Time to Total Knee Arthroplasty (TKA) Post Intra-Articular Injection
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Group Selection
2.2. Outcome Variables
2.3. Demographic Variables
2.4. Data Analyses
3. Results
3.1. Total Population
3.2. Subsequent TKA Population (Case Match 4:1 CS vs. HA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef] [PubMed]
- Berkani, S.; Courties, A.; Eymard, F.; Latourte, A.; Richette, P.; Berenbaum, F.; Sellam, J.; Louati, K. Time to Total Knee Arthroplasty after Intra-Articular Hyaluronic Acid or Platelet-Rich Plasma Injections: A Systematic Literature Review and Meta-Analysis. J. Clin. Med. 2022, 11, 3985. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Levin, G.; Nikolov, N.P.; Abugov, R.; Rothwell, R. Concept End Points Informing Design Considerations for Confirmatory Clinical Trials in Osteoarthritis. Arthritis Care Res. 2022, 74, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Concoff, A.; Niazi, F.; Farrokhyar, F.; Alyass, A.; Rosen, J.; Nicholls, M. Delay to TKA and Costs Associated with Knee Osteoarthritis Care Using Intra-Articular Hyaluronic Acid: Analysis of an Administrative Database. Clinical Medicine Insights. Arthritis Musculoskelet. Disord. 2021, 14, 1179544121994092. [Google Scholar] [CrossRef] [PubMed]
- Jüni, P.; Hari, R.; Rutjes, A.W.; Fischer, R.; Silletta, M.G.; Reichenbach, S.; da Costa, B.R.; da Costa, B.R. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst. Rev. 2015, 2015, CD005328. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Bhandari, M. Cochrane in CORR®: Intra-articular Corticosteroid For Knee Osteoarthritis. Clin. Orthop. Relat. Res. 2018, 476, 1391–1392. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2017, 317, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Runa, M.; Lau, E.; Altman, R.D. Cost-of-illness of knee osteoarthritis: Potential cost savings by not undergoing arthroplasty within the first 2 years. Clin. Outcomes Res. CEOR 2019, 11, 245–255. [Google Scholar] [CrossRef]
- American Academy of Orthopaedic Surgeons. Management of Osteoarthritis of the Knee (Non-Arthroplasty) Evidence-Based Clinical Practice Guideline. 2021. Available online: https://www.aaos.org/globalassets/quality-and-practice-resources/osteoarthritis-of-the-knee/oak3cpg.pdf (accessed on 8 January 2024).
- Berlinberg, E.J.; Swindell, H.; Patel, H.H.; Zabat, M.; Forlenza, E.M.; Cancienne, J.; Forsythe, B. The Epidemiology of Platelet-Rich Plasma Injections From 2010 to 2020 in a Large US Commercial Insurance Claims Database: A Recent Update. J. Am. Acad. Orthop. Surg. 2023, 31, e135–e147. [Google Scholar] [CrossRef]
