Changes in the Lipid Asset of HIV/HCV Patients after a Successful Course of Direct-Acting Antivirals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- -
- A case report form was completed for each patient at baseline that included socio-demographic and epidemiological characteristics (age, sex, HCV genotype, risk factors for HIV and HCV) at baseline, as well as the degree of liver stiffness assessed by liver elastosonography, the HAART in use at the time of enrollment (and the possible switch before starting the DAA therapy), the immune profile (lymphocyte subpopulations), and HIV-RNA and HCV-RNA assays at baseline. Other laboratory investigations, including blood sugar level, parameters of the liver function (AST, ALT, GGT, total bilirubin), renal function parameters (blood urea, creatinine), blood parameters (INR, hemoglobinemia, platelet, and leukocyte counts), and a lipid profile (total cholesterol, high-density lipoprotein [HDL], LDL, and triglycerides) were reported for each patient.
- -
- Laboratory and virologic examinations were scheduled from baseline until completion of DAA treatment and at 12, 24, and 48-week follow-ups.
2.2. Statistical Analysis
3. Results
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c#:~:text=About%202.3%20million%20people%20[6.2,persons%20living%20with%20HIV%20globally (accessed on 20 November 2023).
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial Translocation Is a Cause of Systemic Immune Activation in Chronic HIV Infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Sacks-Davis, R.; Grebely, J.; Dore, G.J.; Osburn, W.; Cox, A.L.; Rice, T.M.; Spelman, T.; Bruneau, J.; Prins, M.; Kim, A.Y.; et al. Hepatitis C Virus Reinfection and Spontaneous Clearance of Reinfection--the InC3 Study. J. Infect Dis. 2015, 212, 1407–1419. [Google Scholar] [CrossRef]
- Chew, K.W.; Bhattacharya, D. Virologic and immunologic aspects of HIV-hepatitis C virus coinfection. AIDS 2016, 30, 2395–2404. [Google Scholar] [CrossRef]
- Ghiglione, Y.; Polo, M.L.; Urioste, A.; Rhodes, A.; Czernikier, A.; Trifone, C.; Quiroga, M.F.; Sisto, A.; Patterson, P.; Salomón, H.; et al. Hepatitis C Virus (HCV) Clearance After Treatment with Direct-Acting Antivirals in Human Immunodeficiency Virus (HIV)-HCV Coinfection Modulates Systemic Immune Activation and HIV Transcription on Antiretroviral Therapy. Open Forum Infect Dis. 2020, 7, ofaa115. [Google Scholar] [CrossRef]
- Maggi, P.; Di Biagio, A.; Rusconi, S.; Cicalini, S.; D’Abbraccio, M.; d’Ettorre, G.; Martinelli, C.; Nunnari, G.; Sighinolfi, L.; Spagnuolo, V.; et al. Cardiovascular risk and dyslipidemia among persons living with HIV: A review. BMC Infect Dis. 2017, 17, 551. [Google Scholar] [CrossRef]
- Riddler, S.A.; Smit, E.; Cole, S.R.; Li, R.; Chmiel, J.S.; Dobs, A.; Palella, F.; Visscher, B.; Evans, R.; Kingsley, L.A. Impact of HIV infection and HAART on serum lipids in men. JAMA 2003, 289, 2978–2982. [Google Scholar] [CrossRef]
