Bone Density Changes at the Origin of the Deltoid Muscle following Reverse Shoulder Arthroplasty
Abstract
1. Introduction
2. Materials and Methods
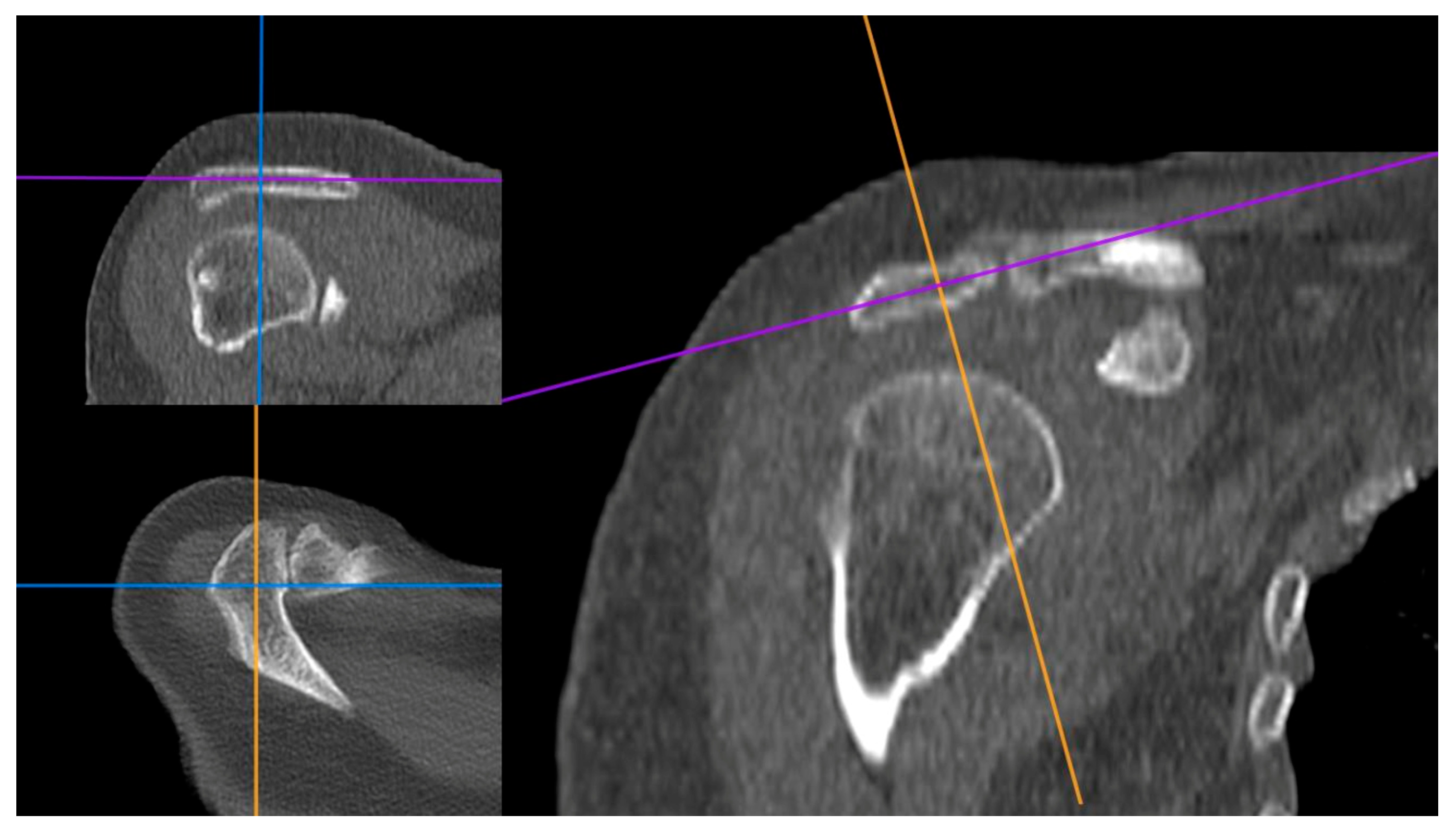
2.1. Image Processing
2.2. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grammont, P.M.; Baulot, E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics 1993, 16, 65–68. [Google Scholar] [CrossRef]
- Avin, K.G.; Bloomfield, S.A.; Gross, T.S.; Warden, S.J. Biomechanical aspects of the muscle-bone interaction. Curr. Osteoporos. Rep. 2015, 13, 1–8. [Google Scholar] [CrossRef]
- Berhouet, J.; Kontaxis, A.; Gulotta, L.V.; Craig, E.; Warren, R.; Dines, J.; Dines, D. Effects of the humeral tray component positioning for onlay reverse shoulder arthroplasty design: A biomechanical analysis. J. Shoulder Elbow Surg. 2015, 24, 569–577. [Google Scholar] [CrossRef]
- Giles, J.W.; Langohr, G.D.; Johnson, J.A.; Athwal, G.S. Implant Design Variations in Reverse Total Shoulder Arthroplasty Influence the Required Deltoid Force and Resultant Joint Load. Clin. Orthop. Relat. Res. 2015, 473, 3615–3626. [Google Scholar] [CrossRef]
- Costantini, O.; Choi, D.S.; Kontaxis, A.; Gulotta, L.V. The effects of progressive lateralization of the joint center of rotation of reverse total shoulder implants. J. Shoulder Elbow Surg. 2015, 24, 1120–1128. [Google Scholar] [CrossRef]
- Fischer, C.; Krammer, D.; Hug, A.; Weber, M.A.; Kauczor, H.U.; Krix, M.; Bruckner, T.; Kunz, P.; Schmidmaier, G.; Zeifang, F. Dynamic contrast-enhanced ultrasound and elastography assess deltoid muscle integrity after reverse shoulder arthroplasty. J. Shoulder Elbow Surg. 2017, 26, 108–117. [Google Scholar] [CrossRef]
- Fischer, C.; Flammer, S.; Kauczor, H.U.; Zeifang, F.; Schmidmaier, G.; Kunz, P. Preoperative deltoid assessment by contrast-enhanced ultrasound (CEUS) as predictor for shoulder function after reverse shoulder arthroplasty: A prospective pilot study. Arch. Orthop. Trauma Surg. 2020, 140, 1001–1012. [Google Scholar] [CrossRef]
- Greiner, S.H.; Back, D.A.; Herrmann, S.; Perka, C.; Asbach, P. Degenerative changes of the deltoid muscle have impact on clinical outcome after reversed total shoulder arthroplasty. Arch. Orthop. Trauma Surg. 2010, 130, 177–183. [Google Scholar] [CrossRef]
- Favard, L.; Levigne, C.; Nerot, C.; Gerber, C.; De Wilde, L.; Mole, D. Reverse prostheses in arthropathies with cuff tear: Are survivorship and function maintained over time? Clin. Orthop. Relat. Res. 2011, 469, 2469–2475. [Google Scholar] [CrossRef]
