Centhaquine Increases Stroke Volume and Cardiac Output in Patients with Hypovolemic Shock
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Echocardiographic Measurements
LVOT Diameter
LVOT VTI (LVOT Velocity Time Integral)
Heart Rate (HR)
SV and CO
LVEF and LVFS
Total Peripheral Resistance (TPR)
2.2. Patient Population, Consent, and Regulatory Oversight
2.3. Safety Evaluation
2.4. Multivariate Imputation by Chained Equations (MICE)
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics, Baseline Characteristics, and the Volume of Fluid Administration during the First 5 h of Resuscitation
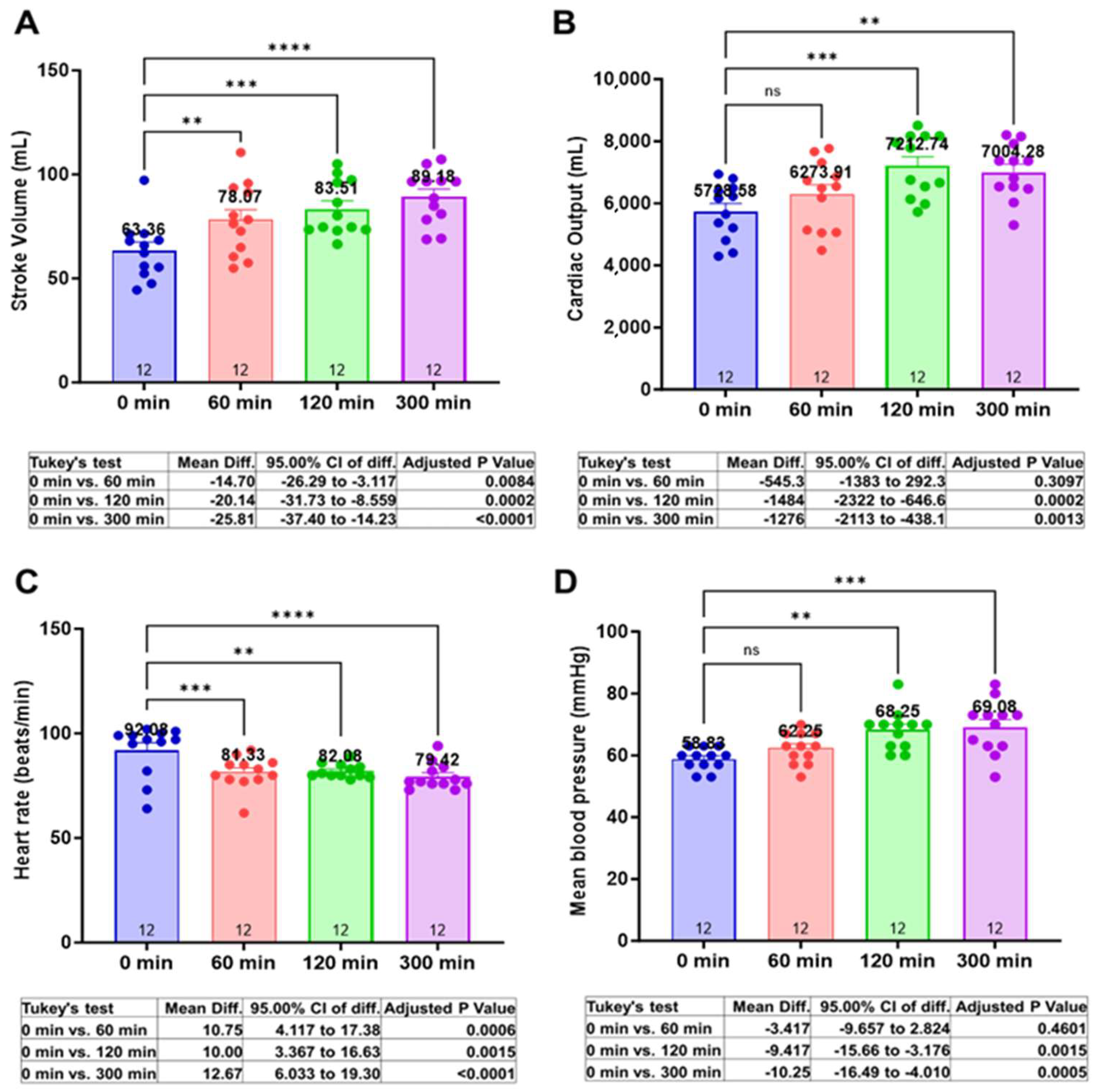
3.2. Centhaquine Increases Stroke Volume, Cardiac Output, and Mean Arterial Pressure
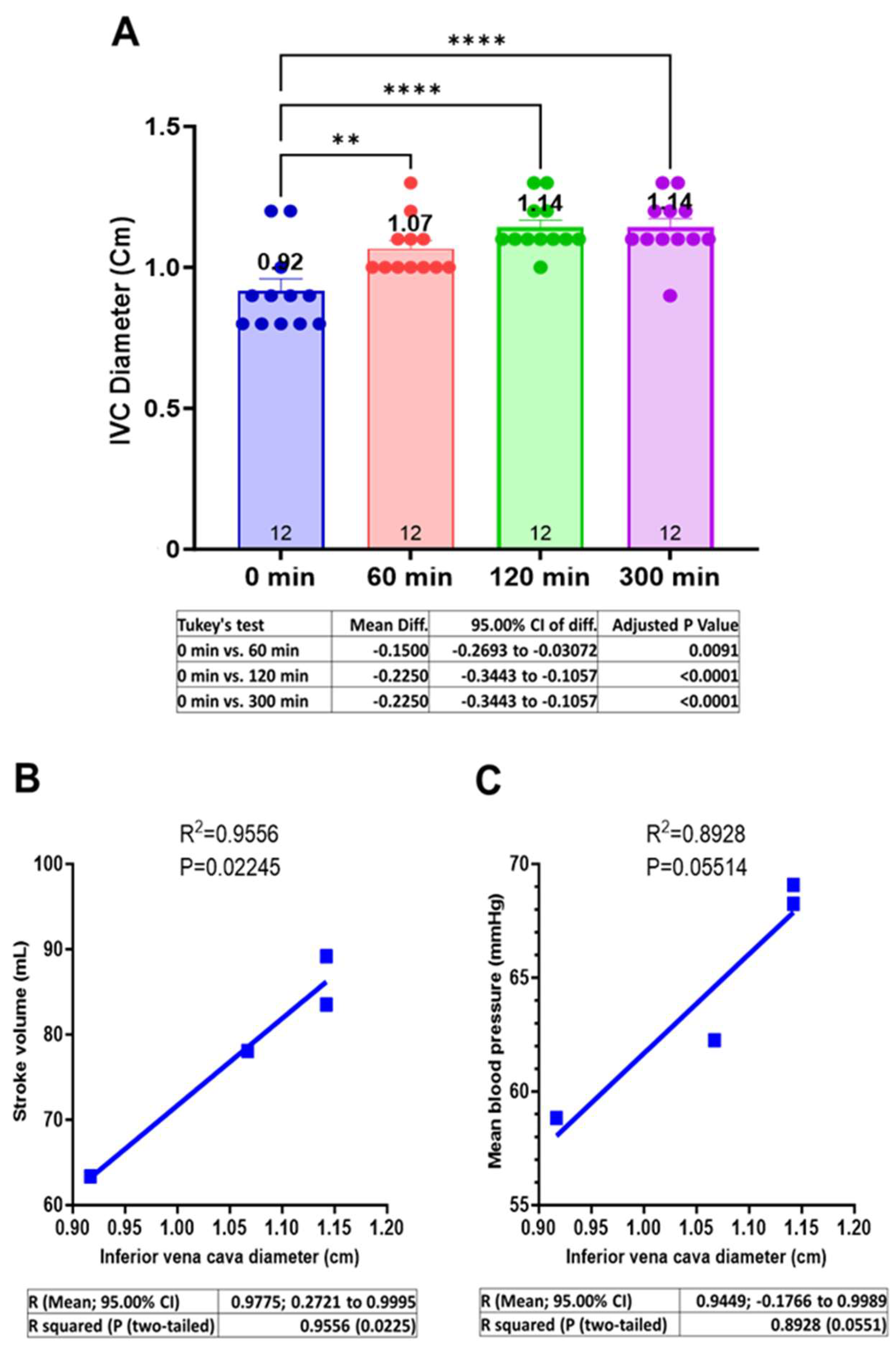
3.3. Centhaquine Increases the Venous Return (Increase in IVC Diameter)
3.4. Centhaquine Increases LVOT-VTI (Blood Flow toward Aortic Annulus/Valve)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Adrenergic receptor |
| SV | Stroke volume |
| CO | Cardiac output |
| LVEF | Left ventricular ejection fraction |
| FS | Fractional shortening (left ventricular) |
| IVC | Inferior vena cava |
| LVOT | Left ventricular outflow tract |
| VTI | Velocity time integral |
| LVDD | Left ventricle diameter at diastole |
| LVDS | Left ventricle diameter at systole |
| DBP | Diastolic blood pressure |
| HR | Heart rate |
| MAP | Mean arterial pressure |
| SVR | Systemic vascular resistance |
| SBP | Systolic blood pressure |
| SOC | Standard of care |
| MICE | Multivariate Imputation by Chained Equations |
| MODS | Multiple Organ Dysfunction Syndrome |
| ARDS | Acute Respiratory Distress Syndrome |
References
- Snyder, E.M., Jr. Management of refractory shock. Calif. Med. 1954, 80, 13–15. [Google Scholar] [PubMed]
- Kalkwarf, K.J.; Cotton, B.A. Resuscitation for Hypovolemic Shock. Surg. Clin. North Am. 2017, 97, 1307–1321. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, A.; Drygalski, T.; Garlicki, J.; Woron, J.; Szpunar, W.; Polak, M.; Dros, J.; Wordliczek, J.; Zajaczkowska, R. The volume of infusion fluids correlates with treatment outcomes in critically ill trauma patients. Front. Med. 2022, 9, 1040098. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Marik, P.E.; Teboul, J.L. Prediction of fluid responsiveness: An update. Ann. Intensive Care 2016, 6, 111. [Google Scholar] [CrossRef]
