Systemic Manifestations of COPD and the Impact of Dual Bronchodilation with Tiotropium/Olodaterol on Cardiac Function and Autonomic Integrity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Blood Biomarkers’ Testing
2.3. Pulmonary Function Tests
2.4. Cardiac Magnetic Resonance Imaging
2.5. Cardiac 123I-Metaiodobenzylguanidine Imaging
2.6. Interventions and Follow-Up
2.7. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
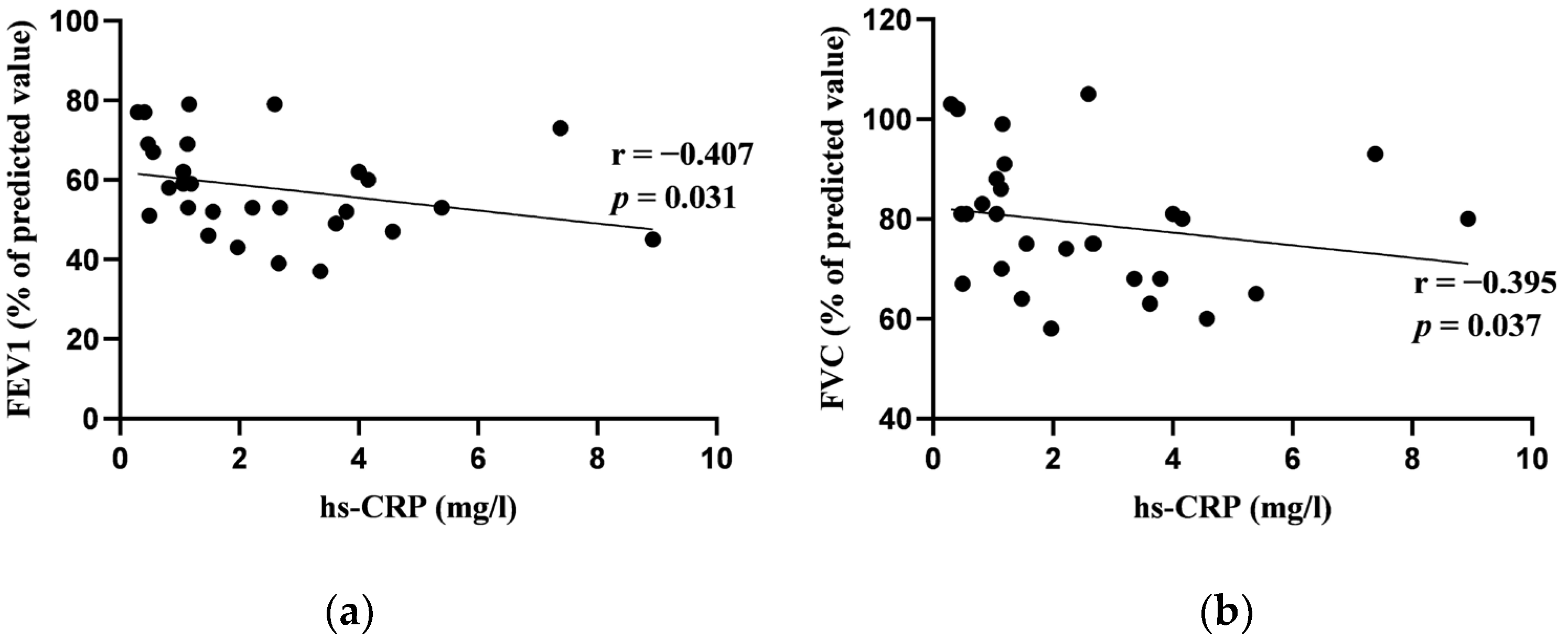
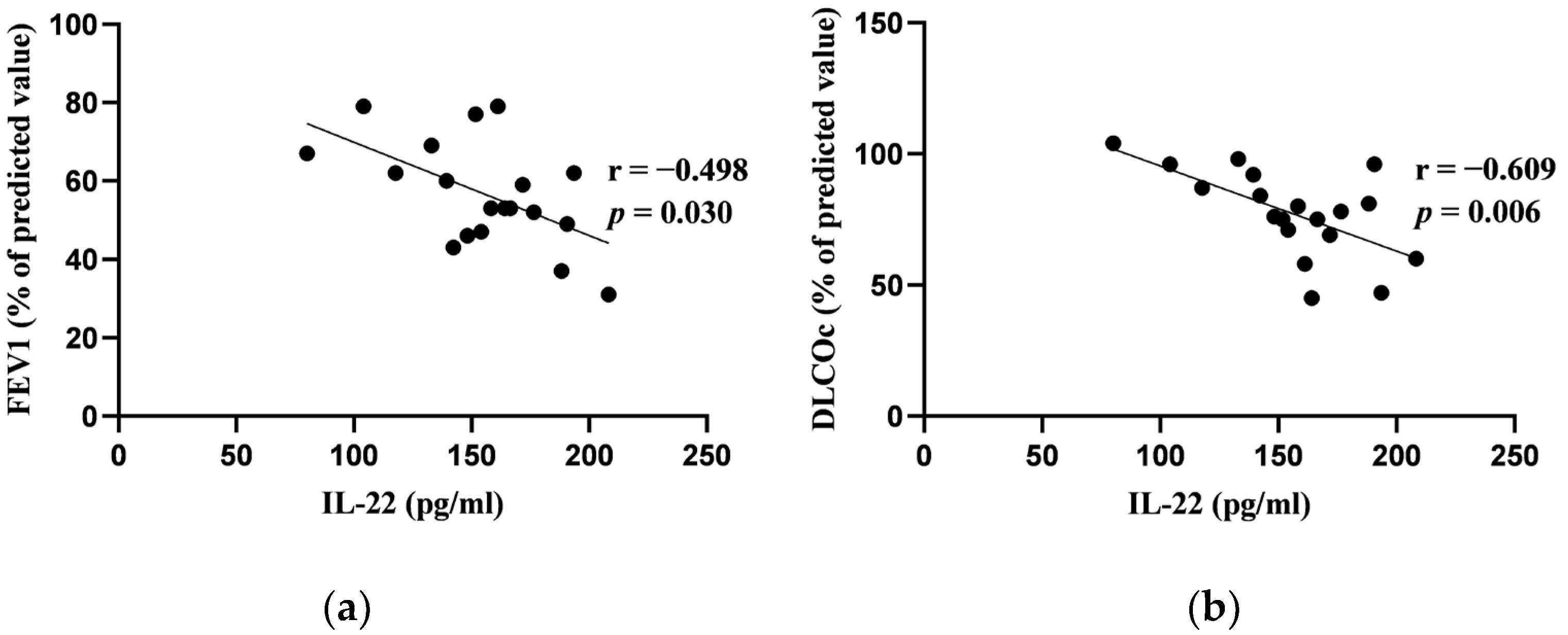
3.2. Correlations between Blood Biomarkers and Pulmonary Function Tests at Baseline
3.3. Correlations between Blood Biomarkers and Cardiac Functional Parameters
3.4. Correlations between Cardiac Magnetic Resonance Imaging Parameters and Pulmonary Function Tests at Baseline
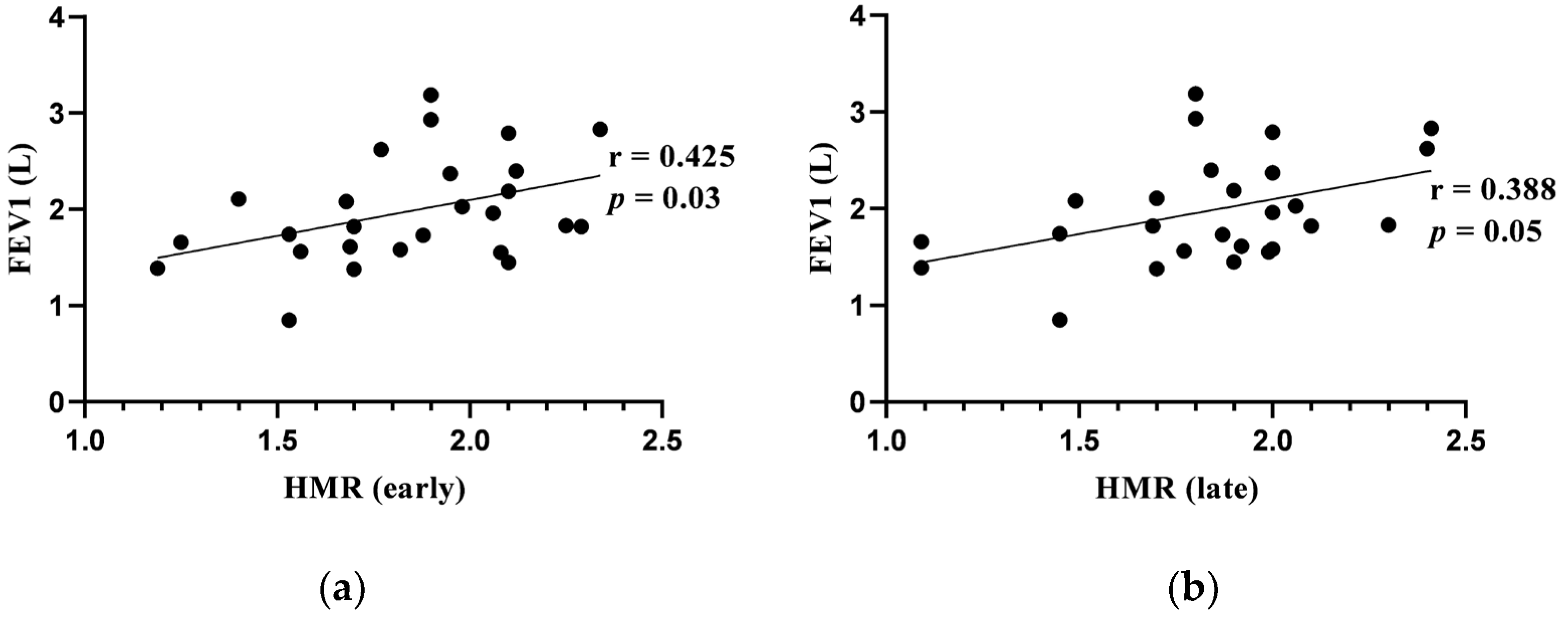
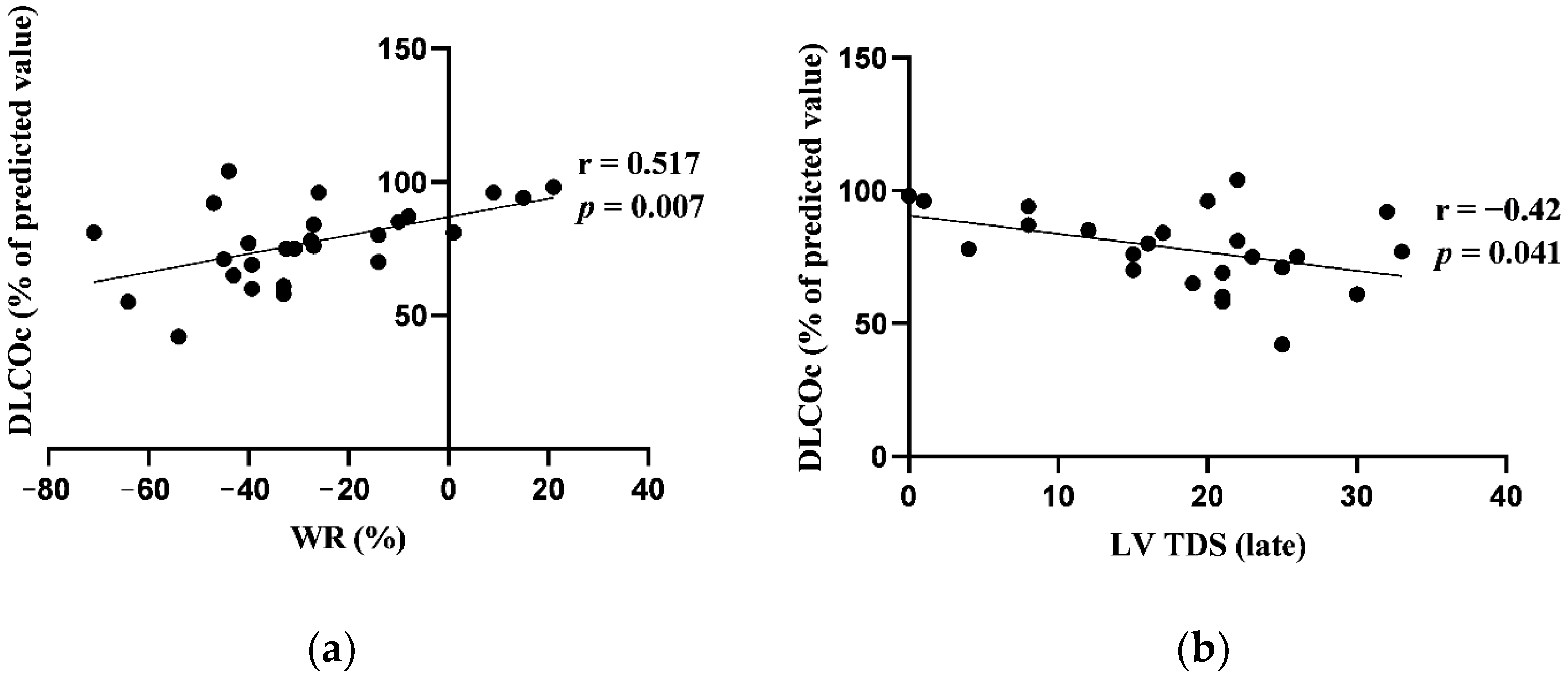
3.5. Correlations between Cardiac 123I-Metaiodobenzylguanidine Imaging Adrenergic Innervation Parameters and Pulmonary Function Tests at Baseline
3.6. Changes in Pulmonary Function Tests from Baseline to 12-Week Follow-Up
3.7. Changes in Cardiac Magnetic Resonance Imaging Parameters from Baseline to 12-Week Follow-Up
3.8. Changes in Cardiac 123I-Metaiodobenzylguanidine Imaging Adrenergic Innervation Parameters from Baseline to 12-Week Follow-Up
4. Discussion
4.1. Blood Biomarkers
4.2. Pulmonary Function Tests
4.3. Cardiac Magnetic Resonance Imaging
4.4. Cardiac 123I-Metaiodobenzylguanidine Imaging
5. Limitations of this Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yamashiro, T.; Moriya, H.; Tsubakimoto, M.; Tsuchiya, N.; Nagatani, Y.; Matsuoka, S.; Murayama, S. Hyperinflated lungs compress the heart during expiration in COPD patients: A new finding on dynamic-ventilation computed tomography. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 3123–3131. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef]
