A Neuronal Network-Based Score Predicting Survival in Patients Undergoing Aortic Valve Intervention: The ABC-AS Score
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection
2.4. Artificial Intelligence Model
2.5. Statistics
3. Results
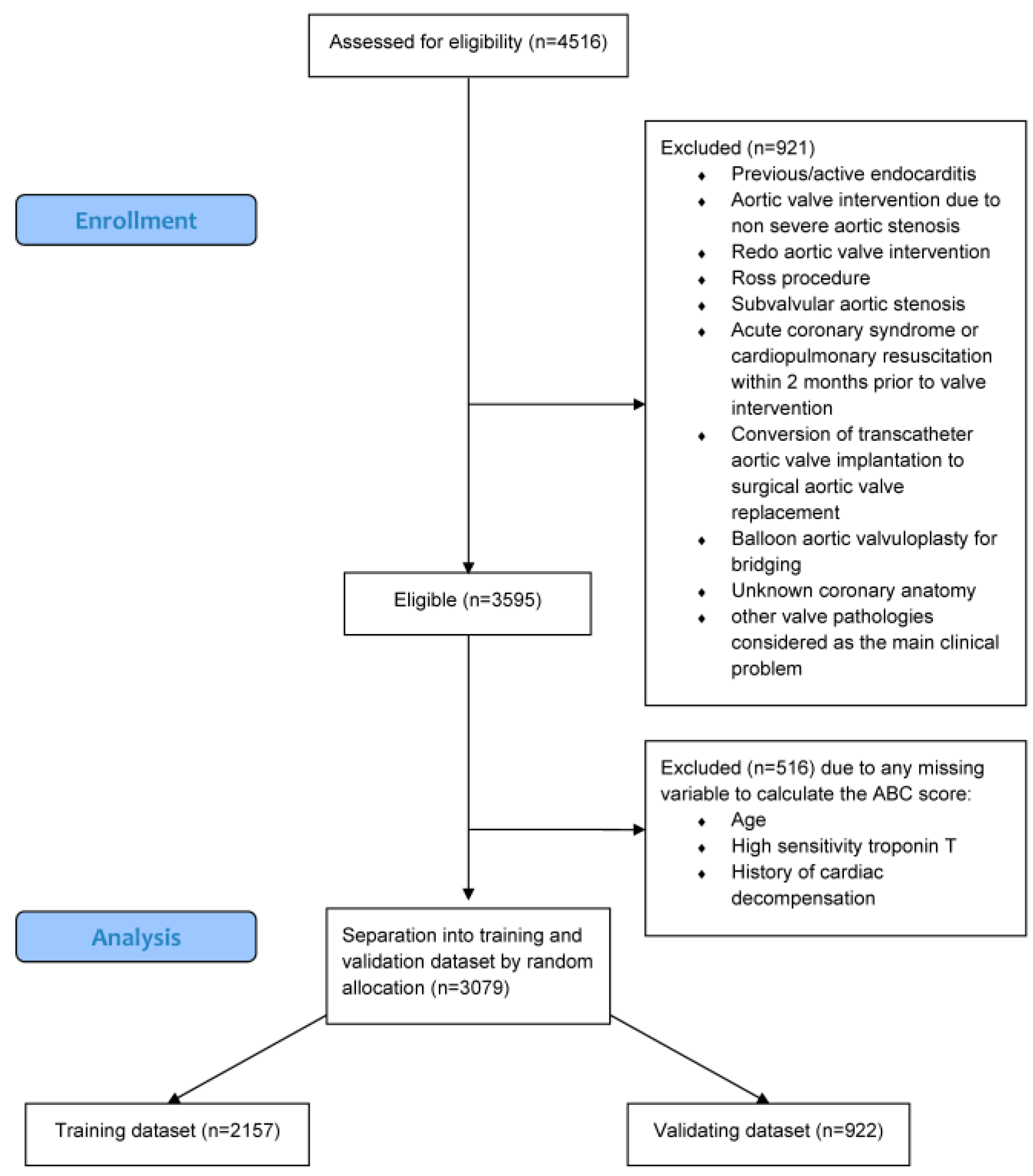
3.1. Study Population
3.2. Baseline Characteristics
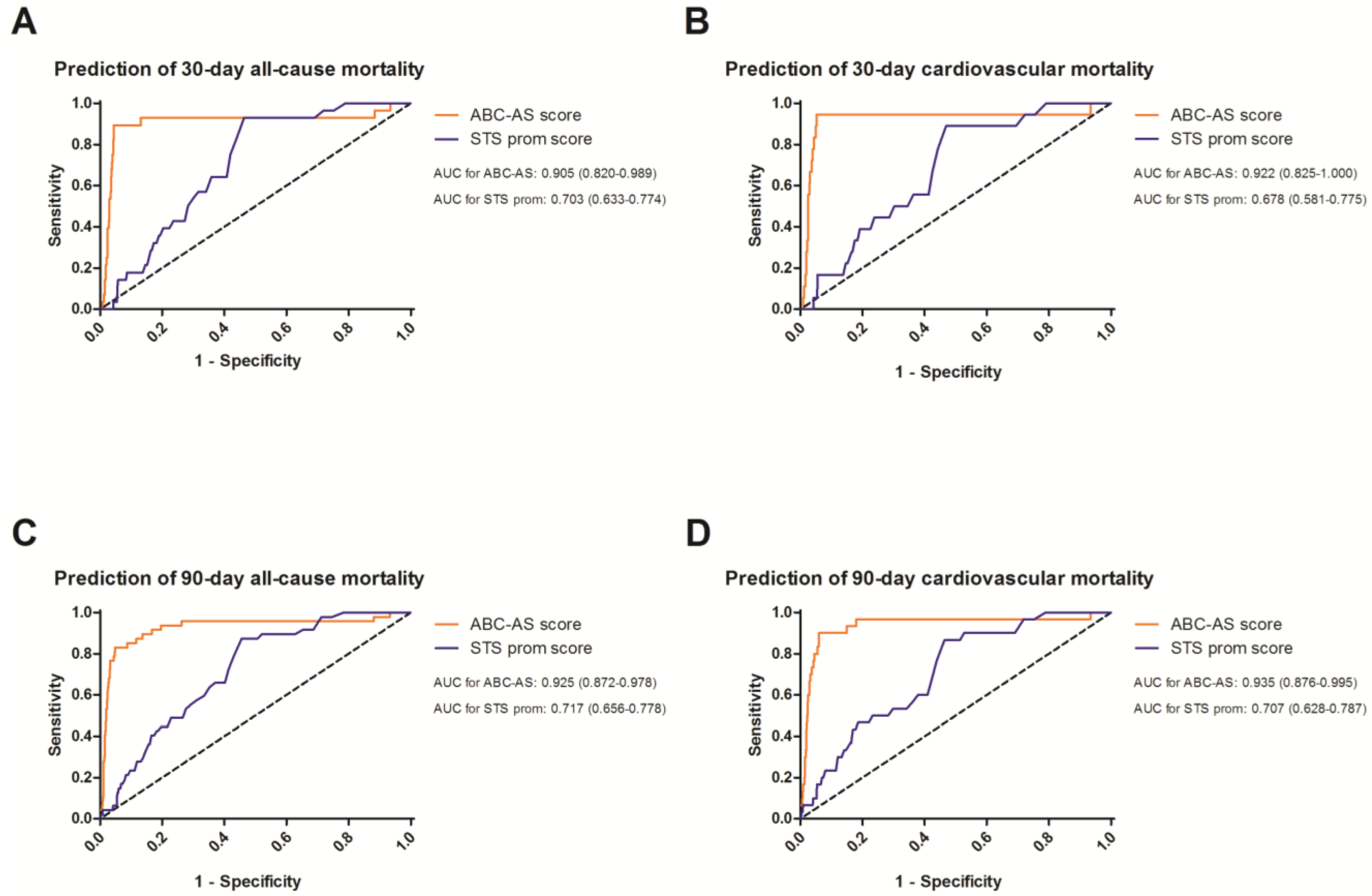
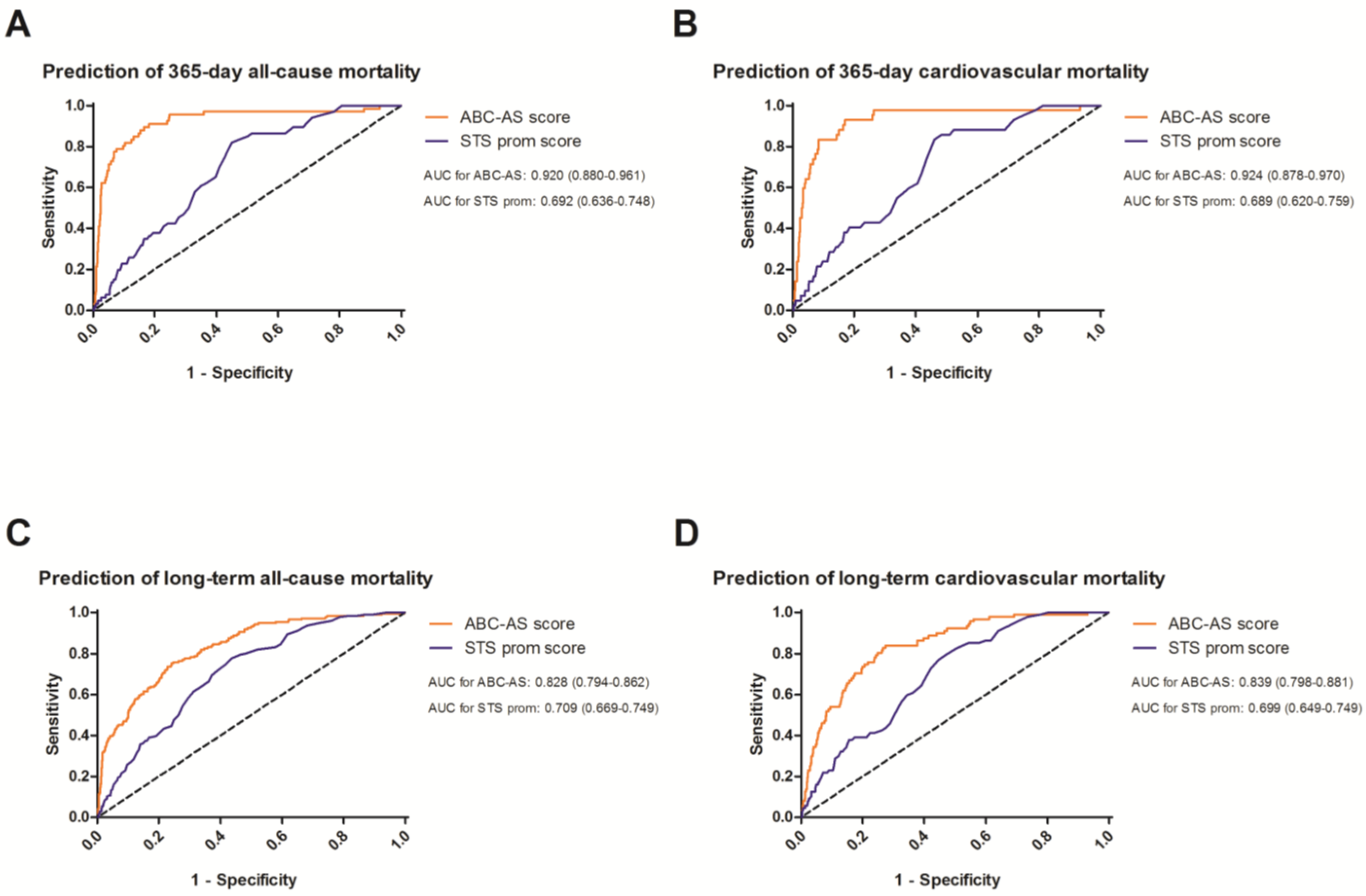
3.3. Receiver Operating Curves to Evaluate the Predictive Value
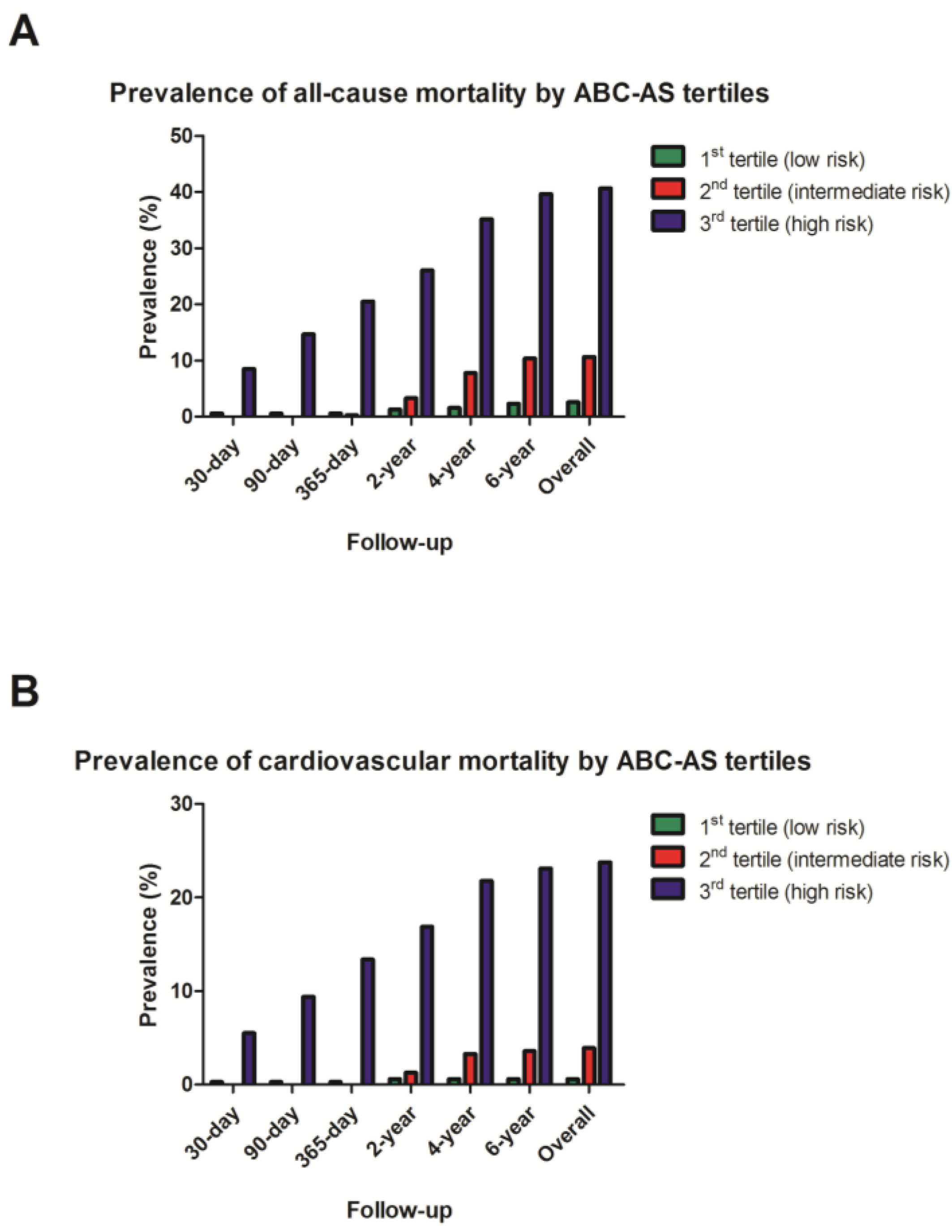
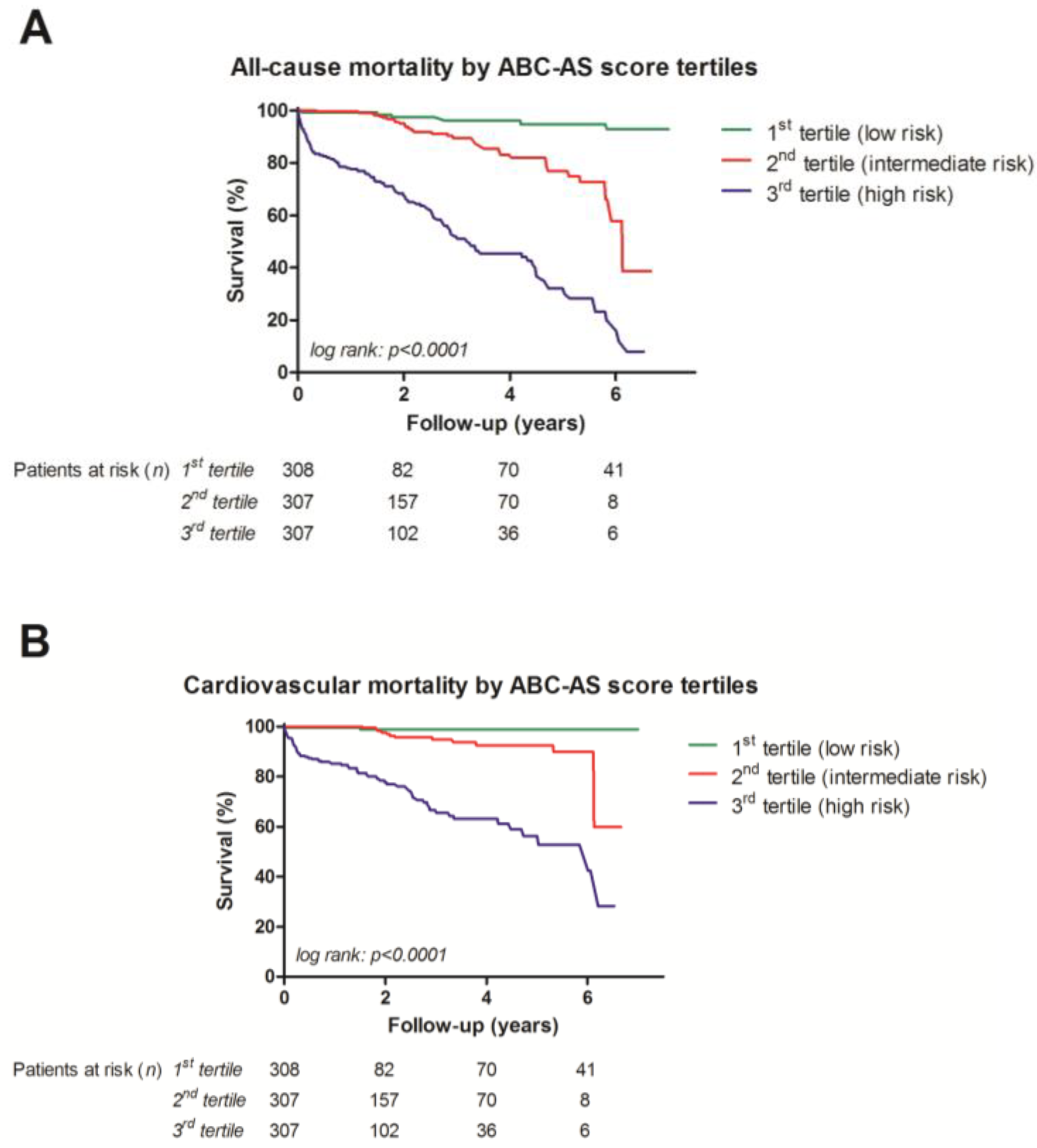
3.4. Univariate Analysis with Kaplan–Meier Curves
3.5. Multivariate Analysis for Direct Comparison
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef] [PubMed]
- Shahian, D.M.; Jacobs, J.P.; Badhwar, V.; Kurlansky, P.A.; Furnary, A.P.; Cleveland, J.C., Jr.; Lobdell, K.W.; Vassileva, C.; Wyler von Ballmoos, M.C.; Thourani, V.H.; et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 1-Background, Design Considerations, and Model Development. Ann. Thorac. Surg. 2018, 105, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.M.; Feng, L.; He, X.; Xian, Y.; Jacobs, J.P.; Badhwar, V.; Kurlansky, P.A.; Furnary, A.P.; Cleveland, J.C., Jr.; Lobdell, K.W.; et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-Statistical Methods and Results. Ann. Thorac. Surg. 2018, 105, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Puskas, J.D.; Kilgo, P.D.; Thourani, V.H.; Lattouf, O.M.; Chen, E.; Vega, J.D.; Cooper, W.; Guyton, R.A.; Halkos, M. The society of thoracic surgeons 30-day predicted risk of mortality score also predicts long-term survival. Ann. Thorac. Surg. 2012, 93, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Barili, F.; Pacini, D.; D’Ovidio, M.; Dang, N.C.; Alamanni, F.; Di Bartolomeo, R.; Grossi, C.; Davoli, M.; Fusco, D.; Parolari, A. The Impact of EuroSCORE II Risk Factors on Prediction of Long-Term Mortality. Ann. Thorac. Surg. 2016, 102, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Nozohoor, S.; Zindovic, I.; Nilsson, J.; Kimblad, P.O.; Sjögren, J. Prediction of 30-day mortality after transcatheter aortic valve implantation: A comparison of logistic EuroSCORE, STS score, and EuroSCORE II. J. Heart Valve Dis. 2014, 23, 567–574. [Google Scholar]
