Uterus Transplantation as Infertility Treatment in Gynecological Cancer Survivors: A Systematic Review
Abstract
1. Introduction
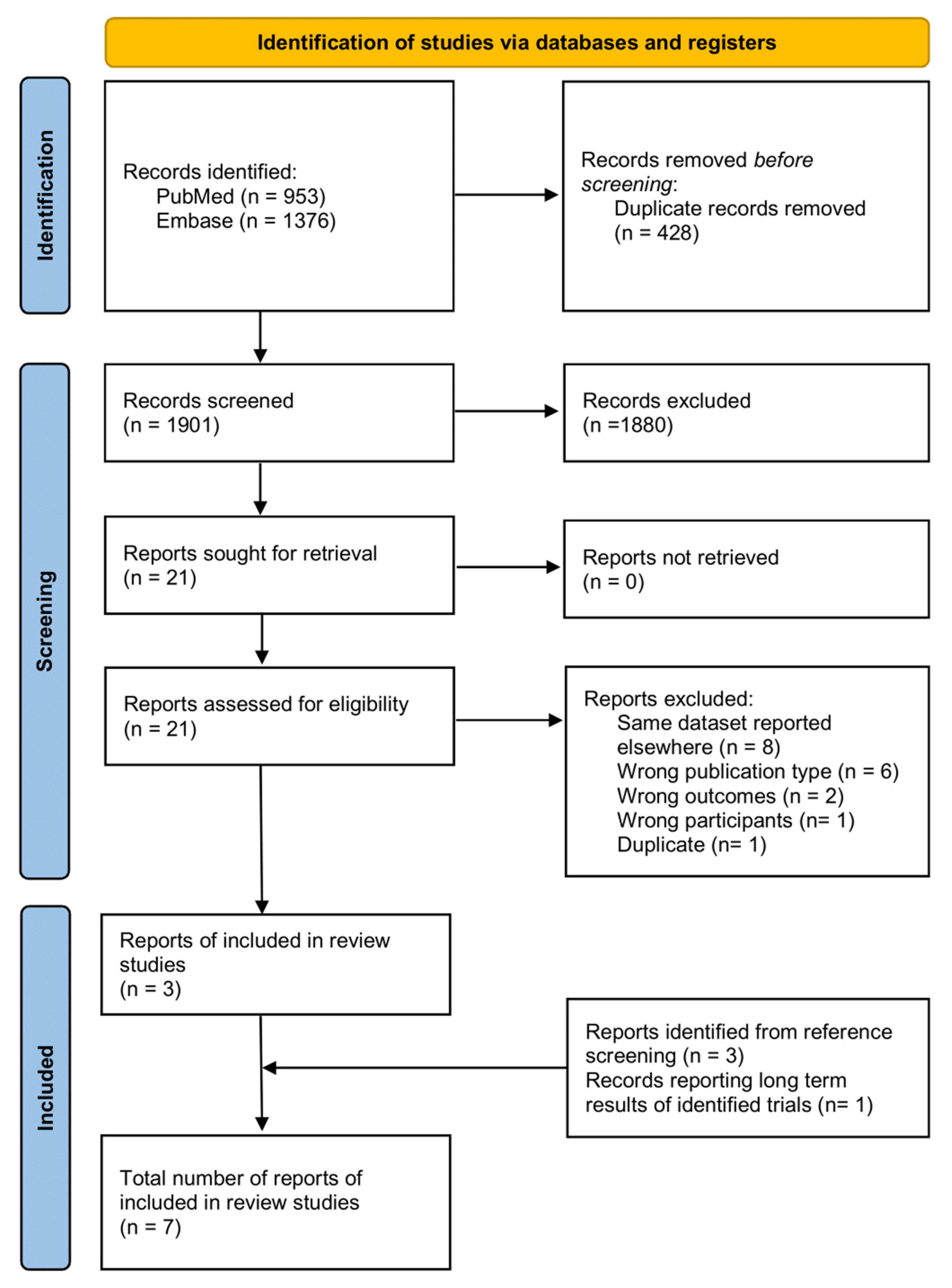
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| (cancer:ti,ab OR neoplas*:ti,ab OR malignan*:ti,ab OR proliferat*:ti,ab OR Neoplasms/exp OR ‘Cancer Survivors’/exp OR ‘Uterine Neoplasms’/exp OR ‘Uterine Cervical Neoplasms’/exp OR ‘Ovarian Neoplasms’/exp) |
| AND |
| (‘uterine transplant*’:ti,ab OR ‘uterus transplant*’:ti,ab OR ((Uterus/exp OR uterus:ti,ab OR Womb:ti,ab OR Uterine:ti,ab) AND (transplantation/exp OR Transplants/exp OR ‘transplant recipient*’/exp OR ‘tissue donor*’/exp OR transplant*:ti,ab OR graft*:ti,ab))) |
| NOT |
| (animals/exp NOT humans/exp) |
References
- Bray Bsc, F.; Laversanne, M.; Sung, H.; Phd, S.; Ferlay, J.; Siegel Mph, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Rob, L.; Skapa, P.; Robova, H. Fertility-Sparing Surgery in Patients with Cervical Cancer. Lancet Oncol. 2011, 12, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Persson, J.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Cervical Cancer-Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Endometrial Carcinoma. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Landoni, F.; Mahner, S.; Mccormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. ESMO Updat. Clin. Pract. Guidel. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.; Matias-Guiu, X.; Amant, F.; Concin, N.; Davidson, B.; Fotopoulou, C.; González-Martin, A.; Gourley, C.; Leary, A.; Lorusso, D.; et al. ESGO-ESMO-ESP Consensus Conference Recommendations on Ovarian Cancer: Pathology and Molecular Biology and Early, Advanced and Recurrent Disease. Ann. Oncol. 2024, 35, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Ann. Oncol. 2015, 27, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Brännström, M.; Johannesson, L.; Dahm-Kähler, P.; Enskog, A.; Mölne, J.; Kvarnström, N.; Diaz-Garcia, C.; Hanafy, A.; Lundmark, C.; Marcickiewicz, J.; et al. First Clinical Uterus Transplantation Trial: A Six-Month Report. Fertil. Steril. 2014, 101, 1228–1236. [Google Scholar] [CrossRef]
- Sallée, C.; Margueritte, F.; Marquet, P.; Piver, P.; Aubard, Y.; Lavoué, V.; Dion, L.; Gauthier, T. Uterine Factor Infertility, a Systematic Review. J. Clin. Med. 2022, 11, 4907. [Google Scholar] [CrossRef]