- Valvi, D.; Mannino, D.M.; Müllerova, H.; Tal-Singer, R. Fibrinogen, chronic obstructive pulmonary disease (COPD) and outcomes in two United States cohorts. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 173–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stockley, R.A.; Halpin, D.M.G.; Celli, B.R.; Singh, D. Chronic Obstructive Pulmonary Disease Biomarkers and Their Interpretation. Am. J. Respir. Crit. Care Med. 2019, 199, 1195–1204. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Fois, A.G.; Sotgia, S.; Mangoni, A.A.; Zinellu, E.; Pirina, P.; Carru, C.; Zinellu, A. The neutrophil-to-lymphocyte ratio as a marker of chronic obstructive pulmonary disease and its exacerbations: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2018, 48, e12984. [Google Scholar] [CrossRef]
- Lee, C.H.; Goag, E.K.; Lee, S.H.; Chung, K.S.; Jung, J.Y.; Park, M.S.; Kim, Y.S.; Kim, S.K.; Chang, J.; Song, J.H. Association of serum ferritin levels with smoking and lung function in the Korean adult population: Analysis of the fourth and fifth Korean National Health and Nutrition Examination Survey. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 3001–3006. [Google Scholar] [CrossRef]
- Kamstrup, P.; Sivapalan, P.; Rønn, C.; Rastoder, E.; Modin, D.; Kristensen, A.K.; Bendstrup, E.; Sørensen, R.; Sørensen, T.B.; Ulrik, C.S.; et al. D-dimer in COPD out-patients: Distribution, association with mortality and effect modification by anticoagulant therapy. A preplanned, published protocol cohort study. Eur. Respir. J. 2023, 62 (Suppl. S67), PA1838. [Google Scholar]
