ROX Index Variation as a Predictor of Outcomes in COVID-19 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. Information Collected
2.3. Analysis Plan
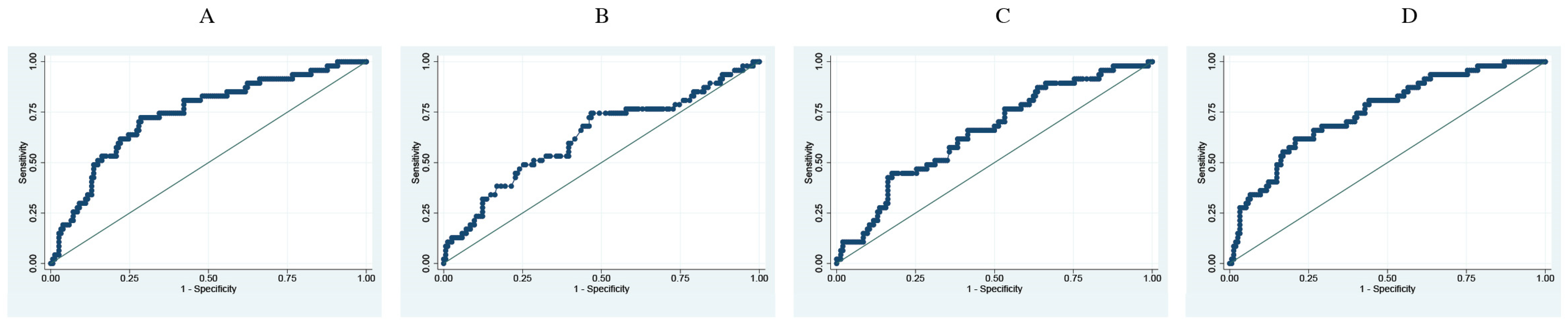
3. Results
3.1. Characteristics of the Population
3.2. Respiratory Associated Factors for Death and Mechanical Ventilation
3.3. Prediction Analysis of Respiratory Parameters and Difference between RI at Entry versus 24 h Later for Risk of Death and Mechanical Ventilation
3.4. Association between 24 h Changes in Respiratory ROX Index and Death
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA—J. Am. Med. Assoc. 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, D.; Fowler, E.J.; Abrams, M.; Collins, S.R. COVID-19—Implications for the Health Care System. N. Engl. J. Med. 2020, 383, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Excess Deaths Associated with COVID-19, January 2020–December 2021 [Internet]. WHO. 2022. Available online: https://www.who.int/data/stories/global-excess-deaths-associated-with-COVID-19-january-2020-december-2021 (accessed on 19 June 2022).
- Torres, I.; Sacoto, F. Localising an asset-based COVID-19 response in Ecuador. Lancet 2020, 395, 1339. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, A.; de Vasconcellos, K.; Abdulatif, M. COVID-19 in Africa: Current difficulties and future challenges considering the ACCCOS study. Anaesth. Crit. Care Pain Med. 2021, 40, 100912. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Center for Systems Science and Engineering. Coronavirus COVID-19 (2019-nCoV). Available online: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467 (accessed on 19 June 2022).