- Gerber, C.; Canonica, S.; Catanzaro, S.; Ernstbrunner, L. Longitudinal observational study of reverse total shoulder arthroplasty for irreparable rotator cuff dysfunction: Results after 15 years. J. Shoulder Elbow Surg. 2018, 27, 831–838. [Google Scholar] [CrossRef]
- Guery, J.; Favard, L.; Sirveaux, F.; Oudet, D.; Mole, D.; Walch, G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J. Bone Jt. Surg. Am. 2006, 88, 1742–1747. [Google Scholar] [CrossRef]
- Mallett, K.; Nguyen, N.T.V.; Giambini, H.; Werthel, J.-D.; Sanchez-Sotelo, J. Changes in deltoid muscle tension after reverse shoulder arthroplasty as quantified by shear wave elastography: Relationship with radiographic parameters and functional outcomes. Semin. Arthroplast. JSES 2021, 31, 751–758. [Google Scholar] [CrossRef]
- Koch, M.; Schmidt, C.; Kerschbaum, M.; Winkler, T.; Pfeifer, C.G.; Greiner, S. Reversed shoulder arthroplasty leads to significant histological changes of the deltoid muscle: A prospective intervention trial. Arch. Orthop. Trauma Surg. 2021, 141, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.H.; Vendries, V.; Bryant, T.J.; Rainbow, M.J.; Ploeg, H.L.; Bicknell, R.T. Trabecular bone density distribution in the scapula of patients undergoing reverse shoulder arthroplasty. JSES Int. 2022, 6, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Hayden, A.; Cotter, E.J.; Hennick, T.; Hetzel, S.; Wollaeger, J.; Anderson, S.; Grogan, B.F. Bone quality in total shoulder arthroplasty: A prospective study correlating computed tomography Hounsfield units with thumb test and fracture risk assessment tool score. JSES Int. 2023, 7, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Yeazell, S.T.; Inacio, J.; Malige, A.; Dailey, H.; Carolan, G.F. Bone density and its relation to the development of acromial stress fracture following reverse total shoulder arthroplasty. Shoulder Elbow 2022, 14, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Soma, D.; Ichiseki, T.; Ueda, S.; Sakurai, M.; Kawahara, N. Radiographic Evaluation and Changes in Bone Density of the Humeral Side after Reverse Total Shoulder Arthroplasty. J. Clin. Med. 2023, 12, 7698. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, L.; Yang, C.; Wei, L.; Li, T.; Zhu, L.; Hu, J. Hounsfield units predicts the occurrence but not the patterns of proximal humerus fracture in the elderly patients. BMC Musculoskelet. Disord. 2023, 24, 342. [Google Scholar] [CrossRef] [PubMed]