- Halkin, A.; Steinvil, A.; Witberg, G.; Barsheshet, A.; Barkagan, M.; Assali, A.; Segev, A.; Fefer, P.; Guetta, V.; Barbash, I.M.; et al. Mortality prediction following transcatheter aortic valve replacement: A quantitative comparison of risk scores derived from populations treated with either surgical or percutaneous aortic valve replacement. The Israeli TAVR Registry Risk Model Accuracy Assessment (IRRMA) study. Int. J. Cardiol. 2016, 215, 227–231. [Google Scholar]
- Wolff, G.; Shamekhi, J.; Al-Kassou, B.; Tabata, N.; Parco, C.; Klein, K.; Maier, O.; Sedaghat, A.; Polzin, A.; Sugiura, A.; et al. Risk modeling in transcatheter aortic valve replacement remains unsolved: An external validation study in 2946 German patients. Clin. Res. Cardiol. 2021, 110, 368–376. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Z.; Lindbäck, J.; Alexander, J.H.; Hanna, M.; Held, C.; Hylek, E.M.; Lopes, R.D.; Oldgren, J.; Siegbahn, A.; Stewart, R.A.; et al. The ABC (age, biomarkers, clinical history) stroke risk score: A biomarker-based risk score for predicting stroke in atrial fibrillation. Eur. Heart J. 2016, 37, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Caravaca, J.M.; Roldán, V.; Esteve-Pastor, M.A.; Valdés, M.; Vicente, V.; Lip, G.Y.H.; Marín, F. Long-Term Stroke Risk Prediction in Patients with Atrial Fibrillation: Comparison of the ABC-Stroke and CHA(2)DS(2)-VASc Scores. J. Am. Heart Assoc. 2017, 6, e006490. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Z.; Oldgren, J.; Lindbäck, J.; Alexander, J.H.; Connolly, S.J.; Eikelboom, J.W.; Ezekowitz, M.D.; Held, C.; Hylek, E.M.; Lopes, R.D.; et al. A biomarker-based risk score to predict death in patients with atrial fibrillation: The ABC (age, biomarkers, clinical history) death risk score. Eur. Heart J. 2018, 39, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Senoner, T.; Adukauskaite, A.; Dobner, S.; Holfeld, J.; Semsroth, S.; Lambert, T.; Zweiker, D.; Theurl, T.; Rainer, P.P.; et al. Long-Term Prognostic Value of High-Sensitivity Troponin T Added to N-Terminal Pro Brain Natriuretic Peptide Plasma Levels Before Valve Replacement for Severe Aortic Stenosis. Am. J. Cardiol. 2019, 124, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Senoner, T.; Adukauskaite, A.; Dobner, S.; Holfeld, J.; Semsroth, S.; Lambert, T.; Zweiker, D.; Theurl, T.; Rainer, P.; et al. Dataset on the prognostic value of cardiac biomarkers used in clinical routine in patients with severe aortic stenosis undergoing valve replacement. Data Brief 2020, 29, 105111. [Google Scholar] [CrossRef]