- La Via, L.; Vasile, F.; Perna, F.; Zawadka, M. Prediction of fluid responsiveness in critical care: Current evidence and future perspective. Trends Anaesth. Crit. Care 2024, 54, 101316. [Google Scholar] [CrossRef]
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.L.; et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef]
- Hollenberg, S.M. Vasoactive drugs in circulatory shock. Am. J. Respir. Crit. Care Med. 2011, 183, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulos, P.P.; Boutsikos, I.; Boutsikos, P.; Iacovidou, N.; Ekmektzoglou, K. Current use and advances in vasopressors and inotropes support in shock. J. Emerg. Crit. Care Med. 2020, 4, 20. [Google Scholar] [CrossRef]
- Fage, N.; Asfar, P.; Radermacher, P.; Demiselle, J. Norepinephrine and Vasopressin in Hemorrhagic Shock: A Focus on Renal Hemodynamics. Int. J. Mol. Sci. 2023, 24, 4103. [Google Scholar] [CrossRef]
- Alexander, R.S. Venomotor tone in hemorrhage and shock. Circ. Res. 1955, 3, 181–190. [Google Scholar] [CrossRef]
- Gulati, A.; Choudhuri, R.; Gupta, A.; Singh, S.; Ali, S.K.N.; Sidhu, G.K.; Haque, P.D.; Rahate, P.; Bothra, A.R.; Singh, G.P.; et al. A Multicentric, Randomized, Controlled Phase III Study of Centhaquine (Lyfaquin®) as a Resuscitative Agent in Hypovolemic Shock Patients. Drugs 2021, 81, 1079–1100. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Jain, D.; Agrawal, N.R.; Rahate, P.; Choudhuri, R.; Das, S.; Dhibar, D.P.; Prabhu, M.; Haveri, S.; Agarwal, R.; et al. Resuscitative Effect of Centhaquine (Lyfaquin®) in Hypovolemic Shock Patients: A Randomized, Multicentric, Controlled Trial. Adv. Ther. 2021, 38, 3223–3265. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A. Shear Stress and Endothelial Mechanotransduction in Trauma Patients with Hemorrhagic Shock: Hidden Coagulopathy Pathways and Novel Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 17522. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Lavhale, M.S.; Garcia, D.J.; Havalad, S. Centhaquin improves resuscitative effect of hypertonic saline in hemorrhaged rats. J. Surg. Res. 2012, 178, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Azur, M.J.; Stuart, E.A.; Frangakis, C.; Leaf, P.J. Multiple imputation by chained equations: What is it and how does it work? Int. J. Methods Psychiatr. Res. 2011, 20, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Anel, R.; Bunnell, E.; Zanotti, S.; Habet, K.; Haery, C.; Marshall, S.; Cheang, M.; Neumann, A.; Ali, A.; et al. Preload-independent mechanisms contribute to increased stroke volume following large volume saline infusion in normal volunteers: A prospective interventional study. Crit. Care 2004, 8, R128–R136. [Google Scholar] [CrossRef] [PubMed]
- Carsetti, A.; Cecconi, M.; Rhodes, A. Fluid bolus therapy: Monitoring and predicting fluid responsiveness. Curr. Opin. Crit. Care 2015, 21, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M. Ideal resuscitation fluid in hypovolemia: The quest is on and miles to go! Int. J. Crit. Illn. Inj. Sci. 2016, 6, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. Vasopressor therapy in critically ill patients with shock. Intensive Care Med. 2019, 45, 1503–1517. [Google Scholar] [CrossRef]
- De Backer, D.; Biston, P.; Devriendt, J.; Madl, C.; Chochrad, D.; Aldecoa, C.; Brasseur, A.; Defrance, P.; Gottignies, P.; Vincent, J.L.; et al. Comparison of dopamine and norepinephrine in the treatment of shock. N. Engl. J. Med. 2010, 362, 779–789. [Google Scholar] [CrossRef]
- Lyon, M.; Blaivas, M.; Brannam, L. Sonographic measurement of the inferior vena cava as a marker of blood loss. Am. J. Emerg. Med. 2005, 23, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Sefidbakht, S.; Assadsangabi, R.; Abbasi, H.R.; Nabavizadeh, A. Sonographic measurement of the inferior vena cava as a predictor of shock in trauma patients. Emerg. Radiol. 2007, 14, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Massalha, M.; Faranish, R.; Romano, S.; Salim, R. Decreased inferior vena cava diameter as an early marker in postpartum hemorrhage. Ultrasound Obstet. Gynecol. 2022, 59, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, D.; Gao, Y.; Wu, Z.; Wang, X.; Lv, C. Effect of VTILVOT variation rate on the assessment of fluid responsiveness in septic shock patients. Medicine 2020, 99, e22702. [Google Scholar] [CrossRef] [PubMed]
- Perez-Manjarrez, A.; Garcia-Cruz, E.; Gopar-Nieto, R.; Jimenez-Rodriguez, G.M.; Lazcano-Diaz, E.; Rojas-Velasco, G.; Manzur-Sandoval, D. Usefulness of the velocity-time integral of the left ventricular outflow tract variability index to predict fluid responsiveness in patients undergoing cardiac surgery. Echo Res. Pract. 