Lung Ultrasound to Evaluate Fluid Status and Optimize Early Volume-Expansion Therapy in Children with Shiga Toxin-Producing Escherichia Coli–Haemolytic Uremic Syndrome: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Anthropometric Data at Tertiary Hospital Admission
2.3. Laboratory Measurement at Tertiary Hospital Admission
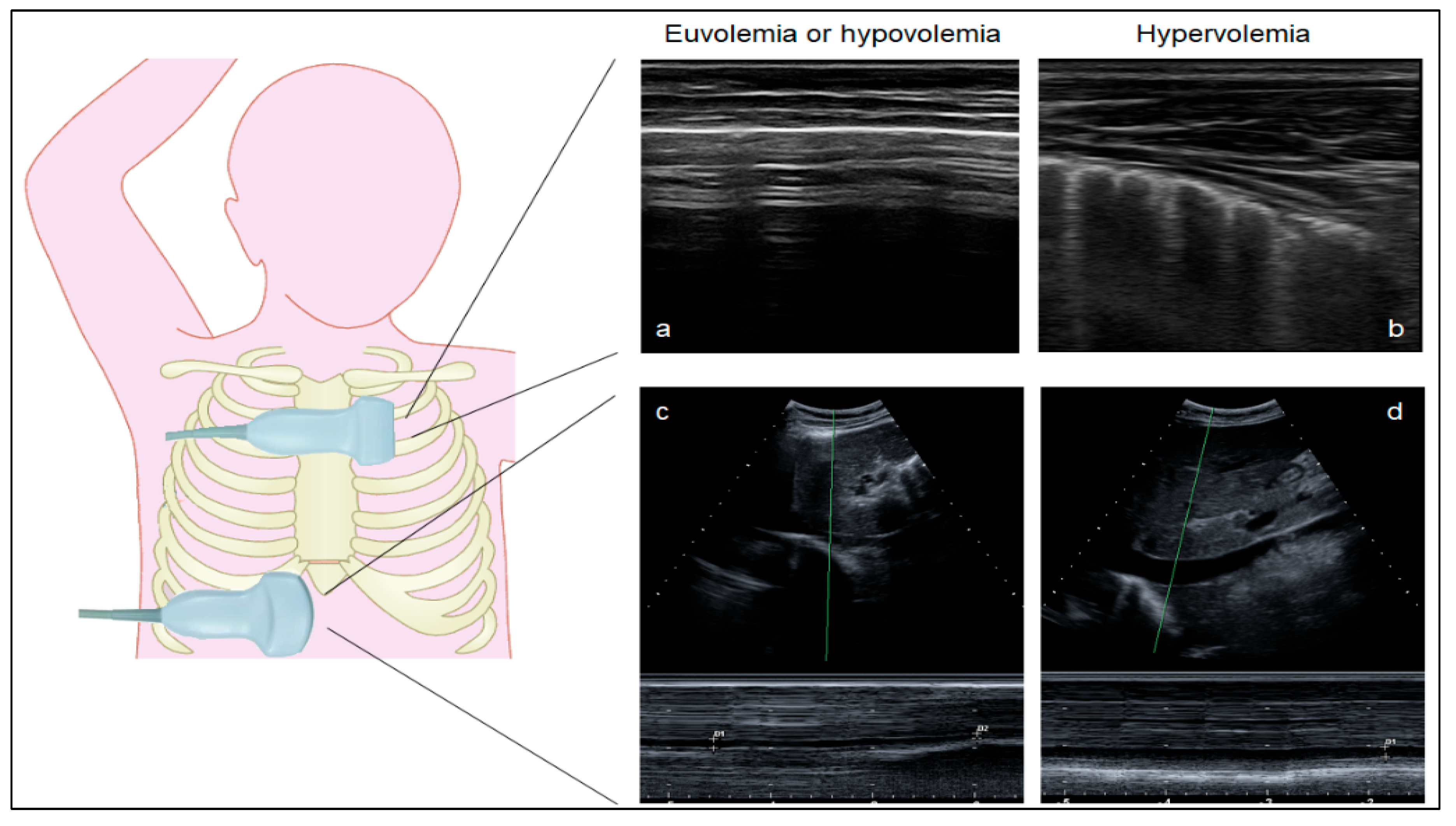
2.4. Ultrasound Measurements
2.5. Controls
2.6. Outcomes
2.7. Statistical Analysis
3. Results
3.1. Lung Ultrasound Can Distinguish Euvolemia and Lung Congestion in Children without STEC-HUS
3.2. Hypovolemia Is Frequent in Children with STEC-HUS, Irrespective of Massive Volume Administration
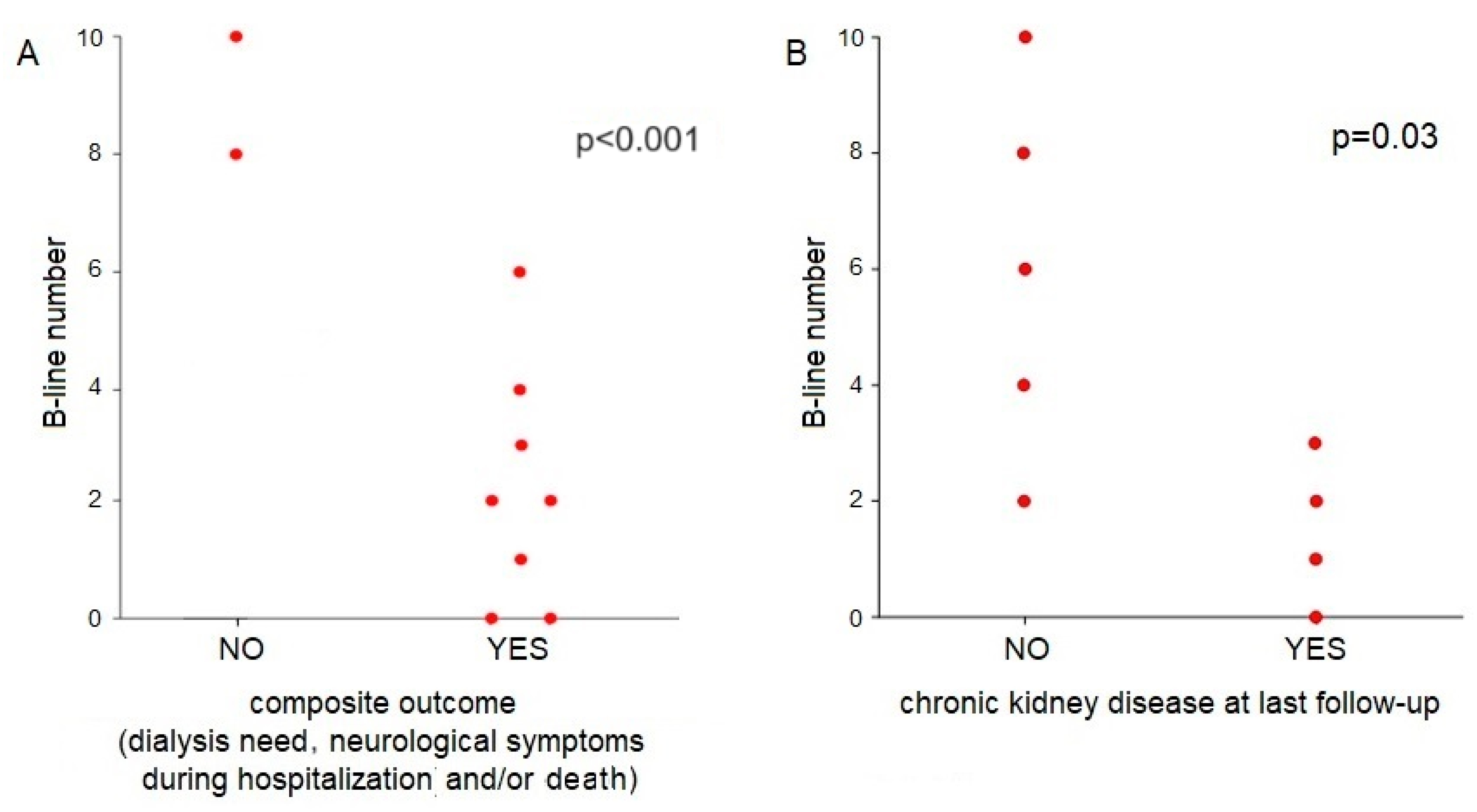
3.3. The Role of Lung Ultrasound and IVC Collapsibility Index in Children with STEC-HUS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fakhouri, F.; Zuber, J.; Frémeaux-Bacchi, V.; Loirat, C. Haemolytic uraemic syndrome. Lancet 2017, 390, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Oakes, R.S.; Siegler, R.L.; Mcreynolds, M.A.; Pysher, T.; Pavia, A.T. Predictors of Fatality in Postdiarrheal Hemolytic Uremic Syndrome. Pediatrics 2006, 117, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Coccia, P.A.; Ramírez, F.B.; Suárez, A.D.C.; Alconcher, L.F.; Balestracci, A.; Chervo, L.A.G.; Principi, I.; Vázquez, A.; Ratto, V.M.; Planells, M.C.; et al. Acute peritoneal dialysis, complications and outcomes in 389 children with STEC-HUS: A multicenter experience. Pediatr. Nephrol. 2021, 36, 1597–1606. [Google Scholar] [CrossRef]
- Garg, A.X.; Suri, R.S.; Barrowman, N.; Rehman, F.; Matsell, D.; Rosas-Arellano, M.P.; Salvadori, M.; Haynes, R.B.; Clark, W.F. Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: A systematic review, meta-analysis, and meta-regression. JAMA 2003, 290, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Grisaru, S.; Xie, J.; Samuel, S.; Hartling, L.; Tarr, P.I.; Schnadower, D.; Freedman, S.B.; Alberta Provincial Pediatric Enteric Infection for the Alberta Provincial Pediatric Enteric Infection Team. Associations between hydration status, intravenous fluid administration, and outcomes of patients infected with shiga toxin-producing escherichia coli a systematic review and meta-analysis. JAMA Pediatr. 2017, 171, 68–76. [Google Scholar] [CrossRef]
