1–4) | 3 (1–4) | 0.865 |
| Serum creatinine (mg/dL) | 0.88 (0.75–1.05) | 0.91 (0.77–1.05) | 0.917 |
| Hemoglobin (g/dL) | 13.7 ± 1.7 | 13.7 ± 2.1 | 0.953 |
| Heart rate (bpm) | 72 ± 17 | 72 ± 14 | 0.884 |
| QRS duration (ms) | 117 ± 36 | 117 ± 38 | 0.981 |
| QRS ≥ 120 ms, n (%) | 34 (51%) | 15 (40%) | 0.946 |
| Systolic BP (mmHg) | 128 ± 17 | 129 ± 16 | 0.854 |
| Diastolic BP (mmHg) | 75 ± 12 | 74 ± 12 | 0.719 |
| Follow-up time (years) | 4.2 (3.2–5.1) | 3.4 (3.0–5.4) | 0.421 |
| Medications, use of | |||
| Beta blockers, n (%) | 34 (56%) | 18 (64%) | 0.447 |
| ACE-Inhibitors/ARBs, n (%) | 47 (71%) | 21 (72%) | 0.905 |
| MRAs, n (%) | 19 (29%) | 9 (30%) | 0.939 |
| Loop diuretics, n (%) | 18 (27%) | 11 (37%) | 0.330 |
| Ivabradine, n (%) | 9 (13%) | 4 (16%) | 0.989 |
| Echocardiography | |||
| Interventricular septum (mm) | 10.9 ± 1.9 | 11.2 ± 2.0 | 0.599 |
| Inferior-lateral wall (mm) | 10.3 ± 2.0 | 9.9 ± 1.6 | 0.262 |
| RWT | 0.36 ± 0.10 | 0.38 ± 0.08 | 0.293 |
| LV EDVi (mL/m2) | 73 (64–97) | 60 (53–70) | <0.001 |
| LVMi (g/m2) | 140 ± 36 | 121 ± 26 | <0.001 |
| Sphericity index | 0.61 (0.55–0.67) | 0.55 (0.52–0.59) | <0.001 |
| LVEF (%) | 36.6 ± 8.5 | 44.8 ± 5.6 | <0.001 |
| LVEF < 40%, n (%) | 42 (63%) | 5 (17%) | <0.001 |
| GLS (%) | −12.6 ± 3.4 | −15.8 ± 3.0 | <0.001 |
| GCS (%) | −16.9 ± 4.4 | −17.9 ± 5.5 | 0.722 |
| TAPSE (mm) | 20 (18–23) | 20 (19–22) | 0.548 |
| RV FWS (%) | −21.5 ± 3.5 | −24.2 ± 3.6 | 0.021 |
| LAVi (mL/m2) | 31 (24–42) | 28 (20–34) | 0.064 |
| LA reservoir strain (%) | 19 (14–24) | 24 (18–29) | 0.045 |
| E wave (m/s) | 0.63 (0.53–0.82) | 0.60 (0.49–0.76) | 0.198 |
| E/A ratio | 0.75 (0.64–1.13) | 0.76 (0.65–1.02) | 0.958 |
| E/e’ ratio* | 11.1 (7.3–14.2) | 11.6 (7.8–19.0) | 0.524 |
| Hemodynamic forces (HDFs) | |||
| HDFs apex-base (%) | 5.4 (4.2–6.2) | 6.6 (4.9–8.1) | 0.001 |
| HDFs lateral-septal (%) | 1.7 (1.3–2.1) | 1.3 (1.0–1.7) | 0.001 |
| HDFs-angle (°) | 66 (62–69) | 73 (71–75) | 0.005 |
| Systolic Thrust (%) | 5.6 (3.7–7.2) | 6.9 (5.2–8.7) | 0.007 |
| LV Suction (%) | −2.5 (−3.2;−1.8) | −3.5 (−4.7;−2.8) | <0.001 |
| Diastolic Deceleration (%) | 2.3 (1.7–3.6) | 2.9 (2.0–4.5) | 0.051 |
| LV Suction Reversal, n. (%) | 34 (51%) | 18 (60%) | 0.398 |
| Systolic Thrust angle (°) | 72 (68–75) | 78 (76–80) | <0.001 |
| LV Suction angle (°) | 57 (51–61) | 67 (62–74) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesareo, M.; Ródenas-Alesina, E.; Guala, A.; Lozano-Torres, J.; Casas, G.; Vallelonga, F.; Airale, L.; Ferreira-González, I.; Milan, A.; Rodriguez-Palomares, J.F. Echocardiography-Derived Hemodynamic Forces Are Associated with Clinical Outcomes in Patients with Non-Ischemic Dilated Cardiomyopathy. J. Clin. Med. 2024, 13, 3862. https://doi.org/10.3390/jcm13133862
Cesareo M, Ródenas-Alesina E, Guala A, Lozano-Torres J, Casas G, Vallelonga F, Airale L, Ferreira-González I, Milan A, Rodriguez-Palomares JF. Echocardiography-Derived Hemodynamic Forces Are Associated with Clinical Outcomes in Patients with Non-Ischemic Dilated Cardiomyopathy. Journal of Clinical Medicine. 2024; 13(13):3862. https://doi.org/10.3390/jcm13133862
Chicago/Turabian StyleCesareo, Marco, Eduard Ródenas-Alesina, Andrea Guala, Jordi Lozano-Torres, Guillem Casas, Fabrizio Vallelonga, Lorenzo Airale, Ignacio Ferreira-González, Alberto Milan, and Jose F. Rodriguez-Palomares. 2024. "Echocardiography-Derived Hemodynamic Forces Are Associated with Clinical Outcomes in Patients with Non-Ischemic Dilated Cardiomyopathy" Journal of Clinical Medicine 13, no. 13: 3862. https://doi.org/10.3390/jcm13133862
APA StyleCesareo, M., Ródenas-Alesina, E., Guala, A., Lozano-Torres, J., Casas, G., Vallelonga, F., Airale, L., Ferreira-González, I., Milan, A., & Rodriguez-Palomares, J. F. (2024). Echocardiography-Derived Hemodynamic Forces Are Associated with Clinical Outcomes in Patients with Non-Ischemic Dilated Cardiomyopathy. Journal of Clinical Medicine, 13(13), 3862. https://doi.org/10.3390/jcm13133862