- Clavel, M.A.; Malouf, J.; Michelena, H.I.; Suri, R.M.; Jaffe, A.S.; Mahoney, D.W.; Enriquez-Sarano, M. B-type natriuretic peptide clinical activation in aortic stenosis: Impact on long-term survival. J. Am. Coll. Cardiol. 2014, 63, 2016–2025. [Google Scholar] [CrossRef]
- Saito, T.; Hojo, Y.; Hirose, M.; Ikemoto, T.; Katsuki, T.; Kario, K. High-sensitivity troponin T is a prognostic marker for patients with aortic stenosis after valve replacement surgery. J. Cardiol. 2013, 61, 342–347. [Google Scholar] [CrossRef]
- Chin, C.W.; Shah, A.S.; McAllister, D.A.; Cowell, J.; Alam, S.; Langrish, J.P.; Strachan, F.E.; Hunter, A.L.; Choy, M.A.; Lang, C.C.; et al. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur. Heart J. 2014, 35, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Stähli, B.E.; Gebhard, C.; Saleh, L.; Falk, V.; Landmesser, U.; Nietlispach, F.; Maisano, F.; Lüscher, T.F.; Maier, W.; Binder, R.K. N-terminal pro-B-type natriuretic peptide-ratio predicts mortality after transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2015, 85, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.; Lambert, T.; Senoner, T.; Dobner, S.; Hoppe, U.C.; Fellner, A.; Pfeifer, B.E.; Feuchtner, G.M.; Friedrich, G.; Semsroth, S.; et al. Impact of route of access and stenosis subtype on outcome after transcatheter aortic valve replacement. Front. Cardiovasc. Med. 2023, 10, 1256112. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, M.; Giallauria, F.; Carrizzo, A.; Visco, V.; Silverio, A.; Cesaro, A.; Calabrò, P.; De Luca, N.; Mancusi, C.; Masarone, D.; et al. Artificial intelligence in cardiovascular prevention: New ways will open new doors. J. Cardiovasc. Med. (Hagerstown) 2023, 24 (Suppl. S2), e106–e115. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef] [PubMed]
- de Hond, A.A.H.; Leeuwenberg, A.M.; Hooft, L.; Kant, I.M.J.; Nijman, S.W.J.; van Os, H.J.A.; Aardoom, J.J.; Debray, T.P.A.; Schuit, E.; van Smeden, M.; et al. Guidelines and quality criteria for artificial intelligence-based prediction models in healthcare: A scoping review. NPJ Digit. Med. 2022, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Kinnebrock, W. Neuronale Netze: Grundlagen, Anwendungen, Beispiele, 1st ed.; Oldenbourg Wissenschaftsverlag: Berlin, Germany; Boston, MA, USA, 1994. [Google Scholar]
- Kaffka, T. Neuronale Netze—Grundlagen: Mit Beispielprogrammen in Java, 1st ed.; mitp Verlag: Kaufering Germany, 2017. [Google Scholar]