- Ardissino, G.; Daccò, V.; Testa, S.; Civitillo, C.F.; Tel, F.; Possenti, I.; Belingheri, M.; Castorina, P.; Bolsa-Ghiringhelli, N.; Tedeschi, S.; et al. Hemoconcentration: A major risk factor for neurological involvement in hemolytic uremic syndrome. Pediatr. Nephrol. 2015, 30, 345–352. [Google Scholar] [CrossRef]
- Ojeda, J.M.; Kohout, I.; Cuestas, E. Dehydration upon admission is a risk factor for incomplete recovery of renal function in children with haemolytic uremic syndrome. Nefrologia 2013, 33, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Loos, S.; Oh, J.; van de Loo, L.; Kemper, M.J.; Blohm, M.; Schild, R. Hemoconcentration and predictors in Shiga toxin-producing E. coli-hemolytic uremic syndrome (STEC-HUS). Pediatr. Nephrol. 2021, 36, 3777–3783. [Google Scholar] [CrossRef]
- Alconcher, L.F.; Coccia, P.A.; Suarez, A.d.C.; Monteverde, M.L.; Gutiérrez, M.G.P.Y.; Carlopio, P.M.; Missoni, M.L.; Balestracci, A.; Principi, I.; Ramírez, F.B.; et al. Hyponatremia: A new predictor of mortality in patients with Shiga toxin-producing Escherichia coli hemolytic uremic syndrome. Pediatr. Nephrol. 2018, 33, 1791–1798. [Google Scholar] [CrossRef]
- Dull, R.O.; Hahn, R.G. Hypovolemia with peripheral edema: What is wrong? Crit. Care 2023, 27, 206. [Google Scholar] [CrossRef]
- Ake, J.A.; Jelacic, S.; Ciol, M.A.; Watkins, S.L.; Murray, K.F.; Christie, D.L.; Klein, E.J.; Tarr, P.I. Relative nephroprotection during Escherichia coli O157:H7 infections: Association with intravenous volume expansion. Pediatrics 2005, 115, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Ardissino, G.; Tel, F.; Possenti, I.; Testa, S.; Consonni, D.; Paglialonga, F.; Salardi, S.; Borsa-Ghiringhelli, N.; Salice, P.; Tedeschi, S.; et al. Early volume expansion and outcomes of hemolytic uremic syndrome. Pediatrics 2016, 137, e20152153. [Google Scholar] [CrossRef] [PubMed]
- Bonany, P.; Bilkis, M.D.; Iglesias, G.; Braun, A.; Tello, J.; Ratto, V.; Vargas, A.; Koch, E.; Jannello, P.; Monteverde, E. Fluid restriction versus volume expansion in children with diarrhea-associated HUS: A retrospective observational study. Pediatr. Nephrol. 2021, 36, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Grisaru, S. Management of hemolytic-uremic syndrome in children. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 231–239. [Google Scholar] [CrossRef] [PubMed]
