Prognostic Impact of Left Ventricular Ejection Fraction Improvement after Transcatheter Aortic Valve Replacement
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Cohort
2.2. Data Collection and Clinical Endpoints
2.3. Statistical Analysis
3. Results
3.1. Patient Cohort and Baseline Characteristics
LVEF Improvement
3.2. Outcomes after TAVR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Aortic stenosis |
| CAD | Coronary artery disease |
| CABG | Coronary arterial bypass graft |
| CCS-Score | Canadian Cardiovascular Society-Score |
| CKD | Chronic kidney disease |
| CVD | Cerebrovascular disease |
| eGFR | Estimated glomerular filtration rate |
| ESC | European Society of Cardiology |
| EuroSCORE | European System for Cardiac Operative Risk Evaluation |
| ICD | Implanted cardioverter defibrillator |
| LVEF | Left ventricular ejection fraction |
| LVEDD | Left ventricular end-diastolic diameter |
| LVESD | Left ventricular end-systolic diameter |
| NYHA | New York Heart Association |
| PCI | Percutaneous coronary intervention |
| STS | Society of Thoracic Surgeons |
| SAVR | Surgical aortic valve replacement |
| TAVR | Transcatheter aortic valve replacement |
| TTE | Transthoracic echocardiography |
| R2 | Coefficient of determination indicating correlation, where a value ≤ 0.35 indicates low or weak correlation |
References
- Osnabrugge, R.L.J.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.J.C.; Piazza, N.; Kappetein, A.P. Aortic Stenosis in the Elderly. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Sharma, R.P.; Cubeddu, R.J.; Aaron, L.; Abdelfattah, O.M.; Koulogiannis, K.P.; Marcoff, L.; Naguib, M.; Kapadia, S.R.; Makkar, R.R.; et al. The Mortality Burden of Untreated Aortic Stenosis. J. Am. Coll. Cardiol. 2023, 82, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Martine, G.; Hélène, E.; Bernard, I.; Patrick, D.-G.; Karine, C.; Jean, F.; Pascal, L.; Alain, L.; Michel, L.; Alain, P.; et al. Registry of Transcatheter Aortic-Valve Implantation in High-Risk Patients. New Engl. J. Med. 2012, 366, 1705–1715. [Google Scholar]
