Prognostic Relevance of Global Myocardial Work Index in Patients with Moderate Aortic Valve Stenosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Follow-Up
2.3. Echocardiography
2.4. Assessment of Aortic Valve Stenosis
2.5. Left Ventricular Function and Morphology
2.6. Left Ventricular Deformation
2.7. Statistical Analysis
3. Results
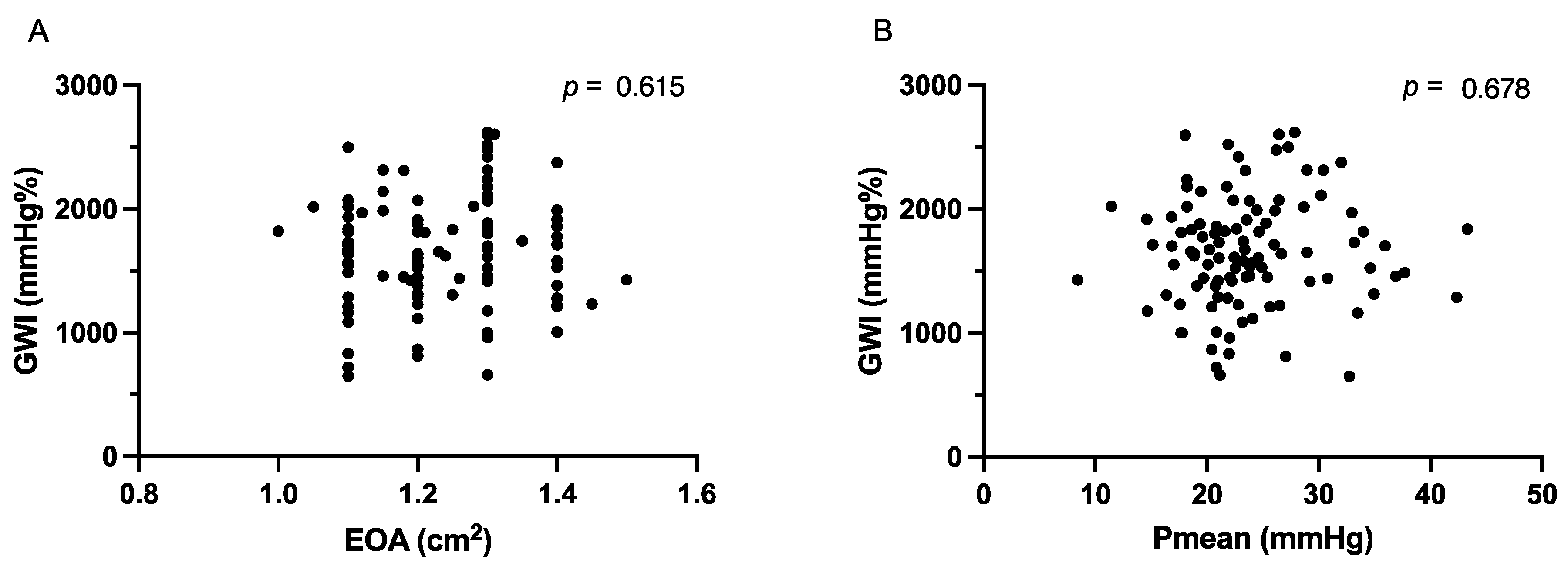
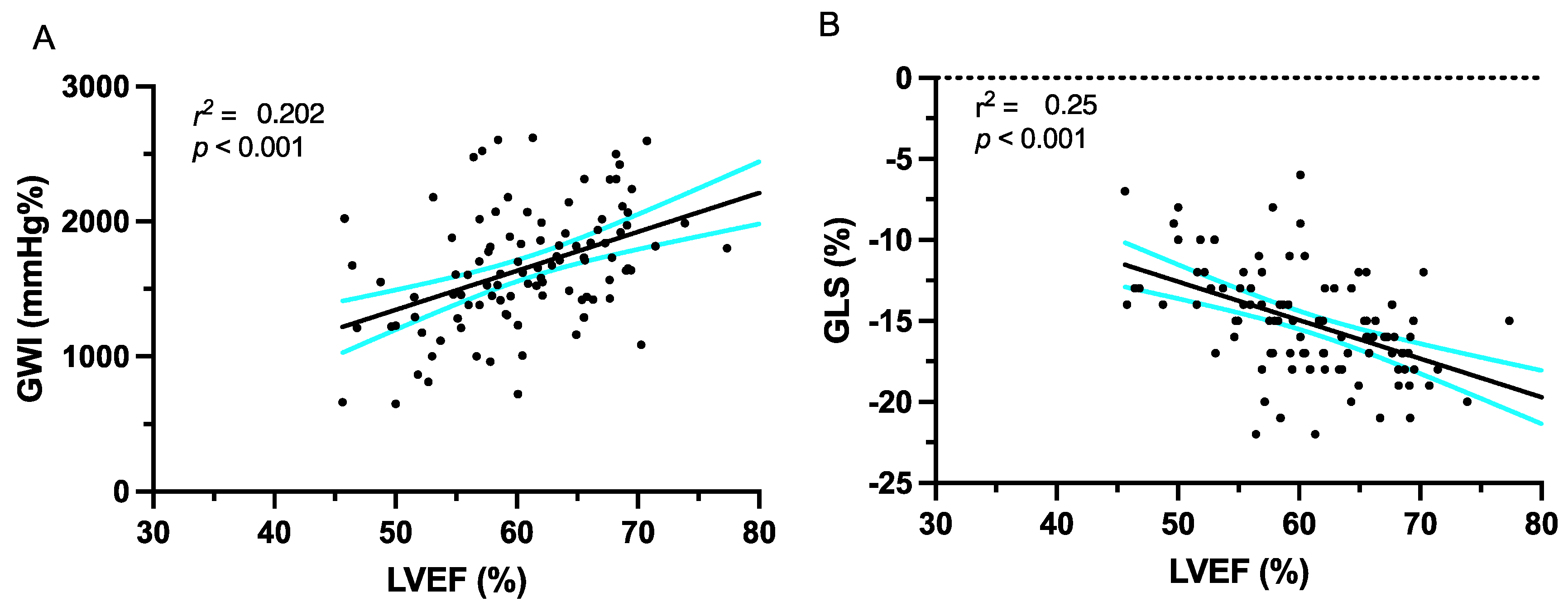
3.1. Echocardiographic Parameters
3.2. Deformation Imaging
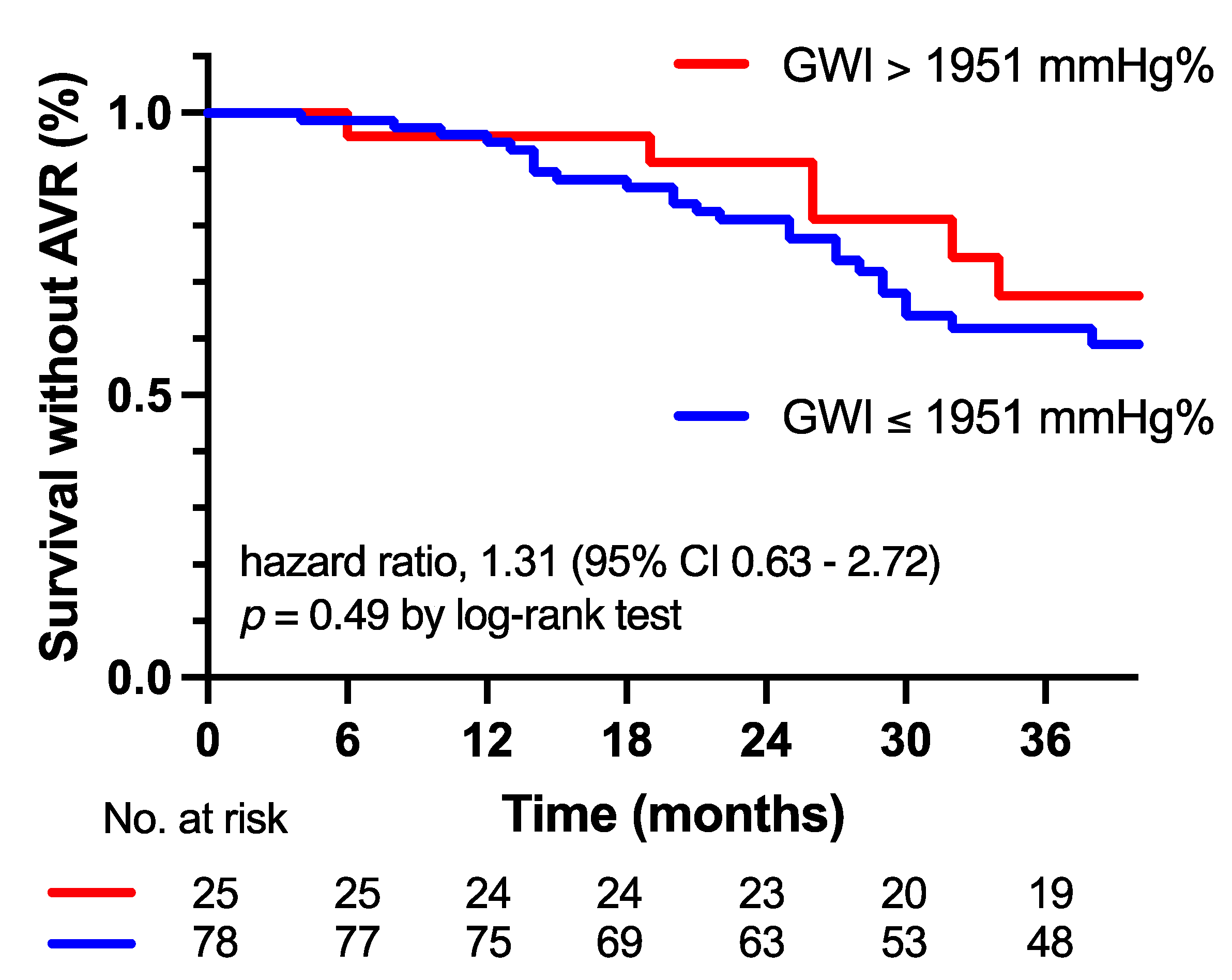
3.3. Progression of AS Severity and Outcome
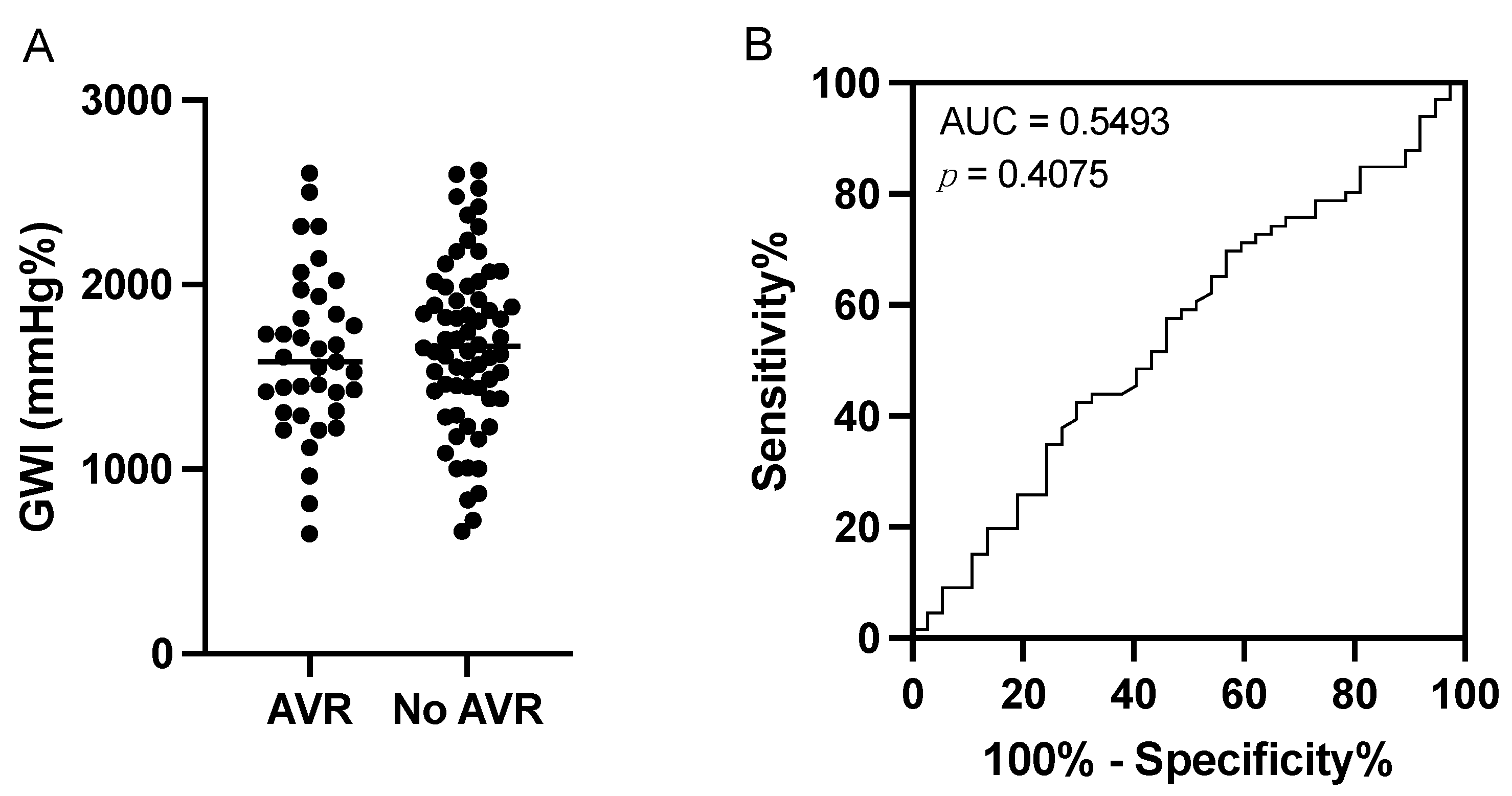
3.4. Sensitivity and Specificity of the Global Work Index
3.5. Intra- and Interobserver Variability
4. Discussion
- (1)
- The GWI could not predict the need for an AVR in moderate AS patients.
- (2)
- There was no difference in the incidence of clinical symptoms between the AVR and non-AVR groups.
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Kvaslerud, A.B.; Santic, K.; Hussain, A.I.; Auensen, A.; Fiane, A.; Skulstad, H.; Aaberge, L.; Gullestad, L.; Broch, K. Outcomes in Asymptomatic, Severe Aortic Stenosis. PLoS ONE 2021, 16, e0249610. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Magne, J.; Dulgheru, R.; Clavel, M.-A.; Donal, E.; Vannan, M.A.; Chambers, J.; Rosenhek, R.; Habib, G.; Lloyd, G.; et al. Outcomes of Patients with Asymptomatic Aortic Stenosis Followed up in Heart Valve Clinics. JAMA Cardiol. 2018, 3, 1060. [Google Scholar] [CrossRef] [PubMed]
- Strange, G.; Stewart, S.; Celermajer, D.; Prior, D.; Scalia, G.M.; Marwick, T.; Ilton, M.; Joseph, M.; Codde, J.; Playford, D. Poor Long-Term Survival in Patients with Moderate Aortic Stenosis. J. Am. Coll. Cardiol. 