Intranasal Immunotherapy with M2 Macrophage Secretome Ameliorates Language Impairments and Autistic-like Behavior in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcomes
2.3. Conditioned Media of M2 Macrophages
2.4. Inhaled Immunotherapy (IIT)
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Children Included in the Study
3.2. Early Effects of Inhaled Immunotherapy
3.3. Long-Term Effects of Inhaled Immunotherapy
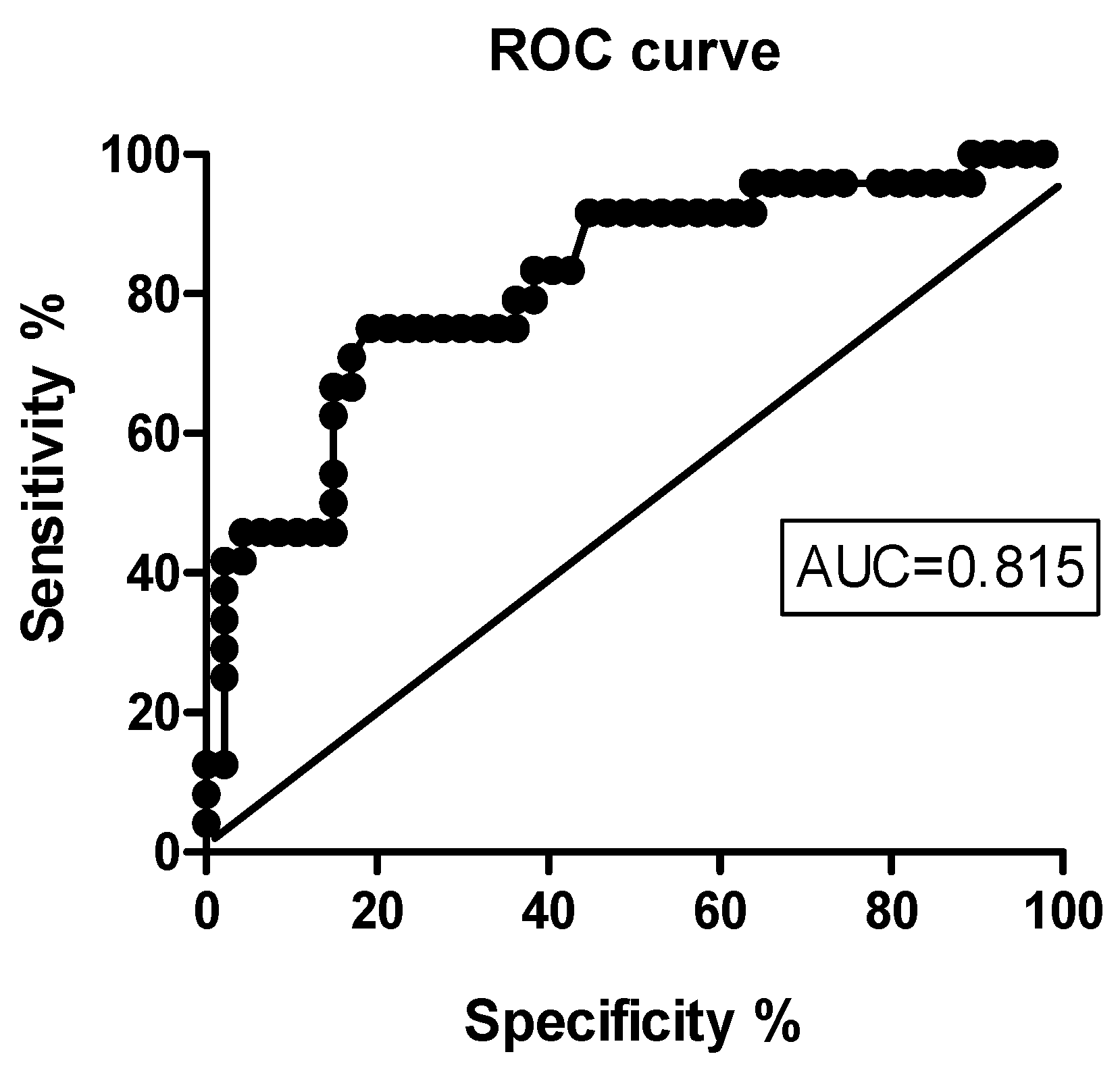
3.4. Predictors of Early Clinical Response to IIT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonneau, D.; Verny, C.; Uze, J. Genetics of specific language impairments. Arch. Pediatr. 2004, 11, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Van Handel, M.; Swaab, H.; de Vries, L.S.; Jongmans, M.J. Long-term cognitive and behavioural consequences of neonatal encephalopathy following perinatal asphyxia: A review. Eur. J. Pediatr. 2007, 166, 645–654. [Google Scholar] [CrossRef] [PubMed]
- McAdams, R.M.; Juul, S.E. The role of cytokines and inflammatory cells in perinatal brain injury. Neurol. Res. Int. 2012, 2012, 561494. [Google Scholar] [CrossRef] [PubMed]
- Amanollahi, M.; Jameie, M.; Heidari, A.; Rezaei, N. The dialogue between neuroinflammation and adult neurogenesis: Mechanisms involved and alterations in neurological diseases. Mol. Neurobiol. 2023, 60, 923–959. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Ying, C.; Zhang, J.; Zhang, H.; Gao, S.; Guo, X.; Lin, J.; Wu, H.; Hong, Y. Stem cells in central nervous system diseases: Promising therapeutic strategies. Exp. Neurol. 2023, 369, 114543. [Google Scholar] [CrossRef] [PubMed]
- Pinho, A.G.; Cibrão, J.R.; Silva, N.A.; Monteiro, S.; Salgado, A.J. Cell secretome: Basic insights and therapeutic opportunities for CNS disorders. Pharmaceuticals 2020, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Alcalá-Barraza, S.R.; Lee, M.S.; Hanson, L.R.; McDonald, A.A.; Frey, W.H.; McLoon, L.K. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J. Drug Target. 2010, 18, 179–190. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, Y.; Xu, G.; Liu, X. Intranasal administration: A potential solution for cross-BBB delivering neurotrophic factors. Histol. Histopathol. 2012, 27, 537–548. [Google Scholar]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H., 2nd. Delivery of IGF-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (Suppl. S3), S5. [Google Scholar] [CrossRef] [PubMed]
- Spetter, M.S.; Hallschmid, M. Intranasal neuropeptide administration to target the human brain in health and disease. Mol. Pharm. 2015, 12, 2767–2780. [Google Scholar] [CrossRef] [PubMed]
- Shevela, E.Y.; Sakhno, L.V.; Kafanova, M.Y.; Davydova, M.N.; Andrushkevich, M.M.; Ostanin, A.A.; Chernykh, E.R. A Method of Obtaining a Conditioned Medium with a Regenerative Potential for Intranasal Administration in the Treatment of Diseases of the Central Nervous System. Patent RF No. 2016121776, 1 June 2016. (In Russian). [Google Scholar]