- Cheung, J.C.H.; Ho, L.T.; Cheng, J.V.; Cham, E.Y.K.; Lam, K.N. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir. Med. 2020, 8, e19. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.; Swamy, L.; Gavin, D.; Theodore, A. Mechanical-Ventilation Supply and Options for the COVID-19 Pandemic Leveraging All Available Resources for a Limited Resource in a Crisis. Ann. Am. Thorac. Soc. 2021, 18, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Qiu, H.; Wan, L.; Ai, Y.; Xue, Z.; Guo, Q.; Deshpande, R.; Zhang, L.; Meng, J.; Tong, C.; et al. Intubation and Ventilation amid the COVID-19 Outbreak. Anesthesiology 2020, 132, 1317–1332. [Google Scholar] [CrossRef] [PubMed]
- Sheata, I.M.; Smith, S.R.; Kamel, H.; Varrassi, G.; Imani, F.; Dayani, A.; Myrcik, D.; Urits, I.; Viswanath, O.; Taha, S.S. Pulmonary Embolism and Cardiac Tamponade in Critical Care Patients with COVID-19; Telemedicine’s Role in Developing Countries: Case Reports and Literature Review. Anesth. Pain Med. 2021, 11, e113752. [Google Scholar]
- Mohammadi, M.; Khamseh, A.K.P.; Varpaei, H.A. Invasive Airway “Intubation” in COVID-19 Patients; Statistics, Causes, and Recommendations: A Review Article. Anesth. Pain Med. 2021, 11, e115868. [Google Scholar] [CrossRef]
- Patel, M.; Chowdhury, J.; Mills, N.; Marron, R.; Gangemi, A.; Dorey-Stein, Z.; Yousef, I.; Zheng, M.; Tragesser, L.; Giutrintano, J.; et al. ROX Index Predicts Intubation in Patients with COVID-19 Pneumonia and Moderate to Severe Hypoxemic Respiratory Failure Receiving High Flow Nasal Therapy. medRxiv 2020. [Google Scholar] [CrossRef]
- Roca, O.; Caralt, B.; Messika, J.; Samper, M.; Sztrymf, B.; Hernández, G.; García-De-Acilu, M.; Frat, J.-P.; Masclans, J.R.; Ricard, J.-D. An Index Combining Respiratory Rate and Oxygenation to Predict Outcome of Nasal High-Flow Therapy. Am. J. Respir. Crit. Care Med. 2019, 199, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-González, A.D.; Vázquez, L.V.; Malmierca, A.B.; Gómez, I.V.; Adán, A.M.; Santana, R.S.D. APACHE II como predictor de mortalidad en una unidad de cuidados intensivos. Rev. Cuba. Med. Intensiv. Emerg. 2020, 19, e739. [Google Scholar]
- Gianstefani, A.; Artesiani, M.L.; Nava, S. Role of ROX Index in the First Assessment of COVID-19 Patients in the Emergency Department. Available online: https://www.researchsquare.com (accessed on 15 January 2022).
- Roca, O.; Messika, J.; Caralt, B.; García-De-Acilu, M.; Sztrymf, B.; Ricard, J.-D.; Masclans, J.R. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: The utility of the ROX index. J. Crit. Care 2016, 35, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Mauri, T.; Carlesso, E.; Spinelli, E.; Turrini, C.; Corte, F.D.; Russo, R.; Ricard, J.-D.; Pesenti, A.; Roca, O.; Grasselli, G. Increasing support by nasal high flow acutely modifies the ROX index in hypoxemic patients: A physiologic study. J. Crit. Care 2019, 53, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.X.; Hsieh, B.; Xiong, Z.; Halsey, K.; Choi, J.W.; Tran, T.M.L.; Pan, I.; Shi, L.