- Huang, L.; Lu, Z.; Zhou, X.; He, L.; You, X.; Chen, C.; Zou, C. U-Shaped Relationship Between Serum Lactate Dehydrogenase with All-Cause Mortality in Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 305–316. [Google Scholar] [CrossRef]
- Ghobadi, H.; Fouladi, N.; Beukaghazadeh, K.; Ansarin, K. Association of High Sensitive CRP Level and COPD Assessment Test Scores with Clinically Important Predictive Outcomes in Stable COPD Patients. Tanaffos 2015, 14, 34–41. [Google Scholar]
- Shafuddin, E.; Fairweather, S.M.; Chang, C.L.; Tuffery, C.; Hancox, R.J. Cardiac biomarkers and long-term outcomes of exacerbations of COPD: A long-term follow-up of two cohorts. ERJ Open Res. 2021, 7, 00531–2020. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, U.; Vanfleteren, L. Troponin as a biomarker for mortality in stable COPD. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef]
- Hawkins, N.M.; Khosla, A.; Virani, S.A.; McMurray, J.J.; FitzGerald, J.M. B-type natriuretic peptides in chronic obstructive pulmonary disease: A systematic review. BMC Pulm. Med. 2017, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Flexeder, C.; McGarrah, R.W., 3rd; Wyss, A.; Morrison, A.C.; North, K.E.; Boerwinkle, E.; Kastenmüller, G.; Gieger, C.; Suhre, K.; et al. Metabolomics Identifies Novel Blood Biomarkers of Pulmonary Function and COPD in the General Population. Metabolites 2019, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Q.; Chen, L.; Dong, L.; Xiong, M.; Xie, X.; Zhao, L.; Xu, J.; Zheng, Z.; Wang, J.; et al. Identification of genetic variants of the IL-22 gene in association with an altered risk of COPD susceptibility. Clin. Respir. J. 2022, 16, 537–545. [Google Scholar] [CrossRef]
- Vanfleteren, L.E.G.W.; Weidner, J.; Franssen, F.M.E.; Gaffron, S.; Reynaert, N.L.; Wouters, E.F.M.; Spruit, M.A. Biomarker-based clustering of patients with chronic obstructive pulmonary disease. ERJ Open Res. 2023, 9, 00301–02022. [Google Scholar] [CrossRef] [PubMed]