- Achila, O.O.; Abrhaley, F.; Kesete, Y.; Tesfaldet, F.; Alazar, F.; Fisshaye, L.; Gebremeskel, L.; Mehari, R.; Andemichael, D. Dyslipidemia and associated risk factors among HIV/AIDS patients on HAART in Asmara, Eritrea. PLoS ONE 2022, 17, e0270838. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, J.; Zhang, W.; Han, N.; Yang, D.; Liu, W.; Zeng, H.; Han, J.; Zhao, H. Cumulative effects of hypertriglyceridemia in HIV-infected patients switching from NNRTIs to PI-based antiretroviral therapy. J. Infect Dev. Ctries. 2022, 16, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, B.; Liang, W.; Chen, T.; Deng, L.; Zhao, M.; Wan, J. The effects of ART on the dynamics of lipid profiles in Chinese Han HIV-infected patients: Comparison between NRTI/NNRTI and NRTI/INSTI. Front Public Health 2023, 11, 1161503. [Google Scholar] [CrossRef]
- Crouchet, E.; Baumert, T.F.; Schuster, C. Hepatitis C virus-apolipoprotein interactions: Molecular mechanisms and clinical impact. Expert Rev. Proteom. 2017, 14, 593–606. [Google Scholar] [CrossRef]
- La Fazia, V.M.; Pierucci, N.; Mohanty, S.; Gianni, C.; Della Rocca, D.G.; Compagnucci, P.; MacDonald, B.; Mayedo, A.; Torlapati, P.G.; Bassiouny, M.; et al. Catheter ablation approach and outcome in HIV+ patients with recurrent atrial fibrillation. J. Cardiovasc Electrophysiol. 2023, 34, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Negro, F.; Sanyal, A.J. Hepatitis C virus, steatosis and lipid abnormalities: Clinical and pathogenic data. Liver Int. 2009, 29, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Osibogun, O.; Ogunmoroti, O.; Michos, E.D.; Spatz, E.S.; Olubajo, B.; Nasir, K.; Maziak, W. A systematic review of the associations between HIV/HCV coinfection and biomarkers of cardiovascular disease. Rev. Med. Virol. 2018, 28, e1953. [Google Scholar] [CrossRef]
- SCORE2 working group and ESC Cardiovascular risk collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef]
- Meissner, E.G.; Lee, Y.J.; Osinusi, A.; Sims, Z.; Qin, J.; Sturdevant, D.; McHutchison, J.; Subramanian, M.; Sampson, M.; Naggie, S.; et al. Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1-infected patients. Hepatology 2015, 61, 790–801. [Google Scholar] [CrossRef]
- Mauss, S.; Berger, F.; Wehmeyer, M.H.; Ingiliz, P.; Hueppe, D.; Lutz, T.; Simon, K.G.; Schewe, K.; Rockstroh, J.K.; Baumgarten, A.; et al. Effect of antiviral therapy for HCV on lipid levels. Antivir. Ther. 2017, 21, 81–88. [Google Scholar] [CrossRef]
- Townsend, K.; Meissner, E.G.; Sidharthan, S.; Sampson, M.; Remaley, A.T.; Tang, L.; Kohli, A.; Osinusi, A.; Masur, H.; Kottilil, S. Interferon-Free Treatment of Hepatitis C Virus in HIV/Hepatitis C Virus-Coinfected Subjects Results in Increased Serum Low-Density Lipoprotein Concentration. AIDS Res. Hum. Retroviruses 2016, 32, 456–462. [Google Scholar] [CrossRef]