- Fageeh, W.; Raffa, H.; Jabbad, H.; Marzouki, A. Transplantation of the Human Uterus. Int. J. Gynecol. Obstet. 2002, 76, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Sanders, S.; Carter, M.; Honeyman, D.; Cleo, G.; Auld, Y.; Booth, D.; Condron, P.; Dalais, C.; Bateup, S.; et al. Improving the Translation of Search Strategies Using the Polyglot Search Translator: A Randomized Controlled Trial. J. Med. Libr. Assoc. JMLA 2020, 108, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Huet, S.; Tardieu, A.; Filloux, M.; Essig, M.; Pichon, N.; Therme, J.F.; Piver, P.; Aubard, Y.; Ayoubi, J.M.; Garbin, O.; et al. Uterus Transplantation in France: For Which Patients? Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 205, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Arian, S.E.; Flyckt, R.L.; Farrell, R.M.; Falcone, T.; Tzakis, A.G. Characterizing Women with Interest in Uterine Transplant Clinical Trials in the United States: Who Seeks Information on This Experimental Treatment? Am. J. Obstet. Gynecol. 2017, 216, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Akar, M.E.; Ozekinci, M.; Alper, O.; Demir, D.; Cevikol, C.; Meric Bilekdemir, A.; Daloglu, A.; Ongut, G.; Senol, Y.; Ozdem, S.; et al. Assessment of Women Who Applied for the Uterine Transplant Project as Potential Candidates for Uterus Transplantation. J. Obstet. Gynaecol. Res. 2015, 41, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, L.; Kvarnström, N.; Mölne, J.; Dahm-Kähler, P.; Enskog, A.; Diaz-Garcia, C.; Olausson, M.; Brännström, M. Uterus Transplantation Trial: 1-Year Outcome. Fertil. Steril. 2015, 103, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Brännström, M.; Dahm-Kähler, P.; Kvarnström, N.; Enskog, A.; Olofsson, J.I.; Olausson, M.; Mölne, J.; Akouri, R.; Järvholm, S.; Nilsson, L.; et al. Reproductive, Obstetric, and Long-Term Health Outcome after Uterus Transplantation: Results of the First Clinical Trial. Fertil. Steril. 2022, 118, 576–585. [Google Scholar] [CrossRef]
- Johannesson, L.; Wallis, K.; Koon, E.C.; McKenna, G.J.; Anthony, T.; Leffingwell, S.G.; Klintmalm, G.B.; Gunby, R.T.; Testa, G. Living Uterus Donation and Transplantation: Experience of Interest and Screening in a Single Center in the United States. Am. J. Obstet. Gynecol. 2018, 218, 331.e1–331.e7. [Google Scholar] [CrossRef]
- Johannesson, L.; Parrott, M.; Wall, A.; Testa, G. Adverse Effects of Immunosuppression in Uterus Transplant Recipients-Dallas Uterus Transplant Study from 22nd Annual State of the Art Winter Symposium. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2022, 22 (Suppl. S1), 66. [Google Scholar] [CrossRef] [PubMed]
- Ayoubi, J.M.; Carbonnel, M.; Racowsky, C.; De Ziegler, D.; Gargiulo, A.; Kvarnström, N.; Dahm-Kähler, P. Evolving Clinical Challenges in Uterus Transplantation. Reprod. BioMed. Online 2022, 45, 947–960. [Google Scholar] [CrossRef]