- Lämmel, U.; Cleve, J. Künstliche Intelligenz: Wissensverarbeitung—Neuronale Netze, 5th ed.; Hanser Fachbuchverlag: Munich, Germany, 2020. [Google Scholar]
- Rey, G.D.; Wender, K.F. Neuronale Netze: Eine Einführung in die Grundlagen, Anwendungen und Datenauswertung, 3rd ed.; Hogrefe Verlag: Göttingen, Germany, 2018. [Google Scholar]
- Zell, A. Simulation Neuronaler Netze, 1st ed.; Oldenbourg Wissenschaftsverlag: Munich, Germany, 1997. [Google Scholar]
- Katus, H.A.; Remppis, A.; Scheffold, T.; Diederich, K.W.; Kuebler, W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am. J. Cardiol. 1991, 67, 1360–1367. [Google Scholar] [CrossRef]
- Solvik, T.A.; Nguyen, T.A.; Tony Lin, Y.H.; Marsh, T.; Huang, E.J.; Wiita, A.P.; Debnath, J.; Leidal, A.M. Secretory autophagy maintains proteostasis upon lysosome inhibition. J. Cell Biol. 2022, 221, e202110151. [Google Scholar] [CrossRef]
- Nicolás-Ávila, J.A.; Lechuga-Vieco, A.V.; Esteban-Martínez, L.; Sánchez-Díaz, M.; Díaz-García, E.; Santiago, D.J.; Rubio-Ponce, A.; Li, J.L.; Balachander, A.; Quintana, J.A.; et al. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 2020, 183, 94–109.e23. [Google Scholar] [CrossRef]
- Canty, J.M., Jr. Myocardial injury, troponin release, and cardiomyocyte death in brief ischemia, failure, and ventricular remodeling. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H1–H15. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.S.; Stein, E.J.; Biersmith, M.; Fearon, W.F.; Elmariah, S.; Kim, J.B.; Clark, D.E.; Patel, J.N.; Gonzales, H.; Baker, M.; et al. Global Longitudinal Strain and Biomarkers of Cardiac Damage and Stress as Predictors of Outcomes After Transcatheter Aortic Valve Implantation. J. Am. Heart Assoc. 2022, 11, e026529. [Google Scholar] [CrossRef] [PubMed]
- Muslimovic, A.; Fridén, V.; Tenstad, O.; Starnberg, K.; Nyström, S.; Wesén, E.; Esbjörner, E.K.; Granholm, K.; Lindahl, B.; Hammarsten, O. The Liver and Kidneys mediate clearance of cardiac troponin in the rat. Sci. Rep. 2020, 10, 6791. [Google Scholar] [CrossRef] [PubMed]
- Fridén, V.; Starnberg, K.; Muslimovic, A.; Ricksten, S.E.; Bjurman, C.; Forsgard, N.; Wickman, A.; Hammarsten, O. Clearance of cardiac troponin T with and without kidney function. Clin. Biochem. 2017, 50, 468–474. [Google Scholar] [CrossRef]
- Takashio, S.; Yamamuro, M.; Izumiya, Y.; Hirakawa, K.; Marume, K.; Yamamoto, M.; Ueda, M.; Yamashita, T.; Ishibashi-Ueda, H.; Yasuda, S.; et al. Diagnostic utility of cardiac troponin T level in patients with cardiac amyloidosis. ESC Heart Fail. 2018, 5, 27–35. [Google Scholar] [CrossRef]