2023, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Geevarghese, M., III; Patel, K.; Gulati, A.; Ranjan, A.K. Role of adrenergic receptors in shock. Front. Physiol. 2023, 14, 1094591. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A.; Laou, E.; Papagiannakis, N.; Spyropoulos, V.; Kouskouni, E.; Theodoraki, K.; Xanthos, T. Assessment of Dynamic Changes in Stressed Volume and Venous Return during Hyperdynamic Septic Shock. J. Pers. Med. 2022, 12, 724. [Google Scholar] [CrossRef]
- Vanhoutte, P.M. The Role of the Systemic Veins: An Update. Phlebology 1987, 2, 61–73. [Google Scholar] [CrossRef]
- Shen, T.; Baker, K. Venous return and clinical hemodynamics: How the body works during acute hemorrhage. Adv. Physiol. Educ. 2015, 39, 267–271. [Google Scholar] [CrossRef]
- Funk, D.J.; Jacobsohn, E.; Kumar, A. Role of the venous return in critical illness and shock: Part II-shock and mechanical ventilation. Crit. Care Med. 2013, 41, 573–579. [Google Scholar] [CrossRef]
- Spiegel, R. Stressed vs. unstressed volume and its relevance to critical care practitioners. Clin. Exp. Emerg. Med. 2016, 3, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Giovannitti, J.A., Jr.; Thoms, S.M.; Crawford, J.J. Alpha-2 adrenergic receptor agonists: A review of current clinical applications. Anesth. Prog. 2015, 62, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Rudner, X.L.; Berkowitz, D.E.; Booth, J.V.; Funk, B.L.; Cozart, K.L.; D’Amico, E.B.; El-Moalem, H.; Page, S.O.; Richardson, C.D.; Winters, B.; et al. Subtype specific regulation of human vascular α1-adrenergic receptors by vessel bed and age. Circulation 1999, 100, 2336–2343. [Google Scholar] [CrossRef]
- Hering, L.; Rahman, M.; Potthoff, S.A.; Rump, L.C.; Stegbauer, J. Role of α2-Adrenoceptors in Hypertension: Focus on Renal Sympathetic Neurotransmitter Release, Inflammation, and Sodium Homeostasis. Front. Physiol. 2020, 11, 566871. [Google Scholar] [CrossRef] [PubMed]

| Age (years) | 37.42 ± 3.68 |
| Body weight (kg) | 58.83 ± 1.57 |
| Height (cm) | 157.67 ± 1.2 |
| Body mass index (kg/m2) | 23.64 ± 0.22 |
| Sex | |
| Men | 9 (75%) |
| Women | 3 (25%) |
| Medical history | |
| Hypertension | 0 |
| Diabetes | 0 |
| Renal disorders | 0 |
| Respiratory disease | 1 (8.33%) |
| Ischemic heart disease | 0 |
| Liver fibrosis | 0 |
| Hepatitis (altered SGPT) | 6 (50%) |
| Pre-eclampsia | 0 |
| Reason for hypovolemic shock | |