- Brännström, M.; Dahm-Kähler, P. Uterus Transplantation and Fertility Preservation. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 55, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Brännström, M. The Swedish Uterus Transplantation Project: The Story behind the Swedish Uterus Transplantation Project. Acta Obstet. Gynecol. Scand. 2015, 94, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Strome, M.; Stein, J.; Esclamado, R.; Hicks, D.; Lorenz, R.R.; Braun, W.; Yetman, R.; Eliachar, I.; Mayes, J. Laryngeal Transplantation and 40-Month Follow-Up. N. Engl. J. Med. 2001, 344, 1676–1679. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Koon, E.C.; Johannesson, L.; McKenna, G.J.; Anthony, T.; Klintmalm, G.B.; Gunby, R.T.; Warren, A.M.; Putman, J.M.; de Prisco, G.; et al. Living Donor Uterus Transplantation: A Single Center’s Observations and Lessons Learned from Early Setbacks to Technical Success. Am. J. Transplant. 2017, 17, 2901–2910. [Google Scholar] [CrossRef] [PubMed]
- Taran, F.A.; Schöller, D.; Rall, K.; Nadalin, S.; Königsrainer, A.; Henes, M.; Bösmüller, H.; Fend, F.; Nikolaou, K.; Notohamiprodjo, M.; et al. Screening and Evaluation of Potential Recipients and Donors for Living Donor Uterus Transplantation: Results from a Single-Center Observational Study. Fertil. Steril. 2019, 111, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Stubblefield, M.D. Radiation Fibrosis Syndrome: Neuromuscular and Musculoskeletal Complications in Cancer Survivors. PM R J. Inj. Funct. Rehabil. 2011, 3, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.W.; Ng, E.H.Y.; Ho, P.C. Ovarian Changes after Abdominal Hysterectomy for Benign Conditions. J. Soc. Gynecol. Investig. 2005, 12, 54–57. [Google Scholar] [CrossRef]
- Piselli, P.; Serraino, D.; Segoloni, G.P.; Sandrini, S.; Piredda, G.B.; Scolari, M.P.; Rigotti, P.; Busnach, G.; Messa, P.; Donati, D.; et al. Risk of de Novo Cancers after Transplantation: Results from a Cohort of 7217 Kidney Transplant Recipients, Italy 1997–2009. Eur. J. Cancer 2013, 49, 336–344. [Google Scholar] [CrossRef]
- Hinten, F.; Meeuwis, K.A.P.; van Rossum, M.M.; de Hullu, J.A. HPV-Related (Pre)Malignancies of the Female Anogenital Tract in Renal Transplant Recipients. Crit. Rev. Oncol./Hematol. 2012, 84, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Acuna, S.A.; Huang, J.W.; Daly, C.; Shah, P.S.; Kim, S.J.; Baxter, N.N. Outcomes of Solid Organ Transplant Recipients With Preexisting Malignancies in Remission: A Systematic Review and Meta-Analysis. Transplantation 2017, 101, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Agraharkar, M.L.; Cinclair, R.D.; Kuo, Y.F.; Daller, J.A.; Shahinian, V.B. Risk of Malignancy with Long-Term Immunosuppression in Renal Transplant Recipients. Kidney Int. 2004, 66, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, L.; Wall, A.; Putman, J.M.; Zhang, L.; Testa, G.; Diaz-Garcia, C. Rethinking the Time Interval to Embryo Transfer after Uterus Transplantation—DUETS (Dallas UtErus Transplant Study). BJOG Int. J. Obstet. Gynaecol. 