- Ternacle, J.; Krapf, L.; Mohty, D.; Magne, J.; Nguyen, A.; Galat, A.; Gallet, R.; Teiger, E.; Côté, N.; Clavel, M.-A.; et al. Aortic Stenosis and Cardiac Amyloidosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2638–2651. [Google Scholar] [CrossRef]
| Variable | Training Cohort (n = 2157) | Validation Cohort (n = 922) | p-Value |
|---|---|---|---|
| Age (years) | 76 (70–82) | 77 (70–82) | n.s. |
| Gender (female), n (%) | 1036 (48.0%) | 436 (47.3%) | n.s. |
| Height (cm) | 168 (160–174) | 168 (162–174) | n.s. |
| Weight (kg) | 75 (65–85) | 75 (65–85) | n.s. |
| Body surface area (DuBois, m2) | 1.84 (1.69–1.98) | 1.84 (1.71–2.00) | n.s. |
| History of cardiac decompensation, n (%) | 319 (18.3) | 215 (23.3) | 0.002 |
| Stable heart failure symptoms, n (%) | 1681 (80.2) | 528 (57.8) | <0.001 |
| Stable angina pectoris, n (%) | 804 (38.4) | 305 (33.4) | 0.009 |
| History of syncope, n (%) | 204 (11.7) | 125 (13.7) | n.s. |
| Asymptomatic, n (%) | 60 (3.4) | 29 (3.1) | n.s. |
| Arterial hypertension, n (%) | 1693 (78.6) | 798 (86.6) | <0.001 |
| Diabetes mellitus, n (%) | 515 (23.9) | 218 (23.6) | n.s. |
| Hypercholesterolemia, n (%) | 939 (53.2) | 609 (66.1) | <0.001 |
| Nicotine | n.s. | ||
| Active smoker, n (%) | 127 (7.3) | 68 (8.4) | |
| Former smoker, n (%) | 274 (15.7) | 122 (15.1) | |
| History of stroke, n (%) | 196 (9.1) | 89 (9.7) | n.s. |
| Atrial fibrillation | n.s. | ||
| Paroxysmal, n (%) | 255 (11.8) | 99 (10.7) | |
| Persistent/permanent, n (%) | 351 (16.3) | 148 (16.1) | |
| Chronic obstructive pulmonary disease, n (%) | 304 (14.1) | 168 (18.3) | 0.003 |
| Carotid stenosis (≥50%), n (%) | 115 (5.3) | 88 (9.7) | <0.001 |
| Coronary artery disease | 0.001 | ||
| No significant coronary artery disease, n (%) | 1374 (63.7) | 535 (58.1) | |
| 1-vessel disease, n (%) | 343 (15.9) | 183 (19.9) | |
| 2-vessel disease, n (%) | 189 (8.8) | 94 (10.2) | |
| 3-vessel disease, n (%) | 173 (8.0) | 91 (9.9) | |
| Left main disease, n (%) | 78 (3.6) | 18 (2.0) | |
| Left ventricular ejection fraction | n.s. | ||
| >50%, n (%) | 1558 (75.3) | 701 (77.9) | |
| 35–50%, n (%) | 430 (20.8) | 171 (19.0) | |
| <35%, n (%) | 80 (3.9) | 28 (3.1) | |
| Aortic valve mean pressure gradient (mmHg) | 50 (41–60) | 46 (40–57) | <0.001 |
| Aortic valve area (cm2) | 0.70 (0.55–0.80) | 0.70 (0.55–0.80) | n.s. |