2019, 74, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Rosenhek, R. Mild and Moderate Aortic Stenosis Natural History and Risk Stratification by Echocardiography. Eur. Heart J. 2004, 25, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Samad, Z.; Vora, A.N.; Dunning, A.; Schulte, P.J.; Shaw, L.K.; Al-Enezi, F.; Ersboll, M.; McGarrah, R.W.; Vavalle, J.P.; Shah, S.H.; et al. Aortic Valve Surgery and Survival in Patients with Moderate or Severe Aortic Stenosis and Left Ventricular Dysfunction. Eur. Heart J. 2016, 37, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Delesalle, G.; Bohbot, Y.; Rusinaru, D.; Delpierre, Q.; Maréchaux, S.; Tribouilloy, C. Characteristics and Prognosis of Patients with Moderate Aortic Stenosis and Preserved Left Ventricular Ejection Fraction. J. Am. Heart Assoc. 2019, 8, e011036. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A Novel Clinical Method for Quantification of Regional Left Ventricular Pressure–Strain Loop Area: A Non-Invasive Index of Myocardial Work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef]
- Hubert, A.; Le Rolle, V.; Leclercq, C.; Galli, E.; Samset, E.; Casset, C.; Mabo, P.; Hernandez, A.; Donal, E. Estimation of Myocardial Work from Pressure–Strain Loops Analysis: An Experimental Evaluation. Eur. Heart J.-Cardiovasc. Imaging 2018, 19, 1372–1379. [Google Scholar] [CrossRef]
- Chan, J.; Edwards, N.F.A.; Khandheria, B.K.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Scalia, G.M. A New Approach to Assess Myocardial Work by Non-Invasive Left Ventricular Pressure–Strain Relations in Hypertension and Dilated Cardiomyopathy. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, 31–39. [Google Scholar] [CrossRef]