- Moat, N.E.; Ludman, P.; De Belder, M.A.; Bridgewater, B.; Cunningham, A.D.; Young, C.P.; Thomas, M.; Kovac, J.; Spyt, T.; MacCarthy, P.A.; et al. Long-Term Outcomes after Transcatheter Aortic Valve Implantation in High-Risk Patients with Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2011, 58, 2130–2138. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev. Esp. Cardiol. (Engl. Ed.) 2022, 75, 524. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.H.; Tzolos, E.; Dweck, M.R. Pathophysiology of aortic stenosis and future perspectives for medical therapy. Cardiol. Clin. 2020, 38, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Vakili, B.A.; Okin, P.M.; Devereux, R.B. Prognostic implications of left ventricular hypertrophy. Am. Heart J. 2001, 141, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Petrov, G.; Dworatzek, E.; Schulze, T.M.; Dandel, M.; Kararigas, G.; Mahmoodzadeh, S.; Knosalla, C.; Hetzer, R.; Regitz-Zagrosek, V. Maladaptive Remodeling Is Associated with Impaired Survival in Women but Not in Men after Aortic Valve Replacement. JACC Cardiovasc. Imaging 2014, 7, 1073–1080. [Google Scholar] [CrossRef]
- Elmariah, S.; Palacios, I.F.; McAndrew, T.; Hueter, I.; Inglessis, I.; Baker, J.N.; Kodali, S.; Leon, M.B.; Svensson, L.; Pibarot, P.; et al. Outcomes of Transcatheter and Surgical Aortic Valve Replacement in High-Risk Patients with Aortic Stenosis and Left Ventricular Dysfunction: Results from the Placement of Aortic Transcatheter Valves (PARTNER) Trial (Cohort A). Circ. Cardiovasc. Interv. 2013, 6, 604–614. [Google Scholar] [CrossRef]
- Attinger-Toller, A.; Ferrari, E.; Tueller, D.; Templin, C.; Muller, O.; Nietlispach, F.; Toggweiler, S.; Noble, S.; Roffi, M.; Jeger, R.; et al. Age-Related Outcomes after Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2021, 14, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Wagener, M.; Reuthebuch, O.; Heg, D.; Tüller, D.; Ferrari, E.; Grünenfelder, J.; Huber, C.; Moarof, I.; Muller, O.; Nietlispach, F.; et al. Clinical Outcomes in High-Gradient, Classical Low-Flow, Low-Gradient, and Paradoxical Low-Flow, Low-Gradient Aortic Stenosis after Transcatheter Aortic Valve Implantation: A Report from the SwissTAVI Registry. JAHA 2023, 12, e029489. [Google Scholar] [CrossRef]
- Okuno, T.; Alaour, B.; Heg, D.; Tueller, D.; Pilgrim, T.; Muller, O.; Noble, S.; Jeger, R.; Reuthebuch, O.; Toggweiler, S.; et al. Long-Term Risk of Stroke after Transcatheter Aortic Valve Replacement: Insights from the SwissTAVI Registry. JACC Cardiovasc. Interv. 2023, 16, 2986–2996. [Google Scholar] [CrossRef] [PubMed]