- Li, A.K.; Stavrakis, A.I.; Photopoulos, C. Platelet-rich plasma use for hip and knee osteoarthritis in the United States. The Knee 2022, 39, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Magruder, M.L.; Caughey, S.; Gordon, A.M.; Capotosto, B.S.S.; Rodeo, S.A. Trends in utilization, demographics, and costs of platelet-rich plasma injections: A ten-year nationwide investigation. Physician Sports Med. 2023, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.C.; Cancienne, J.M.; Browning, R.; Verma, N.N.; Cole, B.J. An Analysis of Current Treatment Trends in Platelet-Rich Plasma Therapy in the Medicare Database. Orthop. J. Sports Med. 2020, 8, 2325967119900811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Fabricant, P.D.; Ishmael, C.R.; Wang, J.C.; Petrigliano, F.A.; Jones, K.J. Utilization of Platelet-Rich Plasma for Musculoskeletal Injuries: An Analysis of Current Treatment Trends in the United States. Orthop. J. Sports Med. 2016, 4, 2325967116676241. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Paterson, K.L.; Metcalf, B.R.; Duong, V.; Eyles, J.; Kasza, J.; Wang, Y.; Cicuttini, F.; Buchbinder, R.; Forbes, A.; et al. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2021, 326, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Costa LA, V.; Lenza, M.; Irrgang, J.J.; Fu, F.H.; Ferretti, M. How Does Platelet-Rich Plasma Compare Clinically to Other Therapies in the Treatment of Knee Osteoarthritis? A Systematic Review and Meta-analysis. Am. J. Sports Med. 2023, 51, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, A.; Sari, A.; Durmus, B. Platelet-Rich Plasma vs Prolotherapy in the Management Of Knee Osteoarthritis: Randomized Placebo-Controlled Trial. Spor. Hekimliği. Dergisi. 2016, 51, 34–43. [Google Scholar] [CrossRef]
- Murray, I.R.; Chahla, J.; Frank, R.M.; Piuzzi, N.S.; Mandelbaum, B.R.; Dragoo, J.L.; Members of the Biologics Association. Rogue stem cell clinics. Bone Jt. J. 2020, 102-B, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Piuzzi, N.S.; Emara, A.; Chahla, J.; Mandelbaum, B.R. Ethical and Practical Considerations for Integrating Cellular (“Stem Cell”) Therapy into Clinical Practice. Curr. Rev. Musculoskelet. Med. 2020, 13, 525–529. [Google Scholar] [CrossRef]
- Smith, C.; Crowley, A.; Munsie, M.; DeMartino, E.S.; Staff, N.P.; Shapiro, S.; Master, Z. Academic physician specialists’ views toward the unproven stem cell intervention industry: Areas of common ground and divergence. Cytotherapy 2021, 23, 348–356. [Google Scholar] [CrossRef]