- Ilardi, F.; Postolache, A.; Dulgheru, R.; Trung, M.-L.N.; de Marneffe, N.; Sugimoto, T.; Go, Y.Y.; Oury, C.; Esposito, G.; Lancellotti, P. Prognostic Value of Non-Invasive Global Myocardial Work in Asymptomatic Aortic Stenosis. J. Clin. Med. 2022, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Fortuni, F.; Butcher, S.C.; van der Kley, F.; Lustosa, R.P.; Karalis, I.; de Weger, A.; Priori, S.G.; van der Bijl, P.; Bax, J.J.; Delgado, V.; et al. Left Ventricular Myocardial Work in Patients with Severe Aortic Stenosis. J. Am. Soc. Echocardiogr. 2021, 34, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.D.; Nishimura, R.A.; Holmes, D.R.; Bailey, K.R. Natural History of Moderate Aortic Stenosis. J. Am. Coll. Cardiol. 1991, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F.; Otto, C.M. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J.-Cardiovasc. Imaging 2017, 18, 254–275. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Voigt, J.-U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef]
- De Rosa, S.; Sabatino, J.; Strangio, A.; Leo, I.; Romano, L.R.; Spaccarotella, C.A.; Mongiardo, A.; Polimeni, A.; Sorrentino, S.; Indolfi, C. Non-Invasive Myocardial Work in Patients with Severe Aortic Stenosis. J. Clin. Med. 2022, 11, 747. [Google Scholar] [CrossRef]
- Truong, V.T.; Vo, H.Q.; Ngo, T.N.M.; Mazur, J.; Nguyen, T.T.H.; Pham, T.T.M.; Le, T.K.; Phan, H.; Palmer, C.; Nagueh, S.F.; et al. Normal Ranges of Global Left Ventricular Myocardial Work Indices in Adults: A Meta-Analysis. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2021, 35, 369–377.e8. [Google Scholar] [CrossRef]
- Lee, H.-F.; Hsu, L.-A.; Chan, Y.-H.; Wang, C.-L.; Chang, C.-J.; Kuo, C.-T. Prognostic Value of Global Left Ventricular Strain for Conservatively Treated Patients with Symptomatic Aortic Stenosis. J. Cardiol. 2013, 62, 301–306. [Google Scholar] [CrossRef]