- Papaporfyriou, A.; Bartziokas, K.; Gompelmann, D.; Idzko, M.; Fouka, E.; Zaneli, S.; Bakakos, P.; Loukides, S.; Papaioannou, A.I. Cardiovascular Diseases in COPD: From Diagnosis and Prevalence to Therapy. Life 2023, 13, 1299. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, M.; Kamusheva, M.; Tachkov, K.; Mitov, K.; Doneva, M.; Pencheva, V.; Petrova, D.; Georgiev, O.; Stoitchkov, J.; Petrova, G. Cardiovascular co-morbidity in patients with COPD in Bulgaria. Biotechnol. Biotechnol. Equip. 2020, 34, 918–924. [Google Scholar] [CrossRef]
- Leitao Filho, F.S.; Sin, D.D. COPD and cardiovascular diseases: Now is the time for action! Thorax 2018, 73, 799–800. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Prevention, Diagnosis and Management of COPD: 2024 Report; GOLD: Deer Park, IL, USA, 2024. [Google Scholar]
- Lahousse, L.; Verhamme, K.M.; Stricker, B.H.; Brusselle, G.G. Cardiac effects of current treatments of chronic obstructive pulmonary disease. Lancet Respir. Med. 2016, 4, 149–164. [Google Scholar] [CrossRef]
- Mammen, M.J.; Pai, V.; Aaron, S.D.; Nici, L.; Alhazzani, W.; Alexander, P.E. Dual LABA/LAMA Therapy versus LABA or LAMA Monotherapy for Chronic Obstructive Pulmonary Disease. A Systematic Review and Meta-analysis in Support of the American Thoracic Society Clinical Practice Guideline. Ann. Am. Thorac. Soc. 2020, 17, 1133–1143. [Google Scholar] [CrossRef]
- Andreas, S.; McGarvey, L.; Bothner, U.; Trampisch, M.; de la Hoz, A.; Fležar, M.; Buhl, R.; Alter, P. Absence of Adverse Effects of Tiotropium/Olodaterol Compared with the Monocomponents on Long-Term Heart Rate and Blood Pressure in Patients with Moderate-to-Very-Severe COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Andreas, S. Effects of LAMA/LABA Alone and in Combination on Cardiac Safety. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1931–1933. [Google Scholar] [CrossRef]