| Gastroenteritis (vomiting, abdominal pain, and/or diarrhea) | 8 (66.67%) |
| Enteric fever | 4 (33.33%) |
| Dengue fever | 2 (16.67%) |
| Falciparum malaria | 1 (8.33%) |
| Acute appendicitis | 1 (8.33%) |
| Clinical factors | |
| Systolic blood pressure (mmHg) | 73.87 ± 2.9 |
| Diastolic blood pressure (mmHg) | 45.33 ± 1.64 |
| Heart rate (beats/min) | 108 ± 4.4 |
| ECG | Normal |
| Random blood glucose (mg/dL) | 104.24 ± 4.75 |
| Shock index | 1.51 ± 0.12 |
| MODS | 2.5 ± 0.38 |
| ARDS | 0.08 ± 0.083 |
| Respiratory rate (breaths/min) | 23.5 ± 0.23 |
| Body temperature (°F) | 102.3 ± 0.36 |
| Blood lactate (mmol/L) | 2.5 ± 0.06 |
| Base deficit (mmol/L) | −1.49 ± 0.06 |
| Hemoglobin (g/dL) | 12.16 ± 0.45 |
| Hematocrit (%) | 37.24 ± 1.26 |
| Creatinine (mg/dL) | 1.13 ± 0.15 |
| Glomerular filtration rate (mL/min/1.73 m2) | 83.43 ± 7.84 |
| pH | 7.21 ± 0.01 |
| pCO2 (mmHg) | 33.17 ± 0.90 |
| PO2/FiO2 | 376.75 ± 6.32 |
| Control (n = 19) | Centhaquine (n = 22) | p Value | |
|---|---|---|---|
| Change in MAP (mmHg) from 0 (baseline) to 24 h | 11.35 ± 2.64 | 25.09 ± 2.74 | 0.0004 |
| Volume (Liters) of fluid administered 0 (baseline) to 24 h | 2.67 ± 2.64 | 2.54 ± 0.18 | 0.3324 |
| Normal Saline (mL) | Normal Saline with Dextrose (mL) | Total Volume (mL) |
|---|---|---|
| 345.7 ± 67.2 | 400.42 ± 32.42 | 746.12 ± 87.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanna, A.; Vaidya, K.; Shah, D.; Ranjan, A.K.; Gulati, A. Centhaquine Increases Stroke Volume and Cardiac Output in Patients with Hypovolemic Shock. J. Clin. Med. 2024, 13, 3765. https://doi.org/10.3390/jcm13133765
Khanna A, Vaidya K, Shah D, Ranjan AK, Gulati A. Centhaquine Increases Stroke Volume and Cardiac Output in Patients with Hypovolemic Shock. Journal of Clinical Medicine. 2024; 13(13):3765. https://doi.org/10.3390/jcm13133765
Chicago/Turabian StyleKhanna, Aman, Krish Vaidya, Dharmesh Shah, Amaresh K. Ranjan, and Anil Gulati. 2024. "Centhaquine Increases Stroke Volume and Cardiac Output in Patients with Hypovolemic Shock" Journal of Clinical Medicine 13, no. 13: 3765. https://doi.org/10.3390/jcm13133765
APA StyleKhanna, A., Vaidya, K., Shah, D., Ranjan, A. K., & Gulati, A. (2024). Centhaquine Increases Stroke Volume and Cardiac Output in Patients with Hypovolemic Shock. Journal of Clinical Medicine, 13(13), 3765. https://doi.org/10.3390/jcm13133765