- Vilchez-Cavazos, F.; Blázquez-Saldaña, J.; Gamboa-Alonso, A.A.; Peña-Martínez, V.M.; Acosta-Olivo, C.A.; Sánchez-García, A.; Simental-Mendía, M. The use of platelet-rich plasma in studies with early knee osteoarthritis versus advanced stages of the disease: A systematic review and meta-analysis of 31 randomized clinical trials. Arch. Orthop. Trauma Surg. 2023, 143, 1393–1408. [Google Scholar] [CrossRef] [PubMed]
- Master, Z.; Matthews KR, W.; Abou-El-Enein, M. Unproven stem cell interventions: A global public health problem requiring global deliberation. Stem Cell Rep. 2021, 16, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Jorquera, C.; Sánchez, P.; Beitia, M.; García-Cano, B.; Guadilla, J.; Delgado, D. Platelet-rich plasma injections delay the need for knee arthroplasty: A retrospective study and survival analysis. Int. Orthop. 2021, 45, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Lim, S.; Steen, R.G.; Dasa, V. Hyaluronic acid injections are associated with delay of total knee replacement surgery in patients with knee osteoarthritis: Evidence from a large US health claims database. PLoS ONE 2015, 10, e0145776. [Google Scholar] [CrossRef]
- Ong, K.L.; Anderson, A.F.; Niazi, F.; Fierlinger, A.L.; Kurtz, S.M.; Altman, R.D. Hyaluronic acid injections in medicare knee osteoarthritis patients are associated with longer time to knee arthroplasty. J. Arthroplast. 2016, 31, 1667–1673. [Google Scholar] [CrossRef]
- Bedard, N.A.; Dowdle, S.B.; Anthony, C.A.; DeMik, D.E.; McHugh, M.A.; Bozic, K.J.; Callaghan, J.J. The AAHKS Clinical Research Award: What Are the Costs of Knee Osteoarthritis in the Year Prior to Total Knee Arthroplasty? J. Arthroplast. 2017, 32, S8–S10.e1. [Google Scholar] [CrossRef]
| Variable | IA-PRP | IA-CS | IA-HA | p-Value * |
|---|---|---|---|---|
| Total N | 3240 | 1,382,572 | 164,000 | - |
| Subsequent TKA N (%) | 71 (2.2) | 81,271 (5.9) | 13,044 (8.0) | - |
| Age Mean ± SD | 63.0 ± 10.0 | 64.8 ± 11.5 | 64.8 ± 11.9 | 0.007932 |
| Age Category N (%) | ||||
| 18 to 19 | 18 (0.6) | 210 (0.02) | 67 (0.04) | <0.0001 |
| 20 to 24 | 40 (1.2) | 1199 (0.1) | 350 (0.2) | <0.0001 |
| 25 to 29 | 31 (1.0) | 2950 (0.2) | 611 (0.4) | <0.0001 |
| 30 to 34 | 54 (1.7) | 7989 (0.6) | 1381 (0.8) | <0.0001 |
| 35 to 39 | 116 (3.6) | 18,805(1.4) | 2829 (1.7) | <0.0001 |
| 40 to 44 | 174 (5.4) | 39,006 (2.8) | 4954(3.0) | <0.0001 |
| 45 to 49 | 275 (8.5) | 71,724 (5.2) | 8603 (5.2) | 1.0 |