| Indexed aortic valve area (DuBois, cm2/m2) | 0.37 (0.30–0.44) | 0.36 (0.30–0.44) | n.s. |
| Stroke volume index (DuBois, mL/m2) | 29.0 (24.0–34.0) | 33.0 (27.0–40.0) | <0.001 |
| Total cholesterol (mg/dL) | 174 (142–207) | 174 (142–203) | n.s. |
| LDL cholesterol (mg/dL) | 102 (79–129) | 96 (71–124) | <0.001 |
| HDL cholesterol (mg/dL) | 54 (43–66) | 56 (45–70) | <0.001 |
| Triglycerides (mg/dL) | 99 (71–139) | 104 (80–141) | 0.001 |
| High-sensitivity troponin T (ng/L) | 18.0 (11.0–33.4) | 17.8 (10.7–30.0) | n.s. |
| N-terminal pro-brain natriuretic peptide (ng/L) | 1284 (512–3005) | 1345 (458–3443) | n.s. |
| Creatinine (mg/dL) | 1.00 (0.80–1.20) | 1.00 (0.85–1.20) | 0.004 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 81.0 (66.9–92.9) | 80.9 (62.5–91.9) | n.s. |
| STS Predicted Risk of Mortality (%) | 2.56 (1.61–4.01) | 2.54 (1.57–3.80) | n.s. |
| Medication | |||
| Betablocker, n (%) | 1064 (50.6) | 487 (53.2) | n.s. |
| Calcium channel blocker, n (%) | 349 (19.8) | 195 (21.4) | n.s. |
| ACE inhibitor/ARB/ARNI, n (%) | 1266 (60.2) | 564 (61.6) | n.s. |
| Acetyl salycilyc acid, n (%) | 1149 (57.1) | 580 (63.3) | 0.001 |
| P2Y12 antagonists, n (%) | 375 (17.8) | 90 (9.8) | <0.001 |
| Vitamin K antagonist, n (%) | 369 (17.5) | 130 (14.2) | 0.023 |
| Direct oral anticoagulants, n (%) | 119 (5.9) | 86 (9.4) | 0.001 |
| Hydrochlorothiazide, n (%) | 499 (28.4) | 234 (25.6) | n.s. |
| Loop diuretic, n (%) | 768 (38.1) | 390 (42.6) | 0.021 |
| Statin, n (%) | 1085 (53.9) | 552 (60.3) | 0.001 |
| Aldosterone antagonist, n (%) | 260 (12.4) | 93 (10.2) | n.s. |
| Insulin, n (%) | 77 (4.4) | 48 (5.3) | n.s. |
| Variable | 1st Tertile (n = 308) | 2nd Tertile (n = 307) | 3rd Tertile (n = 307) |
|---|---|---|---|
| Age (years) | 69 (61–75) | 78 (73–81) | 82 (77–85) |
| Gender (female), n (%) | 140 (45.5%) | 160 (52.1%) | 136 (44.3%) |
| Height (cm) | 168 (162–175) | 168 (162–173) | 168 (160–174) |
| Weight (kg) | 78 (68–88) | 75 (65–85) | 71 (63–83) |
| Body surface area (DuBois, m2) | 1.88 (1.76–2.03) | 1.85 (1.71–1.99) | 1.79 (1.68–1.96) |
| History of cardiac decompensation, n (%) | 20 (6.5) | 64 (20.8) | 131 (42.7) |
| Stable heart failure symptoms, n (%) | 198 (64.3) | 191 (62.2) | 139 (45.3) |
| Stable angina pectoris, n (%) | 127 (41.2) | 94 (30.6) | 84 (27.4) |
| History of syncope, n (%) | 39 (12.7) | 46 (15.0) | 40 (13.0) |
| Asymptomatic, n (%) | 21 (6.8) | 5 (1.6) | 3 (1.0) |