- La Via, L.; Vasile, F.; Perna, F.; Zawadka, M. Prediction of fluid responsiveness in critical care: Current evidence and future perspective. Trends Anaest. Crit. Care 2024, 54, 101316. [Google Scholar] [CrossRef]
- Panuccio, V.; Tripepi, R.; Parlongo, G.; Mafrica, A.; Caridi, G.; Catalano, F.; Marino, F.; Tripepi, G.; Mallamaci, F.; Zoccali, C. Lung ultrasound to detect and monitor pulmonary congestion in patients with acute kidney injury in nephrology wards: A pilot study. J. Nephrol. 2020, 33, 335–341. [Google Scholar] [CrossRef]
- Allinovi, M.; Saleem, M.; Romagnani, P.; Nazerian, P.; Hayes, W. Lung ultrasound: A novel technique for detecting fluid overload in children on dialysis. Nephrol. Dial. Transplant. 2017, 32, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Garnier, A.; Brochard, K.; Kwon, T.; Sellier-Leclerc, A.L.; Lahoche, A.; Launay, E.A.; Nobili, F.; Caillez, M.; Taque, S.; Harambat, J.; et al. Efficacy and Safety of Eculizumab in Pediatric Patients Affected by Shiga Toxin-Related Hemolytic and Uremic Syndrome: A Randomized, Placebo-Controlled Trial. J. Am. Soc. Nephrol. 2023, 34, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Rao, G. Diagnosis, Epidemiology, and Management of Hypertension in Children. Pediatrics 2016, 138, e20153616. [Google Scholar] [CrossRef]
- Allinovi, M.; Saleem, M.A.; Burgess, O.; Armstrong, C.; Hayes, W. Finding covert fluid: Methods for detecting volume overload in children on dialysis. Pediatr. Nephrol. 2016, 31, 2327–2335. [Google Scholar] [CrossRef]
- Haskin, O.; Falush, Y.; Davidovits, M.; Alfandary, H.; Levi, S.; Berant, R. Use of Point-of-Care Ultrasound for Evaluation of Extravascular and Intravascular Fluid Status in Pediatric Patients Maintained on Chronic Hemodialysis. Blood Purif. 2021, 51, 321–327. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, Z.; Fan, J.; Ling, C.; Wang, X.; Liu, X.; Shen, Y. Lung ultrasound methods for assessing fluid volume change and monitoring dry weight in pediatric hemodialysis patients. Pediatr. Nephrol. 2021, 36, 969–976. [Google Scholar] [CrossRef]
- Haciomeroglu, P.; Ozkaya, O.; Gunal, N.; Baysal, K. Venous collapsibility index changes in children on dialysis. Nephrology 2007, 12, 135–139. [Google Scholar] [CrossRef]
- Cosgun, Z.; Dagistan, E.; Cosgun, M.; Ozturk, H. Can Inferior Vena Cava Diameter and Collapsibility Index Be a Predictor in Detecting Preoperative Intravascular Volume Change in Pediatric Patients? J. Cardiovasc. Emergencies 2021, 7, 47–51. [Google Scholar] [CrossRef]