- Zhu, D.; Ito, S.; Miranda, W.R.; Nkomo, V.T.; Pislaru, S.V.; Villarraga, H.R.; Pellikka, P.A.; Crusan, D.J.; Oh, J.K. Left Ventricular Global Longitudinal Strain Is Associated with Long-Term Outcomes in Moderate Aortic Stenosis. Circ. Cardiovasc. Imaging 2020, 13, e009958. [Google Scholar] [CrossRef] [PubMed]
- Stassen, J.; Pio, S.M.; Ewe, S.H.; Singh, G.K.; Hirasawa, K.; Butcher, S.C.; Cohen, D.J.; Généreux, P.; Leon, M.B.; Marsan, N.A.; et al. Left Ventricular Global Longitudinal Strain in Patients with Moderate Aortic Stenosis. J. Am. Soc. Echocardiogr. 2022, 35, 791–800.e4. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Cho, S.W.; Kim, S.M.; Ahn, J.; Carriere, K.; Jeong, D.S.; Lee, S.-C.; Park, S.W.; Choe, Y.H.; Park, P.W.; et al. Assessment of Myocardial Fibrosis Using Multimodality Imaging in Severe Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Oláh, A.; Lux, Á.; Mátyás, C.; Németh, B.T.; Kellermayer, D.; Ruppert, M.; Török, M.; Szabó, L.; Meltzer, A.; et al. Strain and Strain Rate by Speckle-Tracking Echocardiography Correlate with Pressure-Volume Loop-Derived Contractility Indices in a Rat Model of Athlete’s Heart. Am. J. Physiol.-Heart Circ. Physiol. 2015, 308, H743–H748. [Google Scholar] [CrossRef] [PubMed]
- Dahle, G.O.; Stangeland, L.; Moen, C.A.; Salminen, P.-R.; Haaverstad, R.; Matre, K.; Grong, K. The Influence of Acute Unloading on Left Ventricular Strain and Strain Rate by Speckle Tracking Echocardiography in a Porcine Model. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H1330–H1339. [Google Scholar] [CrossRef] [PubMed]
- Ferferieva, V.; Van Den Bergh, A.; Claus, P.; Jasaityte, R.; Veulemans, P.; Pellens, M.; La Gerche, A.; Rademakers, F.; Herijgers, P.; D’Hooge, J. The Relative Value of Strain and Strain Rate for Defining Intrinsic Myocardial Function. Am. J. Physiol.-Heart Circ. Physiol. 2012, 302, H188–H195. [Google Scholar] [CrossRef]