- Fotekova, T.A. Test Method for the Diagnosis of Oral Speech in Primary Schoolchildren; ARKTM: Moscow, Russia, 2000; 55p, ISBN 978-5-8112-2884-3. (In Russian) [Google Scholar]
- Sakhno, L.V.; Shevela, E.Y.; Tikhonova, M.A.; Ostanin, A.A.; Chernykh, E.R. The phenotypic and functional features of human M2 macrophages generated under low serum conditions. Scand. J. Immunol. 2016, 83, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Deng, G.; Wang, S.; Li, S.; Song, P.; Lin, K.; Xu, X.; He, Z. Enhancing regenerative medicine: The crucial role of stem cell therapy. Front. Neurosci. 2024, 18, 1269577. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Kim, S.W.; Seok, S.H. A new era of macrophage-based cell therapy. Exp. Mol. Med. 2023, 55, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Hikawa, N.; Horie, H.; Takenaka, T. Macrophage-enhanced neurite regeneration of adult dorsal root ganglia neurones in culture. Neuroreport 1993, 5, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Chernykh, E.R.; Shevela, E.Y.; Sakhno, L.V.; Tikhonova, M.A.; Petrovsky, Y.L.; Ostanin, A.A. The generation and properties of human M2-like macrophages: Potential candidates for CNS repair? Cell. Ther. Transplant. 2010, 2, e.000080.01. [Google Scholar]
- Rashchupkin, I.M.; Shevela, E.Y.; Maksimova, A.A.; Tikhonova, M.A.; Ostanin, A.A.; Chernykh, E.R. Effect of differently polarized human macrophages on the SH-SY5Y cells damaged by ischemia and hypoxia in vitro. J. Immunol. Res. 2023, 2023, 5595949. [Google Scholar] [CrossRef]
- Markova, E.V.; Shevela, E.Y.; Knyazeva, M.A.; Savkin, I.V.; Serenko, E.V.; Rashchupkin, I.M.; Amstislavskaya, Т.G.; Ostanin, A.A.; Chernykh, E.R. Effect of M2 macrophage-derived soluble factors on behavioural patterns and cytokine production in various brain structures in depression-like mice. Bull. Exp. Biol. Med. 2022, 172, 341–344. [Google Scholar] [CrossRef]
- Maksimova, A.; Shevela, E.; Sakhno, L.; Tikhonova, M.; Ostanin, A.; Chernykh, E. Human Macrophages Polarized by Interaction with Apoptotic Cells Produce Fibrosis-Associated Mediators and Enhance Pro-Fibrotic Activity of Dermal Fibroblasts In Vitro. Cells 2023, 12, 1928. [Google Scholar] [CrossRef] [PubMed]
- Drago, D.; Cossetti, C.; Iraci, N.; Gaude, E.; Musco, G.; Bachi, A.; Pluchino, S. The stem cell secretome and its role in brain repair. Biochimie 2013, 95, 2271–2285. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Zhu, Y.; Sun, Y.; Mao, X.O.; Xie, L.; Greenberg, D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11946–11950. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jin, K.; Mao, X.O.; Greenberg, D.A. Vascular endothelial growth factor promotes proliferation of cortical neuron precursors by regulating E2F expression. FASEB J. 2003, 17, 186–193. [Google Scholar] [PubMed]
- Lange, C.; Storkebaum, E.; de Almodóvar, C.R.; Dewerchin, M.; Carmeliet, P. Vascular endothelial growth factor: A neurovascular target in neurological diseases. Nat. Rev. Neurol. 2016, 12, 439–454. [Google Scholar] [CrossRef]