- Kewcharoen, J.; Trongtorsak, A.; Thangjui, S.; Kanitsoraphan, C.; Prasitlumkum, N. Female Gender Is Associated with an Increased Left Ventricular Ejection Fraction Recovery in Patients with Heart Failure with Reduced Ejection Fraction. Med. Sci. 2022, 10, 21. [Google Scholar] [CrossRef]
- Tastet, L.; Kwiecinski, J.; Pibarot, P.; Capoulade, R.; Everett, R.J.; Newby, D.E.; Shen, M.; Guzzetti, E.; Arsenault, M.; Bédard, É.; et al. Sex-Related Differences in the Extent of Myocardial Fibrosis in Patients with Aortic Valve Stenosis. JACC Cardiovasc. Imaging 2020, 13, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Leu, H.; Chang, H.; Chen, I.; Chen, P.; Lin, S.; Chen, Y. Women had favourable reverse left ventricle remodelling after TAVR. Eur. J. Clin. Investig. 2020, 50, e13183. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Naqvi, S.Y.; Giri, J.; Goldberg, S. Aortic Stenosis: Pathophysiology, Diagnosis, and Therapy. Am. J. Med. 2017, 130, 253–263. [Google Scholar] [CrossRef]
- Granero, V.C.L.; Santos, S.F.; Fernández-Golfín, C.; Martín, M.P.; Faletra, F.F.; Swaans, M.J.; López-Fernández, T.; Mesa, D.; Monzonís, A.M.; Gómez, J.L.Z. Immediate improvement of left ventricular mechanics following transcatheter aortic valve replacement. Cardiol. J. 2018, 25, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Tang, G.H.; Sangiorgi, G.; Pedicino, D.; Enriquez-Sarano, M.; Maisano, F.; Taramasso, M. Lifetime Management of Aortic Stenosis: Transcatheter Versus Surgical Treatment for Young and Low-Risk Patients. Circ. Cardiovasc. Interv. 2022, 15, 915–927. [Google Scholar] [CrossRef]
- Kolte, D.; Bhardwaj, B.; Lu, M.; Alu, M.C.; Passeri, J.J.; Inglessis, I.; Vlahakes, G.J.; Garcia, S.; Cohen, D.J.; Lindman, B.R.; et al. Association Between Early Left Ventricular Ejection Fraction Improvement After Transcatheter Aortic Valve Replacement and 5-Year Clinical Outcomes. JAMA Cardiol. 2022, 7, 934. [Google Scholar] [CrossRef]