- Spaziante, M.; Taliani, G.; Marchetti, G.; Tavelli, A.; Lichtner, M.; Cingolani, A.; Cicalini, S.; Biliotti, E.; Girardi, E.; Antinori, A.; et al. Impact of HCV Eradication on Lipid Metabolism in HIV/HCV Coinfected Patients: Data from ICONA and HepaICONA Foundation Cohort Study. Viruses 2021, 13, 1402. [Google Scholar] [CrossRef]
- Endo, D.; Satoh, K.; Shimada, N.; Hokari, A.; Aizawa, Y. Impact of interferon-free antivirus therapy on lipid profiles in patients with chronic hepatitis C genotype 1b. World J. Gastroenterol. 2017, 23, 2355–2364. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Lo, J.C.; Franklin, A.; Bolger, A.F.; Martin, J.N.; Deeks, S.G.; Waters, D.D. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004, 109, 1603–1608. [Google Scholar] [CrossRef]
- Kohli, P.; Ganz, P.; Ma, Y.; Scherzer, R.; Hur, S.; Weigel, B.; Grunfeld, C.; Deeks, S.; Wasserman, S.; Scott, R.; et al. HIV and Hepatitis C-Coinfected Patients Have Lower Low-Density Lipoprotein Cholesterol Despite Higher Proprotein Convertase Subtilisin Kexin 9 (PCSK9): An Apparent “PCSK9-Lipid Paradox”. J. Am. Heart Assoc. 2016, 5, e002683. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Estep, M.; Negro, F.; Clark, P.J.; Hunt, S.; Song, Q.; Paulson, M.; Stamm, L.M.; Brainard, D.M.; et al. Dysregulation of distal cholesterol biosynthesis in association with relapse and advanced disease in CHC genotype 2 and 3 treated with sofosbuvir and ribavirin. J. Hepatol. 2016, 64, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ryom, L.; Lundgren, J.D.; El-Sadr, W.; Reiss, P.; Kirk, O.; Law, M.; Phillips, A.; Weber, R.; Fontas, E.; d’ Arminio Monforte, A.; et al. Cardiovascular disease and use of contemporary protease inhibitors: The D:A:D international prospective multicohort study. Lancet HIV 2018, 5, e291–e300. [Google Scholar] [CrossRef] [PubMed]
- Abutaleb, A.; Kattakuzhy, S.; Kottilil, S.; O’Connor, E.; Wilson, E. Mechanisms of neuropathogenesis in HIV and HCV: Similarities, differences, and unknowns. J. Neurovirol. 2018, 24, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz, M.M.; Overk, C.; Achim, C.L.; Masliah, E. Pathogenesis of age-related HIV neurodegeneration. J. Neurovirol. 2019, 25, 622–633. [Google Scholar] [CrossRef]
- Salmon-Ceron, D.; Nahon, P.; Layese, R.; Bourcier, V.; Sogni, P.; Bani-Sadr, F.; Audureau, E.; Merchadou, L.; Dabis, F.; Wittkop, L.; et al. Human Immunodeficiency Virus/Hepatitis C Virus [HCV] Co-Infected Patients with Cirrhosis Are No Longer at Higher Risk for Hepatocellular Carcinoma or End-Stage Liver Disease as Compared to HCV Mono-Infected Patients. Hepatology 2019, 70, 939–954. [Google Scholar] [CrossRef]

| Sample size, n | 54 |
| Recruitment period, range of years | 2017–2022 |
| Middle age, years (median ± SD) | 54.2 (56 ± 8.3) |
| Patients in HAART, n (%) | 54 (100) |
| Patients with HIV-RNA < 40 copies/mL, n (%) | 54 (100) |
| CD4, mean ±SD | 958 ± 256 |
| Patients with CD4 > 500/mm3, n (%) | 15 (27.7) |
| Years since HIV diagnosis, ys (IQR) | 18 (14–26) |