- Andreas, S.; Bothner, U.; de la Hoz, A.; Kloer, I.; Trampisch, M.; Alter, P. No Influence on Cardiac Arrhythmia or Heart Rate from Long-Term Treatment with Tiotropium/Olodaterol versus Monocomponents by Holter ECG Analysis in Patients with Moderate-to-Very-Severe COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Rogliani, P.; Matera, M.G.; Cazzola, M. A Systematic Review with Meta-Analysis of Dual Bronchodilation with LAMA/LABA for the Treatment of Stable COPD. Chest 2016, 149, 1181–1196. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Jiang, Y.; Guo, S.; He, J.Q.; Sin, D.D. Combination therapy with long-acting bronchodilators and the risk of major adverse cardiovascular events in patients with COPD: A systematic review and meta-analysis. Eur. Respir. J. 2023, 61, 2200302. [Google Scholar] [CrossRef]
- Chen, C.Y.; Pan, S.W.; Hsu, C.C.; Liu, J.J.; Kumamaru, H.; Dong, Y.H. Comparative cardiovascular safety of LABA/LAMA FDC versus LABA/ICS FDC in patients with chronic obstructive pulmonary disease: A population-based cohort study with a target trial emulation framework. Respir. Res. 2023, 24, 239. [Google Scholar] [CrossRef] [PubMed]
- Kawut, S.M.; Poor, H.D.; Parikh, M.A.; Hueper, K.; Smith, B.M.; Bluemke, D.A.; Lima, J.A.; Prince, M.R.; Hoffman, E.A.; Austin, J.H.; et al. Cor pulmonale parvus in chronic obstructive pulmonary disease and emphysema: The MESA COPD study. J. Am. Coll. Cardiol. 2014, 64, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Watz, H.; Waschki, B.; Meyer, T.; Kretschmar, G.; Kirsten, A.; Claussen, M.; Magnussen, H. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: Role of hyperinflation. Chest 2010, 138, 32–38. [Google Scholar] [CrossRef]