- Zhao, Q.; Cui, C.; Li, Y.; Liu, Y.; Huang, D.; Wang, Y.; Hu, Y.; Liu, R.; Zhu, H.; Liu, L. Evaluation of Myocardial Work in Patients with Hypertrophic Cardiomyopathy and Hypertensive Left Ventricular Hypertrophy Based on Non-Invasive Pressure-Strain Loops. Front. Cardiovasc. Med. 2022, 9, 767875. [Google Scholar] [CrossRef]
- Fan, J.; Ma, C.; Wang, H.; Zhou, B. The Value of Myocardial Work in Patients with Left Ventricular Hypertrophy. Int. J. Cardiovasc. Imaging 2023, 39, 1105–1113. [Google Scholar] [CrossRef]
- Yang, C.; Guo, Y.; Wang, X.; Zhang, R.; Wang, F.; Zhu, H. The Value of Myocardial Work in Assessment of Ventricular Function in Patients with Non-Obstructive Hypertrophic Cardiomyopathy. BMC Cardiovasc. Disord. 2022, 22, 305. [Google Scholar] [CrossRef]
- Urheim, S.; Rabben, S.I.; Skulstad, H.; Lyseggen, E.; Ihlen, H.; Smiseth, O.A. Regional Myocardial Work by Strain Doppler Echocardiography and LV Pressure: A New Method for Quantifying Myocardial Function. Am. J. Physiol.-Heart Circ. Physiol. 2005, 288, H2375–H2380. [Google Scholar] [CrossRef]
- Dobson, L.E.; Fairbairn, T.A.; Musa, T.A.; Uddin, A.; Mundie, C.A.; Swoboda, P.P.; Ripley, D.P.; McDiarmid, A.K.; Erhayiem, B.; Garg, P.; et al. Sex-Related Differences in Left Ventricular Remodeling in Severe Aortic Stenosis and Reverse Remodeling after Aortic Valve Replacement: A Cardiovascular Magnetic Resonance Study. Am. Heart J. 2016, 175, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T. Diagnosing Heart Failure with Preserved Ejection Fraction with Pulmonary Vascular Disease. Heart 2023, 109, 578–580. [Google Scholar] [CrossRef] [PubMed]