| HAART, n (%) | |
| TDF | 22 (40.7) |
| TAF | 3 (5.5) |
| ABC | 8 (14.8) |
| DRV/r, | 11 (20.3) |
| ATV/r | 7 (12.9) |
| Raltegravir | 8 (14.8) |
| CAH HCV, n (%) | 37 (68) |
| Cirrhosis, n (%) | 17 (31) |
| Naïve to DAA, n (%) | 54 (100) |
| HCV-RNA, log10 copies/mL, mean value | 3.38 |
| Genotype 1, n (%) | 30 (55) → 22a, 7b, 1ab |
| Genotype 2, n (%) | 2 (3) |
| Genotype 3, n (%) | 16 (29) |
| Genotype 4, n (%) | 6 (11) |
| DAA, n (%) | |
| Ombitasvir/Paritaprevir/Ritonavir + Dasabuvir and Ribavirin | 3 (5.6) |
| Daclatasvir + Sofosbuvir | 11 (20.4) |
| Elbasvir + Grazoprevir | 2(3.7) |
| Sofosbuvir + Velpatasvir | 18 (33) |
| Glecaprevir + Pibrentasvir | 3(5.6) |
| Ledipasvir + Sofosbuvir | 13 (24.1) |
| Simeprevir | 2(3.7) |
| Sofosbuvir + Ribavirin | 2(3.7) |
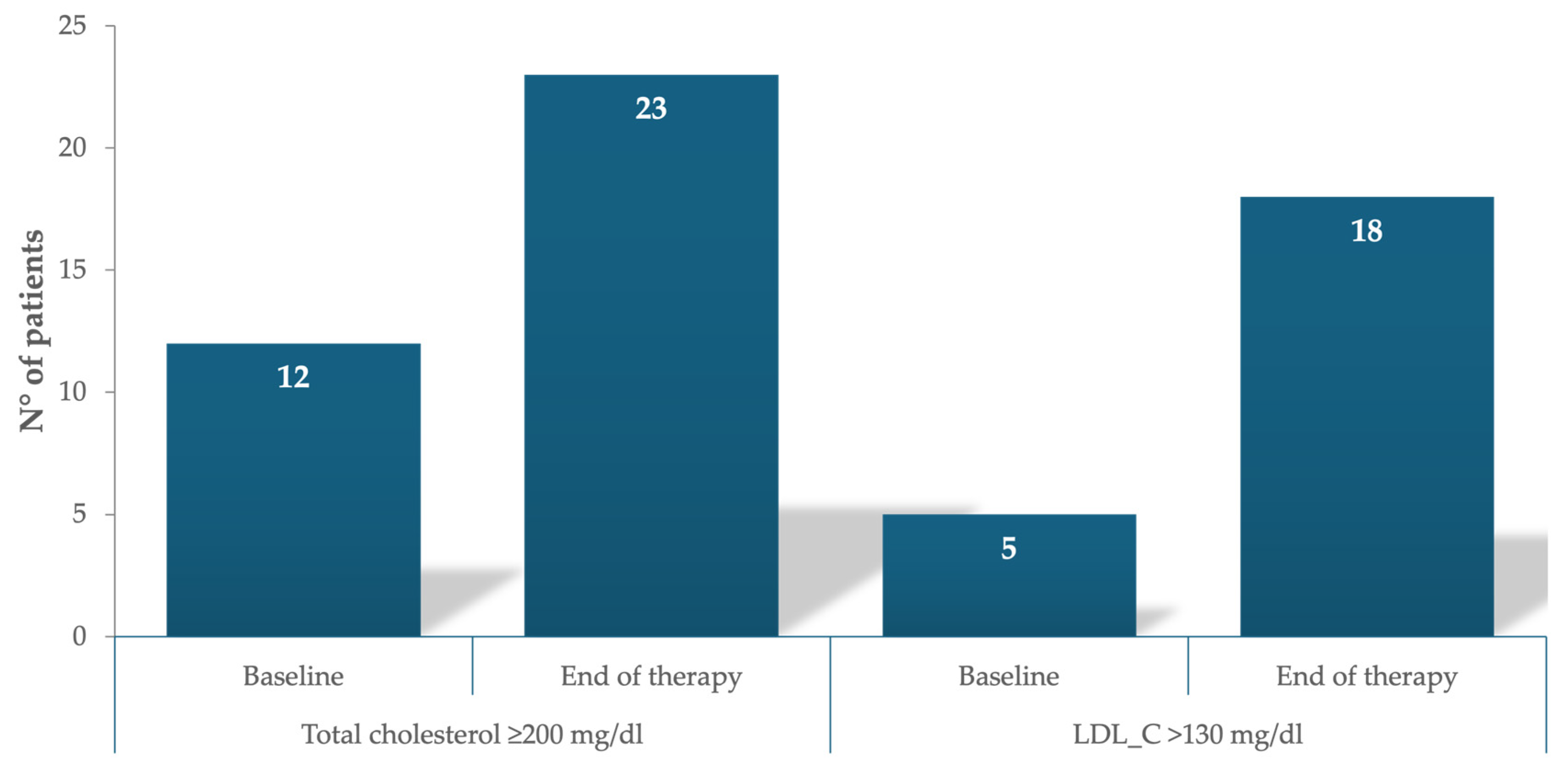
| Pretreatment total cholesterol (<200 mg/dL), n (%) | 42 (78) |
| Pretreatment total cholesterol (>200 mg/dL), n (%) | 12 (22) |
| mg/dL | HDL | p | LDL | p | TG | p | Total Cholesterol | p |
|---|---|---|---|---|---|---|---|---|
| Pretreatment | 48 ± 20 | 86.7 ± 34 | 147.7 ± 143.6 | 165.03 ± 46.5 | ||||
| During treatment (at 4 wks) | 47.9 ± 17 | ns | 105.07 ± 33.9 | ns | 109.4 ± 66 | ns | 174.8 ± 46 | ns |
| At the end of therapy | 51.16 ± 18.6 | ns | 111.4 ± 40.4 | ns | 110.5 ± 80.6 | ns | 184.7 ± 44.9 | 0.0001 |
| 12 wks from SVR | 48.2 ± 19.2 | ns | 108.2 ± 43 | ns | 127.5 ± 93 | ns | 182.46 ± 52.6 | 0.001 |
| 24 wks from SVR | 52.3 ± 21.5 | ns | 103.4 ± 41.38 | 0.0001 | 136.6 ± 108.3 | ns | 183.2 ± 47.3 | 0.002 |
| 48 wks from SVR | 52.7 ± 20.2 | ns | 107.9 ± 41 | ns | 130.5 ± 123 | ns | 186.7 ± 48.7 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spera, A.M.; Conti, V.; Corbi, G.; Ascione, T.; Ciccarelli, M.; Masullo, A.; Franci, G.; Pagliano, P. Changes in the Lipid Asset of HIV/HCV Patients after a Successful Course of Direct-Acting Antivirals. J. Clin. Med. 2024, 13, 3865. https://doi.org/10.3390/jcm13133865
Spera AM, Conti V, Corbi G, Ascione T, Ciccarelli M, Masullo A, Franci G, Pagliano P. Changes in the Lipid Asset of HIV/HCV Patients after a Successful Course of Direct-Acting Antivirals. Journal of Clinical Medicine. 2024; 13(13):3865. https://doi.org/10.3390/jcm13133865
Chicago/Turabian StyleSpera, Anna Maria, Valeria Conti, Graziamaria Corbi, Tiziana Ascione, Michele Ciccarelli, Alfonso Masullo, Gianluigi Franci, and Pasquale Pagliano. 2024. "Changes in the Lipid Asset of HIV/HCV Patients after a Successful Course of Direct-Acting Antivirals" Journal of Clinical Medicine 13, no. 13: 3865. https://doi.org/10.3390/jcm13133865
APA StyleSpera, A. M., Conti, V., Corbi, G., Ascione, T., Ciccarelli, M., Masullo, A., Franci, G., & Pagliano, P. (2024). Changes in the Lipid Asset of HIV/HCV Patients after a Successful Course of Direct-Acting Antivirals. Journal of Clinical Medicine, 13(13), 3865. https://doi.org/10.3390/jcm13133865