- De Almodovar, C.R.; Lambrechts, D.; Mazzone, M.; Carmeliet, P. Role and therapeutic potential of VEGF in the nervous system. Physiol. Rev. 2009, 89, 607–648. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, K.; Xie, L.; Childs, J.; Mao, X.O.; Logvinova, A.; Greenberg, D.A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Investig. 2003, 111, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Louissaint, A., Jr.; Rao, S.; Leventhal, C.; Goldman, S.A. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 2002, 34, 945–960. [Google Scholar] [CrossRef]
- Horie, N.; Pereira, M.P.; Niizuma, K.; Sun, G.; Keren-Gill, H.; Encarnacion, A.; Shamloo, M.; Hamilton, S.A.; Jiang, K.; Huhn, S.; et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells 2015, 29, 274–285. [Google Scholar] [CrossRef]
- Ohm, J.E.; Gabrilovich, D.I.; Sempowski, G.D.; Kisseleva, E.; Parman, K.S.; Nadaf, S.; Carbone, D.P. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 2003, 101, 4878–4886. [Google Scholar] [CrossRef]
- Madathil, S.K.; Evans, H.N.; Saatman, K.E. Temporal and regional changes in IGF-1/IGF-1R signaling in the mouse brain after traumatic brain injury. J. Neurotrauma 2010, 27, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.L.; Xuan, S.; Dragatsis, I.; Efstratiadis, A.; Goldman, J.E. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J. Neurosci. 2003, 23, 7710–7718. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Fawcett, J.R.; Thorne, R.G.; DeFor, T.A.; Frey, W.H., 2nd. Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J. Neurol. Sci. 2001, 187, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, P.; Liao, B.; You, H.; Cai, Y. Effects and action mechanisms of individual cytokines contained in PRP on osteoarthritis. J. Orthop. Surg. Res. 2023, 18, 713. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Wang, J.; Wang, P.; Liu, X.; Liu, P.; Cheng, X.; Cao, L.; Wu, H.; Chen, J.; Zhou, L. Neural stem cell-derived exosomes and regeneration: Cell-free therapeutic strategies for traumatic brain injury. Stem Cell Res. Ther. 2023, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.; Chen, H.; Tang, J.; Jia, X. Exosomes and their microRNA cargo: New players in peripheral nerve regeneration. Neurorehabilit. Neural Repair 2018, 32, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Ma, C.B.; Yuan, H.M.; Cao, B.Y.; Zhu, J.J. Macrophage-derived microvesicles promote proliferation and migration of Schwann cell on peripheral nerve repair. Biochem. Biophys. Res. Commun. 2015, 468, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Si, Q.; Wu, L.; Pang, D.; Jiang, P. Exosomes in brain diseases: Pathogenesis and therapeutic targets. MedComm 2023, 4, e287. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Saha, P.; Kathuria, H.; Pandey, M.M. Intranasal nanotherapeutics for brain targeting and clinical studies in Parkinson’s disease. J. Control. Release 2023, 358, 293–318. [Google Scholar] [CrossRef]