-B.; Wang, D.-C.; Mei, J.; et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology 2020, 296, E46–E54. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, B.T.; Fiksel, J.; Johnson, B.A.; Rennert, L.; Chan, J.; Kho, A.; Akhtar, Z.; Hage, R.; Taljaard, M.; Payette, M.; et al. Severity of COVID-19 respiratory illness and imaging correlation. Nat. Commun. 2020, 11, 5333. [Google Scholar]
- Powell, T.; Christ, K.C.; Birkhead, G.S. Allocation of ventilators in a public health disaster. Disaster Med. Public Health Prep. 2008, 2, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, M.O. Public Health Disasters. In Advancing Global Bioethics; Springer Science and Business Media B.V.: Dordrecht, The Netherlands, 2018; pp. 1–24. [Google Scholar]
- Vega, M.L.; Dongilli, R.; Olaizola, G.; Colaianni, N.; Sayat, M.C.; Pisani, L.; Romagnoli, M.; Spoladore, G.; Prediletto, I.; Montiel, G.; et al. COVID-19 Pneumonia and ROX index: Time to set a new threshold for patients admitted outside the ICU. Pulmonology 2022, 28, 13–17. [Google Scholar] [CrossRef]
- Basoulis, D.; Avramopoulos, P.; Aggelara, M.; Karamanakos, G.; Voutsinas, P.-M.; Karapanou, A.; Psichogiou, M.; Samarkos, M.; Ntziora, F.; Sipsas, N.V. Validation of Sequential ROX-Index Score Beyond 12 Hours in Predicting Treatment Failure and Mortality in COVID-19 Patients Receiving Oxygen via High-Flow Nasal Cannula. Charbonney E., editor. Can. Respir. J. 2023, 2023, 7474564. [Google Scholar] [CrossRef] [PubMed]
- Vanni, G.; Viviani, M.; Barni, S.; Barbensi, E.; Prosperi, D.; Messina, A.; Brogioni, S.; Zatteri, S. The ROX index can support ED clinicians in stratifying COVID-19 patients’ risk of deterioration. Am. J. Emerg. Med. 2021, 49, 146–151. [Google Scholar]
- Bartoletti, M.; Giannasi, G.; Scudeller, L. Evaluation of the ROX Index as a Predictor of Outcome and Intubation in COVID-19 Adult Patients. Pathogens 2021, 10, 1095. [Google Scholar]
- West, J.B. Highest permanent human habitation. Physiology 2002, 17, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Camayo, J.; Mejia, C.R.; Callacondo, D.; Dawson, J.A.; Posso, M.; Galvan, C.A.; Davila-Arango, N.; Bravo, E.A.; Loescher, V.Y.; Padilla-Deza, M.M.; et al. Reference values for oxygen saturation from sea level to the highest human habitation in the Andes in acclimatised persons. Thorax 2018, 73, 776–778. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. High-altitude medicine. Am. J. Respir. Crit. Care Med. 2012, 186, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Chowdhury, Y.S. Fraction of Inspired Oxygen. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- O’Driscoll, B.R.; Howard, L.S.; Earis, J.; Mak, V. British Thoracic Society Guideline for oxygen use in adults in healthcare and emergency settings. BMJ Open Resp. Res. 2017, 4, e000170. [Google Scholar] [CrossRef] [PubMed]