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [CrossRef]
- Marzuillo, P.; Guarino, S.; Apicella, A.; Marotta, R.; Tipo, V.; Perrone, L.; La Manna, A.; Montini, G. Assessment of Volume Status and Appropriate Fluid Replenishment in the Setting of Nephrotic Syndrome. J. Emerg. Med. 2017, 52, e149–e152. [Google Scholar] [CrossRef] [PubMed]
- Trezzi, M.; Torzillo, D.; Ceriani, E.; Costantino, G.; Caruso, S.; Damavandi, P.T.; Genderini, A.; Cicardi, M.; Montano, N.; Cogliati, C. Lung ultrasonography for the assessment of rapid extravascular water variation: Evidence from hemodialysis patients. Intern. Emerg. Med. 2013, 8, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Noble, V.E.; Murray, A.F.; Capp, R.; Sylvia-Reardon, M.H.; Steele, D.J.R.; Liteplo, A. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis: Time course for resolution. Chest 2009, 135, 1433–1439. [Google Scholar] [CrossRef]
- Torino, C.; Gargani, L.; Sicari, R.; Letachowicz, K.; Ekart, R.; Fliser, D.; Covic, A.; Siamopoulos, K.; Stavroulopoulos, A.; Massy, Z.A.; et al. The agreement between auscultation and lung ultrasound in hemodialysis patients: The LUST study. Clin. J. Am. Soc. Nephrol. 2016, 11, 2005–2011. [Google Scholar] [CrossRef]
- Oualha, M.; Pierrepont, S.; Krug, P.; Gitiaux, C.; Hubert, P.; Lesage, F.; Salomon, R. Postdiarrheal hemolytic and uremic syndrome with severe multiorgan involvement and associated early risk factors. Arch. Pediatr. 2018, 25, 118–125. [Google Scholar] [CrossRef]
- Wiedermann, C.J.; Wiedermann, W.; Joannidis, M. Causal relationship between hypoalbuminemia and acute kidney injury. World J. Nephrol. 2017, 6, 176. [Google Scholar] [CrossRef] [PubMed]
- Parri, N.; Allinovi, M.; Giacalone, M.; Corsini, I. To B or not to B. The rationale for quantifying B-lines in pediatric lung diseases. Pediatr. Pulmonol. 2023, 58, 9–15. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 10) | Clinically Hypovolemic Patients (n = 4) | Clinically Euvolemic Patients (n = 6) | |
|---|---|---|---|
| Characteristics at ED admission | |||
| Age (years) | 3.9 (1.4–9.5) | 5.5 (3.2–9.5) | 2.9 (1.4–5.5) |
| Number of B-lines | 3.6 (0–10) | 5.3 (1–10) | 2.5 (0–8) |
| IVCD max (mm) | 6.5 (3–11.2) | 9 (5.5–11.2) | 7.4 (3–10.8) |