- Werthel, J.D.; Walch, G.; Vegehan, E.; Deransart, P.; Sanchez-Sotelo, J.; Valenti, P. Lateralization in reverse shoulder arthroplasty: A descriptive analysis of different implants in current practice. Int. Orthop. 2019, 43, 2349–2360. [Google Scholar] [CrossRef]
- Razi, T.; Niknami, M.; Alavi Ghazani, F. Relationship between Hounsfield Unit in CT Scan and Gray Scale in CBCT. J. Dent. Res. Dent. Clin. Dent. Prospects 2014, 8, 107–110. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; del Rio, A.M.; Bruce, R.J.; Binkley, N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar] [CrossRef]
- Herrmann, M.; Engelke, K.; Ebert, R.; Muller-Deubert, S.; Rudert, M.; Ziouti, F.; Jundt, F.; Felsenberg, D.; Jakob, F. Interactions between Muscle and Bone—Where Physics Meets Biology. Biomolecules 2020, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Agostini, D.; Zeppa Donati, S.; Lucertini, F.; Annibalini, G.; Gervasi, M.; Ferri Marini, C.; Piccoli, G.; Stocchi, V.; Barbieri, E.; Sestili, P. Muscle and Bone Health in Postmenopausal Women: Role of Protein and Vitamin D Supplementation Combined with Exercise Training. Nutrients 2018, 10, 1103. [Google Scholar] [CrossRef]
- Levin, J.M.; Rodriguez, K.; Polascik, B.A.; Zeng, S.; Warren, E., Jr.; Rechenmacher, A.; Helmkamp, J.; Goltz, D.E.; Wickman, J.; Klifto, C.S.; et al. Simple preoperative radiographic and computed tomography measurements predict adequate bone quality for stemless total shoulder arthroplasty. J. Shoulder Elbow Surg. 2022, 31, 2481–2487. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.C.; Chi, D.; Parsons, B.O.; Cagle, P.J., Jr. Acromial spine fracture after reverse total shoulder arthroplasty: A systematic review. J. Shoulder Elbow Surg. 2019, 28, 792–801. [Google Scholar] [CrossRef]
- Moser, T.; Lecours, J.; Michaud, J.; Bureau, N.J.; Guillin, R.; Cardinal, E. The deltoid, a forgotten muscle of the shoulder. Skelet. Radiol. 2013, 42, 1361–1375. [Google Scholar] [CrossRef]
- Fenwick, A.; Reichel, T.; Eden, L.; Schmalzl, J.; Meffert, R.; Plumhoff, P.; Gilbert, F. Deltoid Muscle Tension Alterations Post Reverse Shoulder Arthroplasty: An Investigation Using Shear Wave Elastography. J. Clin. Med. 2023, 12, 6184. [Google Scholar] [CrossRef]