| Death | p-Value | Mechanic Ventilation | p-Value | ||||
|---|---|---|---|---|---|---|---|
| n = 148 | n = 56 | n = 157 | n = 47 | ||||
| No | Yes | No | Yes | ||||
| Age | Mean; SD | 55.8; 1.4 | 60.6; 2.1 | 0.06 | 57.6; 1.4 | 55.6; 1.8 | 0.47 |
| Sex | Male | 83 (56.1) | 40 (71.4) | 0.05 | 89 (56.7) | 34 (72.3) | 0.05 |
| Comorbidities | Diabetes mellitus | 20 (13.5) | 8 (14.3) | 0.88 | 23 (14.7) | 5 (10.6) | 0.48 |
| Hypertension | 33 (22.3) | 11 (19.6) | 0.68 | 34 (21.7) | 10 (21.3) | 0.95 | |
| Lung disease | 6 (4.1) | 3 (5.4) | 0.68 | 5 (3.2) | 4 (8.5) | 0.12 | |
| Hipotiroidism | 6 (4.1) | 3 (5.4) | 0.68 | 5 (3.2) | 4 (8.5) | 0.12 | |
| Renal disease | 6 (4.1) | 3 (5.4) | 0.68 | 5 (3.2) | 4 (8.5) | 0.12 | |
| Surgical history | 5 (3.4) | 0 | na | 5 (3.2) | 0 | 0.21 | |
| Cerebral vascular disease | 2 (1.35) | 1 (1.8) | 0.81 | 2 (1.3) | 1 (2.1) | 0.7 | |
| Obesity | 3 (2) | 2 (3.6) | 0.52 | 3 (1.9) | 2 (4.3) | 0.36 | |
| Any comorbidity | 66 (44.6) | 21 (37.5) | 0.36 | 67 (42.7) | 20(42.5) | 0.98 | |
| Symptoms/signs | Fever | 74 (50) | 32 (57.1) | 0.36 | 75 (47.8) | 23 (49) | 0.89 |
| Cough | 97 (65.5) | 31 (55:4) | 0.18 | 97 (61.8) | 31 (65.9) | 0.6 | |
| Dyspnea | 103 (69.6) | 43 (76.8) | 0.31 | 107 (68.1) | 39 (83) | 0.05 | |
| Myalgias | 68 (45.9) | 25 (44.6) | 0.86 | 77 (49) | 16 (34) | 0.07 | |
| CORADS | Grade 1–2 | 2 (1.4) | 1 (1.8) | 3 (1.9) | 0 | ||
| Grade 3–4 | 9 (6.1) | 2 (3.5) | 9 (5.7) | 2 (4.3) | |||
| Grade 5–6 | 137 (92.6) | 56 (94.7) | 0.76 | 145 (92.4) | 45 (95.7) | 0.6 | |
| FiO2 at Admission | 0.21 | 135 (91.2) | 46 (82.1) | 142 (90.5) | 39 (83) | ||
| 0.22–0.4 | 12 (8.1) | 4 (7.1) | 13 (8.3) | 3 (6.4) | |||
| >0.4 | 1 (0.7) | 6 (10.8) | 0.02 | 2 (1.2) | 5 (10.6) | 0.008 | |
| FiO2 at 24 h | 0.21 | 95 (64.2) | 0 | 89 (56.7) | 6 (12.8) | ||
| 0.22–0.4 | 49 (33.1) | 6 (10.7) | 51.32.5) | 4 (8.5) | |||
| >0.4 | 4 (2.7) | 50 (89.3) | <0.0001 | 17 (10.8) | 37 (78.7) | <0.0001 | |
| Death | Mechanic Ventilation | |||||
|---|---|---|---|---|---|---|
| Survived | Deceased | p Value | Absent | Present | p Value | |
| n = 148 | n = 56 | n = 157 | n = 47 | |||
| Age: mean; SD | 55.8; 16.5 | 60.5; 15.9 | 0.06 | 57.6; 17.3 | 55.6; 12.8 | 0.47 |
| Disease time: mean; SD | 7.3; 4.7 | 7.5; 5.3 | 0.72 | 7.15;5.1 | 7.9; 4.26 | 0.34 |
| Hospitalization time: mean; SD | 9.1; 9.8 | 10.3; 10 | 0.46 | 6.9 (5.8) | 18.3; 14.7 | <0.0001 |
| Sat O2 entry: mean; SD | 79.7; 0.95 | 67.7; 19.8 | 0.0001 | 78.8; 13.3 | 68.4; 18.3 | <0.0001 |
| Sat O2 24 h: mean; SD | 91.6; 2.2 | 82.4; 10.4 | <0.0001 | 89.9; 6.7 | 86.2; 7.6 | 0.0016 |
| Difference sat O2: mean; SD | −11.9; 11.3 | −14.6; 22.1 | 0.37 | −11.1; 13.2 | −17.8; 19.2 | 0.007 |
| FiO2 admission, mean; SD | 0.22; 0.05 | 0.27; 0.17 | 0.04 | 0.22; 0.07 | 0.26; 0.16 | 0.02 |
| FiO2 24 h, mean; SD | 0.24; 0.1 | 0.70; 0.23 | <0.0001 | 0.29; 0.18 | 0.62; 0.27 | <0.0001 |