| Variables | No Aortic Valve Replacement (n = 66) | Aortic Valve Replacement (n = 37) | p-Value |
|---|---|---|---|
| Age (years) | 72.6 ± 10.7 | 71.4 ± 9.6 | 0.562 |
| Male (%) | 44 (67) | 27 (73) | 0.529 |
| Weight (kg) | 80.9 ± 14.6 | 83.6 ± 15.1 | 0.383 |
| Height (cm) | 170.3 ± 9.8 | 172.1 ± 8.0 | 0.315 |
| BSA (m2) | 1.95 ± 0.21 | 1.99 ± 0.20 | 0.342 |
| BMI (kg/m2) | 27.9 ± 4.3 | 28.0 ± 4.3 | 0.911 |
| sBP (mmHg) | 138.8 ± 13.7 | 141.3 ± 17.2 | 0.454 |
| dBP (mmHg) | 78.5 ± 10.5 | 79.3 ± 10.4 | 0.709 |
| HR (1/min) | 69.6 ± 10.2 | 71.0 ± 12.2 | 0.557 |
| Arterial hypertension (%) | 55 (83%) | 24 (65%) | 0.040 |
| Diabetes mellitus (%) | 9 (14%) | 9 (24%) | 0.203 |
| Hypercholesterolemia (%) | 30 (45%) | 23 (62%) | 0.100 |
| COPD (%) | 4 (6%) | 4 (11%) | 0.366 |
| Atrial fibrillation (%) | 12 (18%) | 6 (16%) | 0.798 |
| CAD (%) | 22 (33%) | 15 (41%) | 0.419 |
| Stroke (%) | 14 (21%) | 3 (8%) | 0.088 |
| CKD ≥ 3 (%) | 24 (36%) | 14 (38%) | 0.841 |
| Smoker (%) | 21 (32%) | 9 (24%) | 0.394 |
| Dyspnea (%) | 20 (30%) | 9 (24%) | 0.517 |
| Angina pectoris (%) | 11 (17%) | 7 (19%) | 0.799 |
| Previous syncope (%) | 2 (3%) | 0 (0%) | 0.289 |
| Variables | No Aortic Valve Replacement (n = 66) | Aortic Valve Replacement (n = 37) | p-Value |
|---|---|---|---|
| EOA (cm2) | 1.24 ± 0.11 | 1.23 ± 0.11 | 0.661 |
| Vmax (m/s) | 3.2 ± 0.4 | 3.3 ± 0.5 | 0.301 |
| Pmean (mmHg) | 22.7 ± 4.9 | 25.6 ± 7.5 | 0.039 |
| LVEF (%) | 60.8 ± 7.5 | 60.7 ± 7.2 | 0.947 |
| TAPSE (mm) | 20.8 ± 3.8 | 21.0 ± 3.6 | 0.791 |
| E/E′ | 12.0 ± 4.8 | 13.5 ± 3.1 | 0.058 |
| TRVmax (m/s) | 28.9 ± 7.8 | 30.9 ± 7.2 | 0.193 |
| LVMi (g/m2) | 96.6 ± 35.1 | 113.4 ± 24.9 | 0.006 |
| Variables | No Aortic Valve Replacement (n = 66) | Aortic Valve Replacement (n = 37) | p-Value |