| Arterial hypertension, n (%) | 254 (82.5) | 270 (87.9) | 274 (89.3) |
| Diabetes mellitus, n (%) | 58 (18.8) | 80 (26.1) | 80 (26.1) |
| Hypercholesterolemia, n (%) | 224 (72.7) | 205 (66.8) | 180 (58.6) |
| Nicotine | |||
| Active smoker, n (%) | 24 (7.8) | 22 (7.2) | 22 (7.2) |
| Former smoker, n (%) | 63 (20.5) | 33 (10.7) | 26 (8.5) |
| History of stroke, n (%) | 22 (7.1) | 32 (10.4) | 35 (11.4) |
| Atrial fibrillation | |||
| Paroxysmal, n (%) | 24 (7.8) | 33 (10.7) | 42 (13.7) |
| Persistent/permanent, n (%) | 27 (8.7) | 46 (15.0) | 75 (24.5) |
| Chronic obstructive pulmonary disease, n (%) | 45 (14.6) | 60 (19.5) | 63 (20.5) |
| Carotid stenosis (≥50%), n (%) | 22 (7.2) | 28 (9.4) | 38 (12.6) |
| Coronary artery disease | |||
| No significant coronary artery disease, n (%) | 197 (64.0) | 194 (63.2) | 144 (46.9) |
| 1-vessel disease, n (%) | 53 (17.2) | 60 (19.5) | 70 (22.8) |
| 2-vessel disease, n (%) | 26 (8.4) | 28 (9.1) | 40 (13.0) |
| 3-vessel disease, n (%) | 27 (8.8) | 23 (7.5) | 41 (13.4) |
| Left main disease, n (%) | 5 (1.6) | 2 (0.7) | 11 (3.6) |
| Left ventricular ejection fraction | |||
| >50%, n (%) | 268 (87.0) | 233 (75.9) | 200 (65.1) |
| 35–50%, n (%) | 34 (11.0) | 61 (19.9) | 76 (24.8) |
| <35%, n (%) | 2 (0.6) | 7 (2.3) | 19 (6.2) |
| Aortic valve mean pressure gradient (mmHg) | 45 (40–57) | 47 (41–58) | 46 (40–58) |
| Aortic valve area (cm2) | 0.70 (0.60–0.88) | 0.70 (0.55–0.80) | 0.60 (0.50–0.80) |
| Indexed aortic valve area (DuBois, cm2/m2) | 0.38 (0.32–0.45) | 0.36 (0.30–0.43) | 0.35 (0.28–0.42) |
| Stroke volume index (DuBois, mL/m2) | 33.0 (27.0–40.0) | 35.0 (26.0–42.0) | 31.0 (25.0–38.3) |
| Total cholesterol (mg/dL) | 180 (148–213) | 171 (140–197) | 171 (135–198) |
| LDL cholesterol (mg/dL) | 108 (84–135) | 93 (69–118) | 88 (63–115) |
| HDL cholesterol (mg/dL) | 56 (48–69) | 56 (47–70) | 56 (43–70) |
| Triglycerides (mg/dL) | 106 (81–145) | 106 (78–144) | 101 (79–134) |
| High-sensitivity troponin T (ng/L) | 9.7 (6.0–14.9) | 19.0 (13.0–26.7) | 31.0 (20.0–53.0) |
| N-terminal pro-brain natriuretic peptide (ng/L) | 449 (215–1270) | 1517 (667–3239) | 2827 (1213–6015) |
| Creatinine (mg/dL) | 0.93 (0.80–1.05) | 1.00 (0.84–1.20) | 1.16 (0.96–1.51) |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 90.4 (81.9–101.9) | 78.3 (61.9–87.9) | 66.9 (48.7–82.1) |
| STS Predicted Risk of Mortality (%) | 1.50 (0.95–2.26) | 2.46 (1.79–3.51) | 3.70 (2.84–5.30) |