- Tzani, P.; Aiello, M.; Elia, D.; Boracchia, L.; Marangio, E.; Olivieri, D.; Clini, E.; Chetta, A. Dynamic hyperinflation is associated with a poor cardiovascular response to exercise in COPD patients. Respir. Res. 2011, 12, 150. [Google Scholar] [CrossRef]
- Smith, J.R.; Johnson, B.D.; Olson, T.P. Impaired central hemodynamics in chronic obstructive pulmonary disease during submaximal exercise. J. Appl. Physiol. 2019, 127, 691–697. [Google Scholar] [CrossRef]
- Hohlfeld, J.M.; Vogel-Claussen, J.; Biller, H.; Berliner, D.; Berschneider, K.; Tillmann, H.C.; Hiltl, S.; Bauersachs, J.; Welte, T. Effect of lung deflation with indacaterol plus glycopyrronium on ventricular filling in patients with hyperinflation and COPD (CLAIM): A double-blind, randomised, crossover, placebo-controlled, single-centre trial. Lancet Respir. Med. 2018, 6, 368–378. [Google Scholar] [CrossRef]
- Herth, F.; Hohlfeld, J.M.; Haas, J.; de la Hoz, A.; Jin, X.; Kreitner, K.F.; Vogelmeier, C.; Vogel-Claussen, J.; Watz, H. The effect of tiotropium/olodaterol versus fluticasone propionate/salmeterol on left ventricular filling and lung hyperinflation in patients with COPD. BMJ Open Respir. Res. 2020, 7, e000741. [Google Scholar] [CrossRef]
- Kellerer, C.; Kahnert, K.; Trudzinski, F.C.; Lutter, J.; Berschneider, K.; Speicher, T.; Watz, H.; Bals, R.; Welte, T.; Vogelmeier, C.F.; et al. COPD maintenance medication is linked to left atrial size: Results from the COSYCONET cohort. Respir. Med. 2021, 185, 106461. [Google Scholar] [CrossRef]
- Effect of Dual Bronchodilation with Umeclidinium/Vilanterol on Patients with COPD, Hyperinflation and Heart Failure (CHHEF). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04522596 (accessed on 1 March 2024).