|---|---|---|---|
| GLS (%) | −15.1 ± 3.4 | −15.0 ± 3.4 | 0.887 |
| GWI (mmHg%) | 1674 ± 456 | 1615 ± 441 | 0.522 |
| GCW (mmHg%) | 2103 ± 508 | 2096 ± 455 | 0.943 |
| GWW (mmHg%) | 221 ± 124 | 224 ± 177 | 0.928 |
| GWE (%) | 88.6 ± 5.9 | 88.8 ± 7.0 | 0.884 |
| Variable | Hazard Ratio (95% Confidence Interval) | p-Value |
|---|---|---|
| Age (years) | 1.0 (0.97–1.1) | 0.765 |
| Female sex | 1.1 (0.45–2.5) | 0.846 |
| LVEF (%) | 0.98 (0.94–1.0) | 0.458 |
| Pmean (mmHg) | 1.1 (1.0–1.2) | 0.015 |
| EOA (cm2) | 1.4 (0.039–55) | 0.857 |
| Hypertension | 0.46 (0.21–1.0) | 0.052 |
| Diabetes mellitus | 1.1 (0.46–2.6) | 0.783 |
| Dyspnea (≥NYHA II) | 1.6 (0.60–4.0) | 0.327 |
| Angina | 0.96 (0.33–2.5) | 0.934 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandels, J.; Metze, M.; Hagendorff, A.; Stöbe, S. Prognostic Relevance of Global Myocardial Work Index in Patients with Moderate Aortic Valve Stenosis. J. Clin. Med. 2023, 12, 7694. https://doi.org/10.3390/jcm12247694
Kandels J, Metze M, Hagendorff A, Stöbe S. Prognostic Relevance of Global Myocardial Work Index in Patients with Moderate Aortic Valve Stenosis. Journal of Clinical Medicine. 2023; 12(24):7694. https://doi.org/10.3390/jcm12247694
Chicago/Turabian StyleKandels, Joscha, Michael Metze, Andreas Hagendorff, and Stephan Stöbe. 2023. "Prognostic Relevance of Global Myocardial Work Index in Patients with Moderate Aortic Valve Stenosis" Journal of Clinical Medicine 12, no. 24: 7694. https://doi.org/10.3390/jcm12247694
APA StyleKandels, J., Metze, M., Hagendorff, A., & Stöbe, S. (2023). Prognostic Relevance of Global Myocardial Work Index in Patients with Moderate Aortic Valve Stenosis. Journal of Clinical Medicine, 12(24), 7694. https://doi.org/10.3390/jcm12247694