| Resp. Freq. admission | 27.6; 7.6 | 31.6; 8.9 | 0.004 | 27.9; 7.7 | 31.3; 9.1 | 0.01 |
| Resp. Freq. 24 h | 22.7; 6.2 | 30.2; 7.8 | <0.0001 | 23.5; 5.7 | 28.8; 10.6 | <0.0001 |
| ROX Index admission | 14.3; 4.5 | 9.8: 4.8 | <0.0001 | 14; 4.9 | 10; 4.2 | <0.0001 |
| ROX Index 24 h | 18.54; 4.2 | 4.99; 2.99 | <0.0001 | 17.1; 6 | 7.4; 5.8 | <0.0001 |
| Difference ROX Index mean; SD | −4.3; 5.3 | 4.8: 4.9 | <0.0001 | −3.06; 6.13 | 2.6; 6.2 | <0.0001 |
| Parameter | Death | Mechanic Ventilation | ||||
|---|---|---|---|---|---|---|
| AUC | Sensitivity | Specificity | AUC | Sensitivity | Specificity | |
| O2 sat admission | 0.7410 | 60.71 | 77.70 | 0.7352 | 72.34 | 69.42 |
| FiO2 admission | 0.6761 | 62.5 | 64.19 | 0.6237 | 76.59 | 53.5 |
| Resp. Freq admission | 0.6792 | 91.07 | 39.86 | 0.6434 | 76.59 | 53.5 |
| ROX Index | 0.7868 | 75 | 72.97 | 0.7616 | 74.47 | 70.06 |
| Difference in ROX Index | 0.92 | 0.89 | 0.80 | 0.75 | 57 | 83 |
| Functional Respiratory Category | Admission | 24 h |
|---|---|---|
| n (%) | n (%) | |
| ≥20 Normal | 17 (8.3) | 54 (26.5) |
| 15 to 19.9 Mild | 60 (29.4) | 71 (34.8) |
| 10 to 14.9 Moderate | 64 (31.4) | 24 (11.8) |
| <10 Severe | 63 (30.9) | 55 (26.9) |
| Death | Mechanic Ventilation | |||||||
|---|---|---|---|---|---|---|---|---|
| ROX Index 24 h change | Survive | Death | Crude OR (95%CI) | Adj. OR (95%CI) | no MV | MV | Crude OR (95%CI) | Adj. OR (95%CI) |
| Improvement | 87 (97.7) | 2 (2.3) | 1 | 1 | 81 (91) | 8 (9) | 1 | 1 |
| No change | 46 (58.2) | 33 (41.8) | 31.2 (7.2–135.9) | 46 (9.8–216.2) | 55 (69.6) | 24 (30.4) | 4.4 (1.9–10.5) | 4.4 (1.8–10.7) |
| Deteriorating 1 category | 14 (53.8) | 12 (46.2) | 37.3 (7.5–184.6) | 38 (7.1–199.8) | 17 (65.4) | 9 (34.6) | 5.4 (1.8–15.9) | 5.2 (1.71–15.6) |
| Deteriorating 2 categories | 1 (10) | 9 (90) | 391 (32.2–4753.4) | 674 (47.5–9564) | 4 (40) | 6 (60) | 15.2 (3.5–65.3) | 12.9 (2.9–56.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado, A.; Endara, P.; Abril, P.; Carrión, H.; Largo, C.; Benavides, P. ROX Index Variation as a Predictor of Outcomes in COVID-19 Patients. J. Clin. Med. 2024, 13, 3025. https://doi.org/10.3390/jcm13113025
Maldonado A, Endara P, Abril P, Carrión H, Largo C, Benavides P. ROX Index Variation as a Predictor of Outcomes in COVID-19 Patients. Journal of Clinical Medicine. 2024; 13(11):3025. https://doi.org/10.3390/jcm13113025
Chicago/Turabian StyleMaldonado, Augusto, Pablo Endara, Patricio Abril, Henry Carrión, Carolina Largo, and Patricia Benavides. 2024. "ROX Index Variation as a Predictor of Outcomes in COVID-19 Patients" Journal of Clinical Medicine 13, no. 11: 3025. https://doi.org/10.3390/jcm13113025
APA StyleMaldonado, A., Endara, P., Abril, P., Carrión, H., Largo, C., & Benavides, P. (2024). ROX Index Variation as a Predictor of Outcomes in COVID-19 Patients. Journal of Clinical Medicine, 13(11), 3025. https://doi.org/10.3390/jcm13113025