- Kuneman, J.H.; Butcher, S.C.; Singh, G.K.; Wang, X.; Hirasawa, K.; Van Der Kley, F.; Leon, M.B.; Knuuti, J.; Pibarot, P.; Ajmone Marsan, N.; et al. Prognostic Implications of Change in Left Ventricular Ejection Fraction after Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2022, 177, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Dauerman, H.L.; Reardon, M.J.; Popma, J.J.; Little, S.H.; Cavalcante, J.L.; Adams, D.H.; Kleiman, N.S.; Oh, J.K. Early Recovery of Left Ventricular Systolic Function After CoreValve Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2016, 9, e003425. [Google Scholar] [CrossRef] [PubMed]
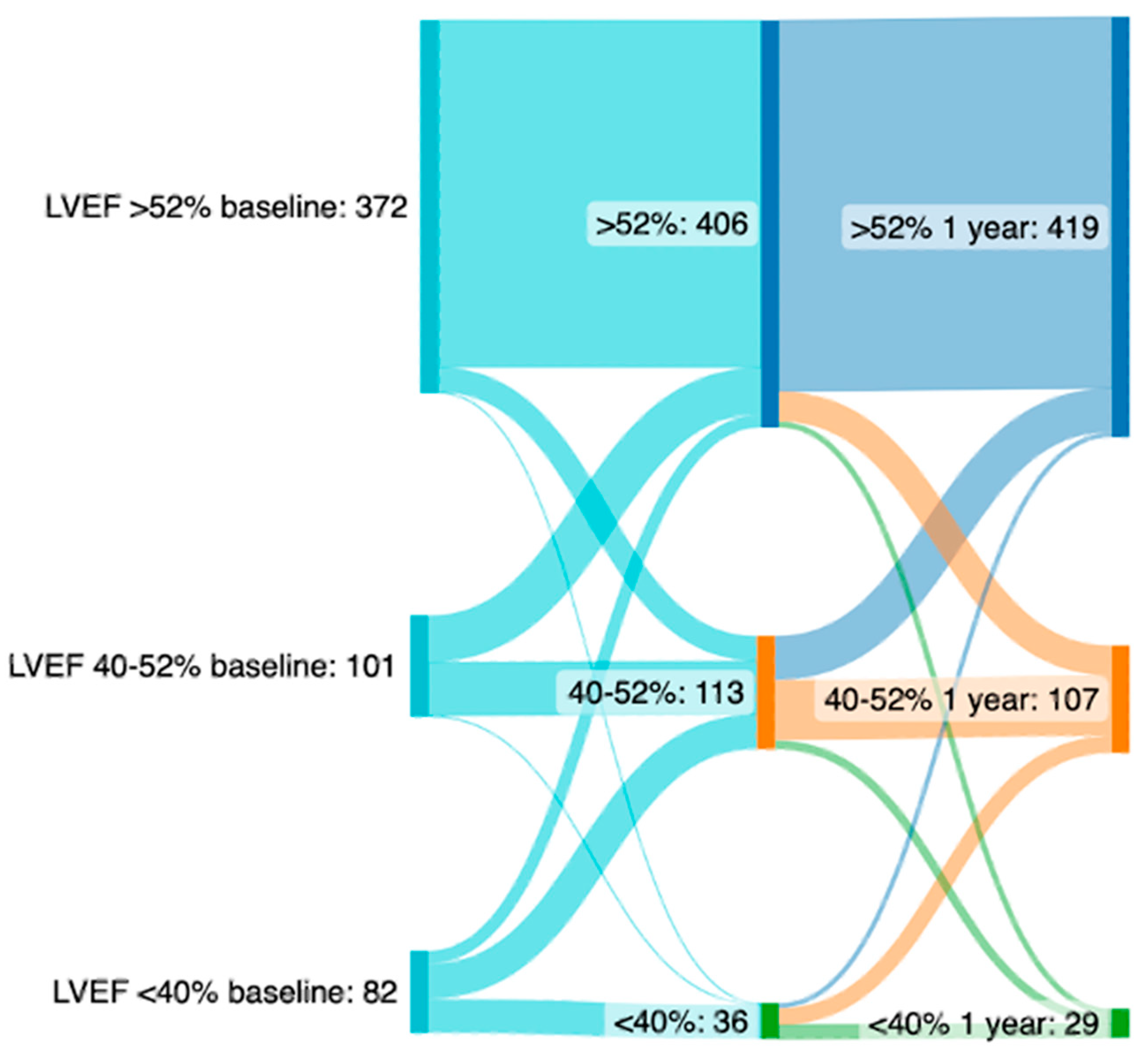


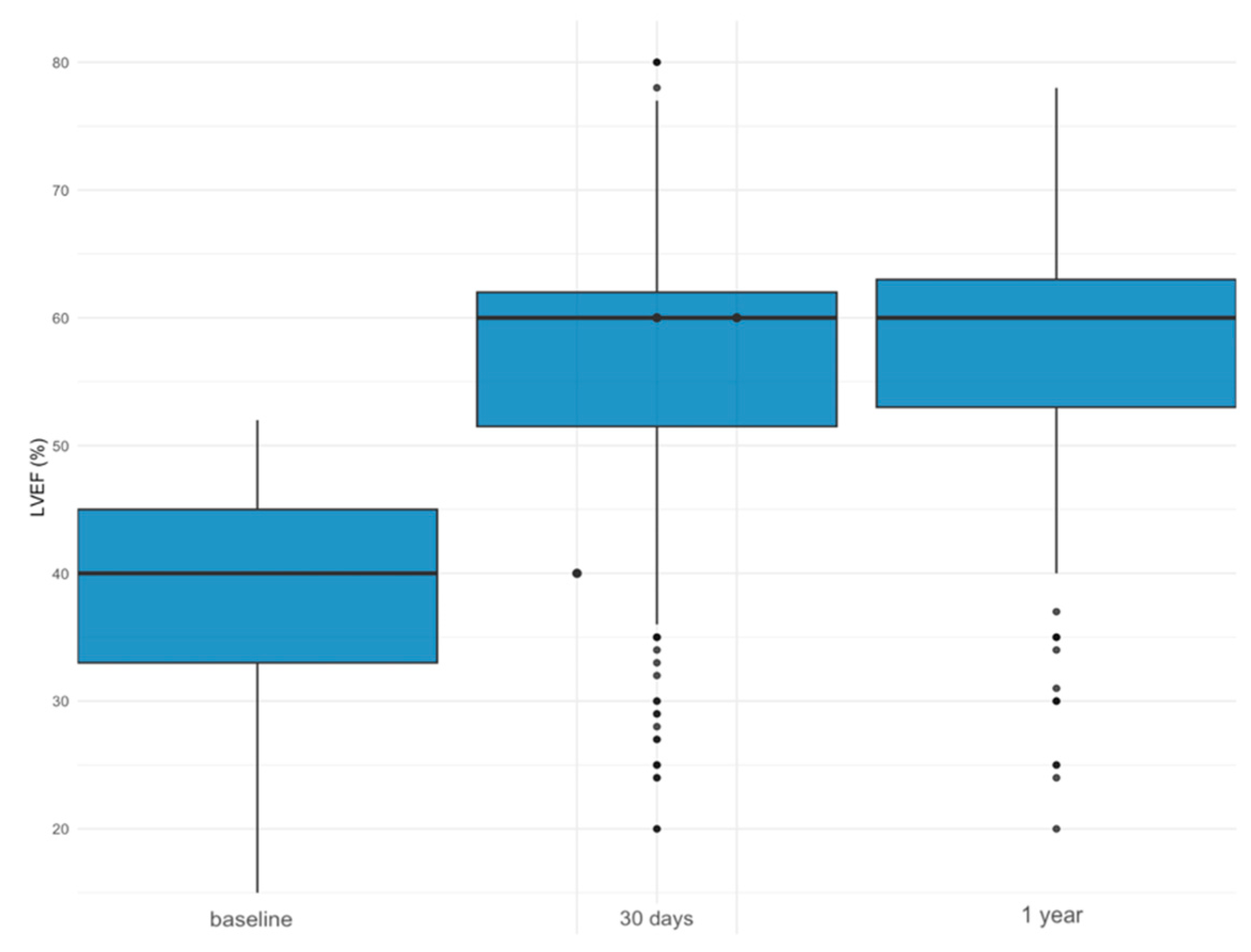
| Severely Reduced LVEF (n = 222) | Moderately Reduced LVEF (n = 303) | Preserved LVEF (n = 1031) | p | |
|---|---|---|---|---|
| Sex | ||||
| Female | 74 (33.3%) | 129 (42.6%) | 547 (53.1%) | <0.001 |
| Male | 148 (66.7%) | 174 (57.4%) | 484 (46.9%) | |
| Age (years) | 82.8 [77.9, 87.0] | 83.5 [79.6, 87.4] | 82.5 [78.8, 85.9] | 0.065 |
| BMI (kg/m2) | 25.1 [23.0, 28.5] | 25.6 [22.9, 28.7] | 26.3 [23.4, 29.6] | 0.013 |
| EuroScore II | 4.9 [2.8, 8.6] | 3.2 [1.8, 5.4] | 1.9 [1.2, 3.5] | <0.001 |
| Society of Thoracic Surgeons (STS) Score | 4.2 [2.6, 6.7] | 3.9 [2.5, 6.7] | 3.2 [2.2, 5.3] | <0.001 |
| Hypertension | 173 (77.9%) | 247 (81.5%) | 832 (80.7%) | 0.560 |
| Diabetes | 84 (37.8%) | 80 (26.4%) | 271 (26.3%) | 0.002 |
| Dyslipidemia | 117 (52.7%) | 181 (59.7%) | 628 (60.9%) | 0.077 |
| Coronary artery disease (CAD) | 144 (64.9%) | 200 (66.0%) | 515 (50.0%) | <0.001 |
| Prior myocardial infarction (MI) | 70 (31.5%) | 66 (21.8%) | 123 (11.9%) | <0.001 |
| Prior percutaneous coronary intervention (PCI) | 85 (38.3%) | 136 (44.9%) | 311 (30.2%) | <0.001 |
| Prior aortic valvuloplasty | 15 (6.8%) | 10 (3.3%) | 28 (2.7%) | 0.011 |
| Prior cardiac surgery | 35 (15.8%) | 46 (15.2%) | 77 (7.5%) | <0.001 |
| Prior implanted cardiac pacemaker | 38 (17.1%) | 39 (12.9%) | 83 (8.1%) | <0.001 |
| Implanted cardiac defibrillator (ICD) | 4 (1.8%) | 1 (0.3%) | 4 (0.4%) | 0.034 |
| Atrial fibrillation (AF) | 68 (30.6%) | 85 (28.1%) | 241 (23.4%) | 0.037 |
| Cerebrovascular disease (Stroke/TIA) | 27 (12.2%) | 38 (12.5%) | 128 (12.4%) | 0.991 |
| Peripheral artery disease (PAD) | 46 (20.7%) | 60 (19.8%) | 160 (15.5%) | 0.066 |
| Chronic kidney disease (CKD) | 170 (77.6%) | 217 (72.1%) | 609 (59.2%) | <0.001 |
| Dialysis | 10 (4.5%) | 9 (3.0%) | 18 (1.7%) | 0.038 |