- Wingrove, J.; Swedrowska, M.; Scherließ, R.; Parry, M.; Ramjeeawon, M.; Taylor, D.; Gauthier, G.; Brown, L.; Amiel, S.; Zelaya, F.; et al. Characterisation of nasal devices for delivery of insulin to the brain and evaluation in humans using functional magnetic resonance imaging. J. Control. Release 2019, 302, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.D.; Frey, W.H., 2nd; Craft, S.; Danielyan, L.; Hallschmid, M.; Schiöth, H.B.; Benedict, C. Intranasal treatment of central nervous system dysfunction in humans. Pharm. Res. 2013, 30, 2475–2484. [Google Scholar] [CrossRef]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef] [PubMed]
- Raghav, M.; Gupta, V.; Awasthi, R.; Singh, A.; Kulkarni, G.T. Nose-to-brain drug delivery: Challenges and progress towards brain targeting in the treatment of neurological disorders. J. Drug Deliv. Sci. Technol. 2023, 86, 104756. [Google Scholar] [CrossRef]
- Sorenson, K.; Kendall, E.; Grell, H.; Kang, M.; Shaffer, C.; Hwang, S. Intranasal oxytocin in pediatric populations: Exploring the potential for reducing irritability and modulating neural responses: A mini review. J. Psychiatr. Brain Sci. 2023, 8, e230008. [Google Scholar]
- Huang, Y.; Huang, X.; Ebstein, R.P.; Yu, R. Intranasal oxytocin in the treatment of autism spectrum disorders: A multilevel meta-analysis. Neurosci. Biobehav. Rev. 2021, 122, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Moerkerke, M.; Daniels, N.; Tibermont, L.; Tang, T.; Evenepoel, M.; van der Donck, S.; Debbaut, E.; Prinsen, J.; Chubar, V.; Claes, S.; et al. Chronic oxytocin administration stimulates the oxytocinergic system in children with autism. Nat. Commun. 2024, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Shpakov, A.O.; Zorina, I.I.; Derkach, K.V. Hot Spots for the Use of Intranasal Insulin: Cerebral Ischemia, Brain Injury, Diabetes Mellitus, Endocrine Disorders and Postoperative Delirium. Int. J. Mol. Sci. 2023, 24, 3278. [Google Scholar] [CrossRef]
- Zwanenburg, R.J.; Bocca, G.; Ruiter, S.A.; Dillingh, J.H.; Flapper, B.C.; van den Heuvel, E.R.; van Ravenswaaij-Arts, C.M. Is there an effect of intranasal insulin on development and behaviour in Phelan-McDermid syndrome? A randomized, double-blind, placebo-controlled trial. Eur. J. Hum. Genet. 2016, 24, 1696–1701. [Google Scholar] [CrossRef]
- Schmidt, H.; Kern, W.; Giese, R.; Hallschmid, M.; Enders, A. Intranasal insulin to improve developmental delay in children with 22q13 deletion syndrome: An exploratory clinical trial. J. Med. Genet. 2009, 46, 217–222. [Google Scholar] [CrossRef] [PubMed]
| Language Development Scale, LDS, Series | Score Me (IQR) | The Test Execution Percentage, PET (%) Me (IQR) |
|---|---|---|
| Series I (speech understanding) (max 33 points) | 5.0 (1.0–21.0) | 15.2 (3.0–63.6) |
| Series II (sensorimotor level of speech) (max 38 points) | 7.5 (2.2–23.7) | 19.7 (5.9–62.5) |
| Series III (grammatical structure of speech) (max 24 points) | 0 (0–5.0) | 0 (0–20.8) |