2019, 126, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Brännström, M.; Racowsky, C.; Richards, E.G.; Flyckt, R.; Stillman, R.J.; O’Brien, J.E.; Ryan, G.L.; de Ziegler, D. Absolute Uterine Infertility a Cornelian Dilemma: Uterine Transplantation or Surrogacy? Fertil. Steril. 2023, 119, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Hammond-Browning, N. UK Criteria for Uterus Transplantation: A Review. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Koplin, J.J.; Kendal, E. Ethical Issues in Uterine Transplantation. Korean J. Transplant. 2020, 34, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, L.; Richards, E.; Reddy, V.; Walter, J.; Olthoff, K.; Quintini, C.; Tzakis, A.; Latif, N.; Porrett, P.; O’Neill, K.; et al. The First 5 Years of Uterus Transplant in the US: A Report From the United States Uterus Transplant Consortium. JAMA Surg. 2022, 157, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.S.; Blake, V. Uterus Transplantation: Ethical and Regulatory Challenges. J. Med. Ethics 2014, 40, 396–400. [Google Scholar] [CrossRef]
- Oktem, O.; Kim, S.S.; Selek, U.; Schatmann, G.; Urman, B. Ovarian and Uterine Functions in Female Survivors of Childhood Cancers. Oncologist 2018, 23, 214–224. [Google Scholar] [CrossRef]
| (“cancer”[tiab] OR “neoplas*”[tiab] OR “malignan*”[tiab] OR “proliferat*”[tiab] OR “Neoplasms”[Mesh] OR “Cancer Survivors”[Mesh] OR “Uterine Neoplasms”[Mesh] OR “Uterine Cervical Neoplasms”[Mesh] OR “Ovarian Neoplasms”[Mesh]) |
| AND |
| (“uterine transplant*”[tiab] OR “uterus transplant*”[tiab] OR ((“Uterus”[Mesh] OR “uterus” [tiab] OR “Womb”[tiab] OR “Uterine”[tiab]) AND (“transplantation”[MeSH] OR “Transplants”[Mesh] OR “transplant recipient*”[MeSH] OR “tissue donor*”[MeSH] OR “transplant*”[tiab] OR “graft*”[tiab]))) |
| NOT |
| (animals [mh] NOT humans [mh]) |
| Original Study | Country | Type of Study | Sample Size | Number of Gynecological Cancer Survivors | Gynecological Cancer | Cancer Therapies | Examined Outcomes | Findings | |
|---|---|---|---|---|---|---|---|---|---|
| Peer-reviewed papers | Huet et al., 2016 [15] | France | Observational Study | 39 | 10 | Ovarian cancer (n = 4), cervical cancer (n = 3), bladder sarcoma (n = 1), and choriocarcinoma (n = 1) | Hysterectomy before 8.37 years (range 3–23); bilateral adnexectomy in five patients | Proportion of gynecological cancer survivors among uterus transplantation applicants | 7.20% |
| Arian et al., 2017 [16] | USA | Observational Study | 239 | 39 | Not specified | Not specified | Proportion of gynecological cancer survivors among uterus transplantation applicants | 16.30% | |