| COPD | 16 (7.2%) | 32 (10.6%) | 95 (9.2%) | 0.421 |
| Severely Reduced LVEF (n = 222) | Moderately Reduced LVEF (n = 303) | Preserved LVEF (n = 1031) | p | |
|---|---|---|---|---|
| LVEF at baseline (%) | 31.0 [27.0, 35.0] | 45.0 [42.0, 50.0] | 60.0 [58.0, 65.0] | <0.001 |
| Left ventricular hypertrophy at baseline | 46 (20.7%) | 54 (17.8%) | 79 (7.6%) | <0.001 |
| Aortic valve area (cm2) | 0.7 [0.6, 0.8] | 0.7 [0.6, 0.9] | 0.8 [0.6, 0.9] | <0.001 |
| Indexed aortic valve area (cm2/m2) | 0.3 [0.2, 0.3] | 0.3 [0.2, 0.3] | 0.3 [0.2, 0.3] | <0.001 |
| Peak aortic valve gradient (mmHg) | 49.0 [36.0, 68.0] | 61.0 [44.0, 76.0] | 70.0 [54.0, 83.0] | <0.001 |
| Mean aortic valve gradient (mmHg) | 34.0 [25.0, 43.0] | 42.0 [34.0, 52.0] | 45.0 [40.0, 53.5] | <0.001 |
| Right ventricular dysfunction at baseline | 72 (35.6%) | 64 (23.5%) | 51 (5.6%) | <0.001 |
| Pulmonary hypertension at baseline | 46 (22.8%) | 54 (19.9%) | 79 (8.6%) | <0.001 |
| Left ventricular mass (g) | 228.0 [191.0, 256.5] | 204.0 [175.0, 238.0] | 185.0 [150.0, 223.0] | <0.001 |
| Indexed left ventricular mass (g/m2) | 123.5 [107.0, 141.0] | 116.5 [96.0, 139.0] | 104.0 [88.0, 122.0] | <0.001 |
| Left ventricular end-diastolic diameter (LVEDD, mm) | 54.0 [48.0, 58.0] | 48.0 [43.0, 53.0] | 44.0 [39.0, 48.0] | <0.001 |
| Left ventricular end-systolic diameter (LVESD, mm) | 45.0 [38.0, 49.0] | 35.0 [30.0, 40.0] | 28.0 [24.0, 33.0] | <0.001 |
| Valve prothesis size (mm) | 27.0 [26.0, 29.0] | 27.0 [25.0, 29.0] | 26.0 [25.0, 29.0] | <0.001 |
| Expandable valve type | ||||
| Mechanical-expandable | 6 (2.7%) | 21 (6.9%) | 57 (5.6%) | 0.001 |
| Self-expandable | 129 (58.4%) | 186 (61.4%) | 702 (68.4%) | |
| Balloon-expandable | 86 (38.9%) | 96 (31.7%) | 268 (26.1%) | |
| Vascular access for TAVR | 0.377 | |||
| Transfemoral access | 192 (86.5%) | 253 (83.5%) | 911 (88.4%) | |
| Transapical access | 23 (10.4%) | 40 (13.2%) | 94 (9.2%) | |
| Subclavian access | 6 (2.7%) | 8 (2.6%) | 24 (2.3%) | |
| Direct aortic access | 1 (0.4%) | 2 (0.7%) | 2 (0.1%) |
| Improvement (n = 155) | No Improvement (n = 229) | p | |
|---|---|---|---|
| Sex | |||
| Female | 74 (47.7%) | 74 (32.3%) | 0.003 |
| Male | 81 (52.3%) | 155 (67.7%) | |
| Age | 82.5 (±6.6) | 82.1 (±6.9) | 0.496 |