- Bacle, G.; Nove-Josserand, L.; Garaud, P.; Walch, G. Long-Term Outcomes of Reverse Total Shoulder Arthroplasty: A Follow-up of a Previous Study. J. Bone Jt. Surg. Am. 2017, 99, 454–461. [Google Scholar] [CrossRef]
- Walker, D.R.; Struk, A.M.; Matsuki, K.; Wright, T.W.; Banks, S.A. How do deltoid muscle moment arms change after reverse total shoulder arthroplasty? J. Shoulder Elbow Surg. 2016, 25, 581–588. [Google Scholar] [CrossRef]



| ACROMION | CLAVICLE | SCAPULAR SPINE | ||||
|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | PRE | POST | |
| Number of values | 44 | 44 | 44 | 44 | 44 | 44 |
| Mean | 155.9 | 179.9 | 117.3 | 95.94 | 347 | 385.2 |
| Std. Deviation | 122.9 | 105.5 | 72.34 | 62.76 | 163.8 | 161.2 |
| Lower 95% CI of mean | 118.5 | 147.8 | 95.34 | 76.86 | 297.2 | 334.9 |
| Upper 95% CI of mean | 193.3 | 212 | 139.3 | 115 | 396.8 | 435.4 |
| Coefficient of variation | 78.85% | 58.66% | 61.65% | 65.41% | 47.21% | 41.84% |
| Wilcoxon matched pairs signed rank test | ||||||
| Sum of signed ranks (W) | 522 | −444 | 587 | |||
| p value | 0.0019 (*) | 0.0088 (*) | 0.0001 (*) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldaria, A.; Giovannetti de Sanctis, E.; Saccone, L.; Baldari, A.; Azzolina, D.; La Verde, L.; Palumbo, A.; Franceschi, F. Bone Density Changes at the Origin of the Deltoid Muscle following Reverse Shoulder Arthroplasty. J. Clin. Med. 2024, 13, 3695. https://doi.org/10.3390/jcm13133695
Caldaria A, Giovannetti de Sanctis E, Saccone L, Baldari A, Azzolina D, La Verde L, Palumbo A, Franceschi F. Bone Density Changes at the Origin of the Deltoid Muscle following Reverse Shoulder Arthroplasty. Journal of Clinical Medicine. 2024; 13(13):3695. https://doi.org/10.3390/jcm13133695
Chicago/Turabian StyleCaldaria, Antonio, Edoardo Giovannetti de Sanctis, Luca Saccone, Angelo Baldari, Danila Azzolina, Luca La Verde, Alessio Palumbo, and Francesco Franceschi. 2024. "Bone Density Changes at the Origin of the Deltoid Muscle following Reverse Shoulder Arthroplasty" Journal of Clinical Medicine 13, no. 13: 3695. https://doi.org/10.3390/jcm13133695
APA StyleCaldaria, A., Giovannetti de Sanctis, E., Saccone, L., Baldari, A., Azzolina, D., La Verde, L., Palumbo, A., & Franceschi, F. (2024). Bone Density Changes at the Origin of the Deltoid Muscle following Reverse Shoulder Arthroplasty. Journal of Clinical Medicine, 13(13), 3695. https://doi.org/10.3390/jcm13133695