| Akar et al., 2015 [17] | Turkey | Observational Study | 144 | 0 | Not applicable | Not applicable | Proportion of gynecological cancer survivors among uterus transplantation applicants | 0% | |
| Johannesson et al., 2018 [20] | USA | Observational Study | 179 | 11 | Not specified | Hysterectomy | Proportion of gynecological cancer survivors among uterus transplantation applicants | 6.10% | |
| Johannesson et al., 2015 [18] | Sweden | Clinical Trial | 7 | 1 | Cervical Cancer (stage 1B1) | Radical hysterectomy and pelvic lymph node dissection | Post-transplantation menstruation, uterine artery blood flow, rejection episodes, and relevant adverse effects of immunosuppression post-transplantation | Menstruation within two months post-transplantation, normal Doppler studies of uterine arteries, and no rejection episodeCIN II in one out of seven patients (14.3%) | |
| Brännström et al., 2022 [19] | Sweden | Clinical Trial | 7 | 1 | Cervical Cancer (stage 1B1) | Radical hysterectomy and pelvic lymph node dissection | Pregnancy rates and live birth rates per embryo transfer post-transplantation | First pregnancy leading to live birth after six embryo transfers (rate 17%) Second pregnancy leading to live birth after two embryo transfers (rate 50%) | |
| Poster | Johannesson et al., 2022 [21] | USA | Clinical Trial | 20 | 0 | Not applicable | Not applicable | Relevant adverse effects of immunosuppression post-transplantation | CIN I in 1/20 patients (5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsarna, E.; Eleftheriades, A.; Matsas, A.; Triantafyllidou, O.; Christopoulos, P. Uterus Transplantation as Infertility Treatment in Gynecological Cancer Survivors: A Systematic Review. J. Clin. Med. 2024, 13, 3172. https://doi.org/10.3390/jcm13113172
Tsarna E, Eleftheriades A, Matsas A, Triantafyllidou O, Christopoulos P. Uterus Transplantation as Infertility Treatment in Gynecological Cancer Survivors: A Systematic Review. Journal of Clinical Medicine. 2024; 13(11):3172. https://doi.org/10.3390/jcm13113172
Chicago/Turabian StyleTsarna, Ermioni, Anna Eleftheriades, Alkis Matsas, Olga Triantafyllidou, and Panagiotis Christopoulos. 2024. "Uterus Transplantation as Infertility Treatment in Gynecological Cancer Survivors: A Systematic Review" Journal of Clinical Medicine 13, no. 11: 3172. https://doi.org/10.3390/jcm13113172
APA StyleTsarna, E., Eleftheriades, A., Matsas, A., Triantafyllidou, O., & Christopoulos, P. (2024). Uterus Transplantation as Infertility Treatment in Gynecological Cancer Survivors: A Systematic Review. Journal of Clinical Medicine, 13(11), 3172. https://doi.org/10.3390/jcm13113172