| BMI | 25.5 [23.0, 28.4] | 26.4 [23.4, 29.1] | 0.316 |
| EuroScore II | 4.1 [2.0, 5.8] | 3.7 [2.0, 6.0] | 0.857 |
| Society of Thoracic Surgeons (STS) Score | 4.0 [2.6, 6.5] | 3.7 [2.4, 6.3] | 0.583 |
| Hypertension | 119 (76.8%) | 188 (82.1%) | 0.251 |
| Diabetes | 49 (31.6%) | 69 (30.1%) | 0.845 |
| Dyslipidemia | 78 (50.3%) | 132 (57.6%) | 0.190 |
| Coronary artery disease (CAD) | 95 (61.3%) | 155 (67.7%) | 0.238 |
| Prior myocardial infarction (MI) | 40 (25.8%) | 52 (22.7%) | 0.564 |
| Prior percutaneous coronary intervention (PCI) | 61 (39.4%) | 101 (44.1%) | 0.413 |
| Prior aortic valvuloplasty | 3 (1.9%) | 15 (6.6%) | 0.064 |
| Prior cardiac surgery | 14 (9.0%) | 38 (16.6%) | 0.049 |
| Prior implanted cardiac pacemaker | 19 (12.3%) | 41 (17.9%) | 0.176 |
| Implanted cardiac defibrillator (ICD) | 1 (0.6%) | 2 (0.9%) | 1.000 |
| Atrial fibrillation (AF) | 37 (23.9%) | 74 (32.3%) | 0.094 |
| Cerebrovascular disease (Stroke/TIA) | 19 (12.3%) | 23 (10.0%) | 0.606 |
| Peripheral artery disease (PAD) | 24 (15.5%) | 46 (20.1%) | 0.312 |
| Chronic kidney disease (CKD) | 105 (68.2%) | 168 (74.3%) | 0.233 |
| Dialysis | 3 (1.9%) | 11 (4.8%) | 0.233 |
| COPD | 9 (5.8%) | 20 (8.7%) | 0.385 |
| Improvement (n = 155) | No Improvement (n = 229) | p | |
|---|---|---|---|
| LVEF at baseline (%) | 38.0 [30.0, 44.5] | 42.0 [35.0, 47.0] | <0.001 |
| Left ventricular hypertrophy at baseline | 26 (16.7%) | 48 (20.96%) | 0.37 |
| Aortic valve area (cm2) | 0.7 [0.5, 0.8] | 0.8 [0.6, 0.9] | 0.008 |
| Indexed aortic valve area (cm2/m2) | 0.2 [0.2, 0.3] | 0.3 [0.2, 0.3] | 0.033 |
| Peak aortic valve gradient (mmHg) | 60.0 [45.0, 78.0] | 53.0 [37.0, 71.0] | 0.011 |
| Mean aortic valve gradient (mmHg) | 41.0 [31.0, 50.1] | 38.0 [27.0, 48.0] | 0.049 |
| Right ventricular dysfunction at baseline | 22 (14.2%) | 71 (31.0%) | <0.001 |
| Pulmonary hypertension at baseline | 26 (18.6%) | 48 (23.9%) | 0.300 |
| Left ventricular mass (g) | 225.5 [189.0, 256.0] | 218.0 [178.5, 250.5] | 0.428 |
| Indexed left ventricular mass (g/m2) | 124.5 [112.2, 140.0] | 114.0 [98.0, 140.0] | 0.101 |
| Left ventricular end-diastolic diameter (LVEDD, mm) | 50.0 [44.0, 54.2] | 50.0 [45.0, 56.0] | 0.242 |
| Left ventricular end-systolic diameter (LVESD, mm) | 38.0 [33.0, 45.0] | 39.0 [32.0, 45.0] | 0.965 |