| Medication | |||
| Betablocker, n (%) | 147 (47.7) | 167 (54.4) | 173 (56.4) |
| Calcium channel blocker, n (%) | 63 (20.5) | 68 (22.1) | 64 (20.8) |
| ACE inhibitor/ARB/ARNI, n (%) | 187 (60.7) | 190 (61.9) | 187 (60.9) |
| Acetyl salycilyc acid, n (%) | 195 (63.3) | 197 (64.2) | 188 (61.2) |
| P2Y12 antagonists, n (%) | 16 (5.2) | 29 (9.4) | 45 (14.7) |
| Vitamin K antagonist, n (%) | 28 (9.1) | 41(13.4) | 61 (19.9) |
| Direct oral anticoagulants, n (%) | 15 (4.9) | 31 (10.1) | 40 (13.0) |
| Hydrochlorothiazide, n (%) | 79 (25.6) | 84 (27.4) | 71 (23.1) |
| Loop diuretic, n (%) | 57 (18.5) | 140 (45.6) | 193 (62.9) |
| Statin, n (%) | 194 (63.0) | 192 (62.5) | 166 (54.1) |
| Aldosterone antagonist, n (%) | 14 (4.5) | 33 (10.7) | 46 (15.0) |
| Insulin, n (%) | 11 (3.6) | 17 (5.5) | 20 (6.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbieri, F.; Pfeifer, B.E.; Senoner, T.; Dobner, S.; Spitaler, P.; Semsroth, S.; Lambert, T.; Zweiker, D.; Neururer, S.B.; Scherr, D.; et al. A Neuronal Network-Based Score Predicting Survival in Patients Undergoing Aortic Valve Intervention: The ABC-AS Score. J. Clin. Med. 2024, 13, 3691. https://doi.org/10.3390/jcm13133691
Barbieri F, Pfeifer BE, Senoner T, Dobner S, Spitaler P, Semsroth S, Lambert T, Zweiker D, Neururer SB, Scherr D, et al. A Neuronal Network-Based Score Predicting Survival in Patients Undergoing Aortic Valve Intervention: The ABC-AS Score. Journal of Clinical Medicine. 2024; 13(13):3691. https://doi.org/10.3390/jcm13133691
Chicago/Turabian StyleBarbieri, Fabian, Bernhard Erich Pfeifer, Thomas Senoner, Stephan Dobner, Philipp Spitaler, Severin Semsroth, Thomas Lambert, David Zweiker, Sabrina Barbara Neururer, Daniel Scherr, and et al. 2024. "A Neuronal Network-Based Score Predicting Survival in Patients Undergoing Aortic Valve Intervention: The ABC-AS Score" Journal of Clinical Medicine 13, no. 13: 3691. https://doi.org/10.3390/jcm13133691
APA StyleBarbieri, F., Pfeifer, B. E., Senoner, T., Dobner, S., Spitaler, P., Semsroth, S., Lambert, T., Zweiker, D., Neururer, S. B., Scherr, D., Schmidt, A., Feuchtner, G. M., Hoppe, U. C., Adukauskaite, A., Reinthaler, M., Landmesser, U., Müller, S., Steinwender, C., & Dichtl, W. (2024). A Neuronal Network-Based Score Predicting Survival in Patients Undergoing Aortic Valve Intervention: The ABC-AS Score. Journal of Clinical Medicine, 13(13), 3691. https://doi.org/10.3390/jcm13133691