| 50 to 54 | 376 (11.6) | 121,847 (8.8) | 13,595 (8.3) | <0.0001 |
| 55 to 59 | 505 (15.6) | 172,608 (12.5) | 18,254 (11.1) | <0.0001 |
| 60 to 64 | 535 (16.5) | 208,619 (15.1) | 21,615 (13.2) | <0.0001 |
| 65 to 69 | 415 (12.8) | 203,381 (14.7) | 26,138 (15.9) | <0.0001 |
| 70 to 74 | 344 (10.6) | 188,465 (13.6) | 24,692 (15.1) | <0.0001 |
| 75 to 79 | 256 (7.9) | 223,009 (16.1) | 28,323 (17.3) | <0.0001 |
| 80 to 84 | 101 (3.1) | 122,760 (8.9) | 12,588 (7.7) | <0.0001 |
| Male Gender N (%) | 1485 (45.8) | 500,034 (36.2) | 62,669 (38.2) | <0.0001 |
| Region N (%) | ||||
| Midwest | 674 (20.8) | 400,644 (30.0) | 42,907 (26.2) | <0.0001 |
| Northeast | 682 (21.0) | 294,311 (21.3) | 40,686 (24.8) | <0.0001 |
| South | 1508 (46.5) | 513,096 (37.1) | 57,523 (35.1) | <0.0001 |
| West | 363 (11.2) | 166,619 (12.1) | 22,065 (13.5) | <0.0001 |
| Unknown | 13 (0.4) | 7902 (0.6) | 819 (0.5) | <0.0001 |
| Service Location N (%) | ||||
| Clinic | 0 (0) | 12,959 (0.9) | 499 (0.3) | <0.0001 |
| Office | 1213 (37.4) | 1,213,283 (87.8) | 145,371 (88.6) | <0.0001 |
| Inpatient | 495 (15.3) | 165 (0.01) | 0 (0) | 0.0001 |
| Other | 0 (0) | 1281 (0.09) | 45 (0.03) | <0.0001 |
| Outpatient | 1519 (46.9) | 148,276 (10.7) | 17,024 (10.4) | <0.0001 |
| Comorbidities N (%) | ||||
| Alcohol Use? | 195 (6.0) | 85,494 (6.2) | 8202 (5.0) | <0.0001 |
| Cancer | 441 (13.6) | 251,909 (18.2) | 31,248 (19.1) | <0.0001 |
| Coronary Artery Disease | 725 (22.4) | 414,040 (29.9) | 48,522 (29.6) | 0.002596 |
| Chronic Kidney Disease | 383 (11.8) | 273,475 (19.8) | 29,931 (18.3) | <0.0001 |
| COPD | 812 (25.1) | 425,993 (30.8) | 48,574 (29.6) | <0.0001 |
| Congestive Heart Failure | 134 (4.1) | 98,897 (7.2) | 11,193 (6.8) | <0.0001 |
| Depression | 1220 (37.7) | 570,067 (41.2) | 60,753 (37.0) | <0.0001 |
| Diabetes | 1074 (33.1) | 596,587 (43.2) | 70,136 (42.8) | 0.002964 |
| Diabetes Complicated | 519 (16.0) | 330,672 (23.9) | 37,989 (23.2) | <0.0001 |
| Diabetes Uncomplicated | 880 (27.2) | 486,913 (35.2) | 57,285 (34.9) | 0.02107 |
| Hypertension | 2056 (63.5) | 1,097,128 (79.4) | 125,741 (76.7) | <0.0001 |
| Hypothyroidism | 898 (27.7) | 418,494 (30.3) | 50,314 (30.7) | 0.0006423 |
| Liver Disease | 544 (16.8) | 263,193 (19.0) | 29,546 (18.0) | <0.0001 |
| Obesity | 1488 (45.9) | 693,586 (51.2) | 76,982 (46.9) | <0.0001 |
| Renal Disease | 395 (12.2) | 280,691 (20.3) | 30,801 (18.8) | <0.0001 |
| Renal Failure | 395 (12.2) | 280,409 (20.3) | 30,766 (18.8) | <0.0001 |
| Rheumatoid Arthritis | 119 (3.7) | 80,601 (5.8) | 7651 (4.7) | <0.0001 |