| Valve prothesis size (mm) | 26.0 [25.0, 29.0] | 27.0 [26.0, 29.0] | 0.016 |
| Expandable valve type | 0.405 | ||
| Mechanical-expandable | 8 (5.2%) | 13 (5.6%) | |
| Self-expandable | 88 (56.8%) | 144 (62.9%) | |
| Balloon-expandable | 59 (38.0%) | 72 (31.4%) | |
| Vascular access for TAVR | 0.042 | ||
| Transfemoral access | 143 (92.3%) | 198 (86.5%) | |
| Transapical access | 7 (4.5%) | 27 (11.8%) | |
| Subclavian access | 5 (3.2%) | 3 (1.3%) | |
| Direct aortic access | 0 | 1 (0.4%) |
| Variable | R2 | p |
|---|---|---|
| Age | 0.06 | 0.64 |
| Sex | <0.001 | 0.34 |
| Arterial hypertension | 0.008 | 0.07 |
| Diabetes | 0.002 | 0.31 |
| CKD | 0.003 | 0.29 |
| CAD | 0.001 | 0.49 |
| Prior PCI | 0.002 | 0.42 |
| Prior heart surgery | 0.003 | 0.28 |
| Prior myocardial infarction | <0.005 | 0.75 |
| Atrial fibrillation | 0.002 | 0.49 |
| Cerebrovascular disease | 0.006 | 0.10 |
| Implanted pacemaker | 0.013 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reichl, J.J.; Stolte, T.; Tang, S.; Boeddinghaus, J.; Wagener, M.; Leibundgut, G.; Kaiser, C.A.; Nestelberger, T. Prognostic Impact of Left Ventricular Ejection Fraction Improvement after Transcatheter Aortic Valve Replacement. J. Clin. Med. 2024, 13, 3639. https://doi.org/10.3390/jcm13133639
Reichl JJ, Stolte T, Tang S, Boeddinghaus J, Wagener M, Leibundgut G, Kaiser CA, Nestelberger T. Prognostic Impact of Left Ventricular Ejection Fraction Improvement after Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine. 2024; 13(13):3639. https://doi.org/10.3390/jcm13133639
Chicago/Turabian StyleReichl, Jakob Johannes, Thorald Stolte, Shihui Tang, Jasper Boeddinghaus, Max Wagener, Gregor Leibundgut, Christoph Ado Kaiser, and Thomas Nestelberger. 2024. "Prognostic Impact of Left Ventricular Ejection Fraction Improvement after Transcatheter Aortic Valve Replacement" Journal of Clinical Medicine 13, no. 13: 3639. https://doi.org/10.3390/jcm13133639
APA StyleReichl, J. J., Stolte, T., Tang, S., Boeddinghaus, J., Wagener, M., Leibundgut, G., Kaiser, C. A., & Nestelberger, T. (2024). Prognostic Impact of Left Ventricular Ejection Fraction Improvement after Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine, 13(13), 3639. https://doi.org/10.3390/jcm13133639