| Series IV (vocabulary and word formation skills) (max 17 points) | 0.7 (0–6.0) | 4.4 (0–35.3) |
| Series V (coherent speech) (max 25 points) | 0 (0.0–2.5) | 0 (0–10.0) |
| Series VI (general and fine motor skills) (max 23 points) | 11.0 (5.0–16.0) | 47.8 (21.7–69.6) |
| Total score (max 160 points) | 38.0 (16.0–65.0) | 23.9 (10.0–40.8) |
| Parameters Me (IQR) | Visit 1 (at Baseline, n = 71) | Visit 2 (in 1 Month, n = 71) | PU |
|---|---|---|---|
| LDS, series | |||
| Series I (speech understanding) (max 33 points) | 5.0 (1.0–21.0) | 5.2 (1.5–22.0) | 0.47 |
| Series II (sensorimotor level of speech) (max 38 points) | 7.5 (2.2–23.7) | 11.7 (3.0–27.7) | 0.26 |
| Series III (grammatical structure of speech) (max 24 points) | 0 (0–5.0) | 0 (0–8.0) | 0.55 |
| Series IV (vocabulary and word formation skills) (max 17 points) | 0.7 (0–6.0) | 1.0 (0–7.0) | 0.48 |
| Series V (coherent speech) (max 25 points) | 0 (0.0–2.5) | 0 (0–2.5) | 0.95 |
| Series VI (general and fine motor skills) (max 23 points) | 11.0 (5.0–16.0) | 12.5 (6.0–18.0) | 0.23 |
| Total score (max 160 points) | 38.0 (16.0–65.0) | 42.7 (18.2–75.7) | 0.29 |
| PET, % | 23.9 (10.0–40.8) | 26.7 (11.4–47.3) | 0.29 |
| CARS, points | 33.0 (28.0–39.0) | 29.0 (24.0–33.0) | 0.002 |
| SNAP-IV, points | 34.5 (31.0–39.0) | 28.5 (24.0–32.0) | 0.011 |
| What Are the Most Striking Positive Symptoms, in Your Opinion, That Appeared after Treatment: | n | % |
|---|---|---|
| Language impairments | ||
| 38/71 | 53 |
| 30/71 | 42 |
| 27/71 | 38 |
| 42/71 | 59 |
| 13/71 | 18 |
| 15/71 | 21 |
| 10/71 | 14 |
| 17/71 | 24 |
| 8/71 | 11 |
| 30/71 | 42 |
| Autism-like symptoms | ||
| 38/71 | 53 |
| 38/71 | 53 |
| 38/71 | 53 |
| 17/71 | 24 |
| 21/71 | 29 |
| 34/71 | 48 |
| 30/71 | 42 |
| 30/71 | 42 |
| 25/71 | 35 |
| 23/71 | 32 |
| 27/71 | 35 |
| 25/71 | 35 |
| 15/71 | 21 |
| 34/71 | 48 |
| Parameters Me (IQR) | Visit 1 (at Baseline) | Visit 2 (1 Month) | Visit 3 (6 Month) |
|---|---|---|---|
| LDS series | |||
| Series I (speech understanding) (max 33 points) | 17.0 (2.0–25.0) | 18.0 (3.0–33.0) | 21.0 (4.0–33.0) |
| Series II (sensorimotor level of speech) (max 38 points) | 10.7 (2.7–22.2) | 13.0 (5.7–27.5) | 18.5 (7.0–29.5) |
| Series III (grammatical structure of speech) (max 24 points) | 0 (0–5.0) | 1.2 (0–8.0) | 2.7 (0–10.0) |
| Series IV (vocabulary and word formation skills) (max 17 points) | 2.2 (0–8.0) | 3.5 (0–8.0) | 5.0 (0–10.2) |
| Series V (coherent speech) (max 25 points) | 0 (0–3.5) | 0 (0–5.0) | 0 (0–5.0) |
| Series VI (general and fine motor skills) (max 23 points) | 10.0 (5.0–14.7) | 12.5 (7.2–17.2) | 14.0 * (9.0–18.5) |
| Total score (max 160 points) | 45.5 (24.0–68.5) | 58.7 (26.7–83.2) | 64.5 * (33.2–89.0) |
| PET (%) | 28.4 (15.0–42.8) | 36.7 (16.7–52.0) | 40.3 * (20.8–55.6) |
| CARS, points | 35.0 (27.0–41.0) | 28.0 * (23.0–34.0) | 27.0 ** (21.0–33.0) |
| SNAP-IV, points | 32.5 (27.0–38.0) | 29.0 (22.0–35.0) | 28.0 * (19.0–31.0) |