| Tobacco Use | 1081 (33.4) | 524,869 (38.0) | 55,114 (33.6) | <0.0001 |
| Variable | IA-CS (4:1 Case Match) | IA-HA (4:1 Case Match) |
|---|---|---|
| Total N | 45,124 | 11,492 |
| Time to TKA Mean ± SD | 370.0 ± 348.9 | 377.8 ± 349.2 |
| Male Gender N (%) | 16,645 (36.9) | 4273 (37.2) |
| Age Mean ± SD | 68.0 ± 8.6 | 67.9 ± 8.7 |
| Comorbidities | ||
| Alcohol Abuse | 954 (2.1) | 312 (2.7) |
| Cancer | 8668 (19.2) | 2283 (19.9) |
| Coronary Artery Disease | 14,106 (31.3) | 3583 (31.2) |
| Chronic Kidney Disease | 8425 (18.7) | 2099 (18.3) |
| COPD | 13,246 (29.4) | 3407 (29.6) |
| Congestive Heart Failure | 2685 (6.0) | 674 (5.9) |
| Depression | 17,663 (39.1) | 4412 (38.4) |
| Diabetes | 19,226 (42.6) | 4917 (42.8) |
| Diabetes Complicated | 9829 (21.8) | 2506 (21.8) |
| Diabetes Uncomplicated | 15,316 (33.9) | 3949 (34.4) |
| Hypertension | 37,945 (84.1) | 9527 (82.9) |
| Hypothyroidism | 14,172 (31.4) | 3697 (32.2) |
| Liver Disease | 7425 (16.5) | 1922 (16.7) |
| Obesity | 24,241 (53.7) | 6171 (53.7) |
| Renal Disease | 9335 (20.7) | 2157 (18.8) |
| Renal Failure | 9337 (20.7) | 2155 (18.8) |
| Rheumatoid Arthritis | 2646 (5.9) | 579 (5.0) |
| Tobacco Use | 17,027 (37.7) | 4383 (38.1) |
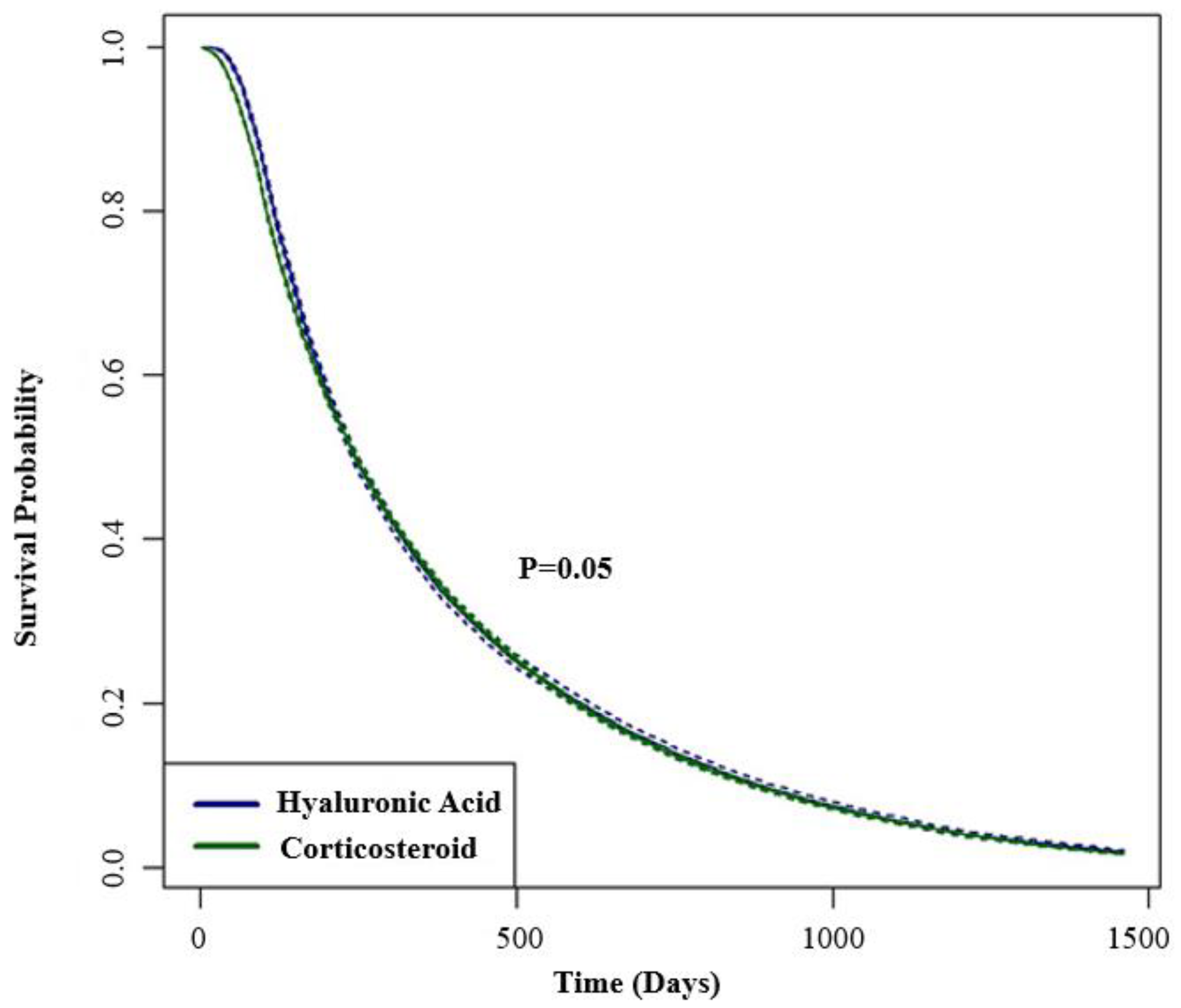
| IA-CS (95% CI) N = 45,124 | IA-HA (95% CI) N = 11,492 | |
|---|---|---|
| 6 months | 60.4 (59.9–60.8) | 61.6 (60.7–62.5) |
| 1 year | 35.7 (35.3–36.2) | 35.1 (34.2–35.9) |
| 2 year | 14.3 (14.0–14.7) | 14.8 (14.2–15.5) |
| 3 year | 5.6 (5.4–5.9) | 5.8 (5.4–6.3) |
| 4 year | 1.7 (1.6–1.8) | 1.9 (1.7–2.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gesheff, M.G.; Scalzitti, D.A.; Bains, S.S.; Dubin, J.; Delanois, R.E. Time to Total Knee Arthroplasty (TKA) Post Intra-Articular Injection. J. Clin. Med. 2024, 13, 3764. https://doi.org/10.3390/jcm13133764
Gesheff MG, Scalzitti DA, Bains SS, Dubin J, Delanois RE. Time to Total Knee Arthroplasty (TKA) Post Intra-Articular Injection. Journal of Clinical Medicine. 2024; 13(13):3764. https://doi.org/10.3390/jcm13133764
Chicago/Turabian StyleGesheff, Martin G., David A. Scalzitti, Sandeep S. Bains, Jeremy Dubin, and Ronald E. Delanois. 2024. "Time to Total Knee Arthroplasty (TKA) Post Intra-Articular Injection" Journal of Clinical Medicine 13, no. 13: 3764. https://doi.org/10.3390/jcm13133764
APA StyleGesheff, M. G., Scalzitti, D. A., Bains, S. S., Dubin, J., & Delanois, R. E. (2024). Time to Total Knee Arthroplasty (TKA) Post Intra-Articular Injection. Journal of Clinical Medicine, 13(13), 3764. https://doi.org/10.3390/jcm13133764