| IVCD min (mm) | 4.5 (0.5–7.5) | 4.7 (1.5–7.3) | 4.5 (0.5–7.5) |
| IVC-CI (%) | 49 (25–83) | 50 (35–73) | 48 (25–83) |
| EV+ | 6 (60) | 2 (50) | 4 (66) |
| Hypertension | 4 (40) | 0 | 4 (66) |
| Oligoanuria | 8 (80) | 4 (100) | 4 (66) |
| Serum laboratory parameters at onset | |||
| creatinine (mg/dL) | 3.5 (0.7–11.4) | 4.9 (0.8–11.4) | 2.6 (0.7–3.6) |
| urea/creatinine ratio | 73.6 (28–122) | 68 (47–120) | 78 (28–122) |
| Na (mEq/L) | 134 (126–142) | 131 (126–138) | 135 (131–142) |
| Albumin (g/dL) | 2.4 (1.6–3.3) | 2.3 (1.6–3.1) | 2.5 (1.8–3.3) |
| Complications | |||
| Dialysis need during hospitalization | 8 (80) | 3 (75) | 5 (83) |
| Death during hospitalization | 1 (10) | 0 | 1 (17) |
| Neurological involvement during hospitalization | 3 (33) | 2 (50) | 1 (17) |
| CKD at last follow-up | 4/9 (44) | 3/4 (75) | 1/5 (20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allinovi, M.; Farella, I.; Giacalone, M.; Lugli, G.; Cirillo, L.; Parri, N.; Becherucci, F. Lung Ultrasound to Evaluate Fluid Status and Optimize Early Volume-Expansion Therapy in Children with Shiga Toxin-Producing Escherichia Coli–Haemolytic Uremic Syndrome: A Pilot Study. J. Clin. Med. 2024, 13, 3024. https://doi.org/10.3390/jcm13113024
Allinovi M, Farella I, Giacalone M, Lugli G, Cirillo L, Parri N, Becherucci F. Lung Ultrasound to Evaluate Fluid Status and Optimize Early Volume-Expansion Therapy in Children with Shiga Toxin-Producing Escherichia Coli–Haemolytic Uremic Syndrome: A Pilot Study. Journal of Clinical Medicine. 2024; 13(11):3024. https://doi.org/10.3390/jcm13113024
Chicago/Turabian StyleAllinovi, Marco, Ilaria Farella, Martina Giacalone, Gianmarco Lugli, Luigi Cirillo, Niccolò Parri, and Francesca Becherucci. 2024. "Lung Ultrasound to Evaluate Fluid Status and Optimize Early Volume-Expansion Therapy in Children with Shiga Toxin-Producing Escherichia Coli–Haemolytic Uremic Syndrome: A Pilot Study" Journal of Clinical Medicine 13, no. 11: 3024. https://doi.org/10.3390/jcm13113024
APA StyleAllinovi, M., Farella, I., Giacalone, M., Lugli, G., Cirillo, L., Parri, N., & Becherucci, F. (2024). Lung Ultrasound to Evaluate Fluid Status and Optimize Early Volume-Expansion Therapy in Children with Shiga Toxin-Producing Escherichia Coli–Haemolytic Uremic Syndrome: A Pilot Study. Journal of Clinical Medicine, 13(11), 3024. https://doi.org/10.3390/jcm13113024