| What Are the Most Striking Positive Symptoms, in Your Opinion, That Appeared after Treatment: | n | % |
|---|---|---|
| Language impairments | ||
| 32/39 | 82 |
| 26/39 | 66 |
| 32/39 | 82 |
| 32/39 | 82 |
| 23/39 | 59 |
| 10/39 | 25 |
| 10/39 | 25 |
| 20/39 | 51 |
| 10/39 | 25 |
| 23/39 | 59 |
| Autism-like symptoms | ||
| 23/39 | 59 |
| 35/39 | 90 |
| 23/39 | 59 |
| 6/39 | 15 |
| 16/39 | 41 |
| 19/39 | 49 |
| 19/39 | 49 |
| 19/39 | 49 |
| 13/39 | 33 |
| 23/39 | 59 |
| 29/39 | 74 |
| 23/39 | 59 |
| 6/39 | 15 |
| 23/39 | 59 |
| Parameters Me (IQR) | Responders (n = 47) | Non-Responders (n = 24) | PU |
|---|---|---|---|
| Age, years | 6.0 (4.4–7.0) | 6.0 (5.0–7.0) | 0.71 |
| CARS, points | 33.0 (27.0–38.0) | 35.0 (30.2–40.2) | 0.14 |
| SNAP-IV, points | 36.0 (26.0–39.0) | 34.0 (28.5–36.5) | 0.80 |
| Language development scale | |||
| Series I (max 33 points) | 16.0 (4.5–24.5) | 1.0 (0.1–2.0) | 0.0001 |
| Series II (max 38 points) | 16.0 (6.0–25.5) | 2.9 (1.2–13.7) | 0.004 |
| Series III (max 24 points) | 2.0 (0–10.2) | 0 (0.0–0.6) | 0.016 |
| Series IV (max 17 points) | 3.0 (0–8.0) | 0 (0–0) | 0.0001 |
| Series V (max 25 points) | 0 (0–5.0) | 0 (0–0) | 0.005 |
| Series VI (max 23 points) | 12.0 (6.0–16.0) | 6.5 (3.0–13.6) | 0.08 |
| Total score (max 160 points) | 54.0 (30.0–82.0) | 18.5 (4.7–31.0) | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevela, E.Y.; Loginova, T.A.; Munkuev, A.S.; Volskaya, T.E.; Sergeeva, S.A.; Rashchupkin, I.M.; Kafanova, M.Y.; Degtyareva, V.G.; Sosnovskaya, A.V.; Ostanin, A.A.; et al. Intranasal Immunotherapy with M2 Macrophage Secretome Ameliorates Language Impairments and Autistic-like Behavior in Children. J. Clin. Med. 2024, 13, 3079. https://doi.org/10.3390/jcm13113079
Shevela EY, Loginova TA, Munkuev AS, Volskaya TE, Sergeeva SA, Rashchupkin IM, Kafanova MY, Degtyareva VG, Sosnovskaya AV, Ostanin AA, et al. Intranasal Immunotherapy with M2 Macrophage Secretome Ameliorates Language Impairments and Autistic-like Behavior in Children. Journal of Clinical Medicine. 2024; 13(11):3079. https://doi.org/10.3390/jcm13113079
Chicago/Turabian StyleShevela, Ekaterina Ya., Tatiana A. Loginova, Alexandr S. Munkuev, Tatiana E. Volskaya, Svetlana A. Sergeeva, Ivan M. Rashchupkin, Marina Yu. Kafanova, Valentina G. Degtyareva, Anastasia V. Sosnovskaya, Alexandr A. Ostanin, and et al. 2024. "Intranasal Immunotherapy with M2 Macrophage Secretome Ameliorates Language Impairments and Autistic-like Behavior in Children" Journal of Clinical Medicine 13, no. 11: 3079. https://doi.org/10.3390/jcm13113079
APA StyleShevela, E. Y., Loginova, T. A., Munkuev, A. S., Volskaya, T. E., Sergeeva, S. A., Rashchupkin, I. M., Kafanova, M. Y., Degtyareva, V. G., Sosnovskaya, A. V., Ostanin, A. A., & Chernykh, E. R. (2024). Intranasal Immunotherapy with M2 Macrophage Secretome Ameliorates Language Impairments and Autistic-like Behavior in Children. Journal of Clinical Medicine, 13(11), 3079. https://doi.org/10.3390/jcm13113079