- Stewart, A.G.; Waterhouse, J.C.; Howard, P. Cardiovascular autonomic nerve function in patients with hypoxaemic chronic obstructive pulmonary disease. Eur. Respir. J. 1991, 4, 1207–1214. [Google Scholar] [CrossRef]
- Heindl, S.; Lehnert, M.; Criée, C.P.; Hasenfuss, G.; Andreas, S. Marked sympathetic activation in patients with chronic respiratory failure. Am. J. Respir. Crit. Care Med. 2001, 164, 597–601. [Google Scholar] [CrossRef]
- Patakas, D.; Louridas, G.; Kakavelas, E. Reduced baroreceptor sensitivity in patients with chronic obstructive pulmonary disease. Thorax 1982, 37, 292–295. [Google Scholar] [CrossRef]
- van Gestel, A.J.; Steier, J. Autonomic dysfunction in patients with chronic obstructive pulmonary disease (COPD). J. Thorac. Dis. 2010, 2, 215–222. [Google Scholar] [CrossRef]
- Nissinen, S.I.; Mäkikallio, T.H.; Seppänen, T.; Tapanainen, J.M.; Salo, M.; Tulppo, M.P.; Huikuri, H.V. Heart rate recovery after exercise as a predictor of mortality among survivors of acute myocardial infarction. Am. J. Cardiol. 2003, 91, 711–714. [Google Scholar] [CrossRef]
- Cole, C.R.; Foody, J.M.; Blackstone, E.H.; Lauer, M.S. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann. Intern. Med. 2000, 132, 552–555. [Google Scholar] [CrossRef]
- Lacasse, M.; Maltais, F.; Poirier, P.; Lacasse, Y.; Marquis, K.; Jobin, J.; LeBlanc, P. Post-exercise heart rate recovery and mortality in chronic obstructive pulmonary disease. Respir. Med. 2005, 99, 877–886. [Google Scholar] [CrossRef]
- Merlet, P.; Valette, H.; Dubois-Randé, J.L.; Moyse, D.; Duboc, D.; Dove, P.; Bourguignon, M.H.; Benvenuti, C.; Duval, A.M.; Agostini, D.; et al. Prognostic value of cardiac metaiodobenzylguanidine imaging in patients with heart failure. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1992, 33, 471–477. [Google Scholar]
- Peix, A.; Mesquita, C.T.; Paez, D.; Pereira, C.C.; Felix, R.; Gutierrez, C.; Jaimovich, R.; Ianni, B.M.; Soares, J., Jr.; Olaya, P.; et al. Nuclear medicine in the management of patients with heart failure: Guidance from an expert panel of the International Atomic Energy Agency (IAEA). Nucl. Med. Commun. 2014, 35, 818–823. [Google Scholar] [CrossRef]
- Verschure, D.O.; Nakajima, K.; Verberne, H.J. Cardiac (123)I-mIBG Imaging in Heart Failure. Pharmaceuticals 2022, 15, 656. [Google Scholar] [CrossRef]
- Carrió, I.; Cowie, M.R.; Yamazaki, J.; Udelson, J.; Camici, P.G. Cardiac sympathetic imaging with mIBG in heart failure. JACC Cardiovasc. Imaging 2010, 3, 92–100. [Google Scholar] [CrossRef]
- Sakamaki, F.; Satoh, T.; Nagaya, N.; Kyotani, S.; Nakanishi, N.; Ishida, Y. Abnormality of left ventricular sympathetic nervous function assessed by (123)I-metaiodobenzylguanidine imaging in patients with COPD. Chest 1999, 116, 1575–1581. [Google Scholar] [CrossRef]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef]
- Flotats, A.; Carrió, I.; Agostini, D.; Le Guludec, D.; Marcassa, C.; Schäfers, M.; Somsen, G.A.; Unlu, M.; Verberne, H.J. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1802–1812. [Google Scholar] [CrossRef]
- Pinto-Plata, V.M.; Müllerova, H.; Toso, J.F.; Feudjo-Tepie, M.; Soriano, J.B.; Vessey, R.S.; Celli, B.R. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 2006, 61, 23–28. [Google Scholar] [CrossRef]
- Aksu, F.; Capan, N.; Aksu, K.; Ofluoğlu, R.; Canbakan, S.; Yavuz, B.; Akin, K.O. C-reactive protein levels are raised in stable Chronic obstructive pulmonary disease patients independent of smoking behavior and biomass exposure. J. Thorac. Dis. 2013, 5, 414–421. [Google Scholar] [CrossRef]
- Agarwal, R.; Zaheer, M.S.; Ahmad, Z.; Akhtar, J. The relationship between C-reactive protein and prognostic factors in chronic obstructive pulmonary disease. Multidiscip. Respir. Med. 2013, 8, 63. [Google Scholar] [CrossRef]
- Hogg, J.C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004, 364, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.; Vestbo, J.; Lange, P.; Bojesen, S.E.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 175, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Leuzzi, G.; Galeone, C.; Taverna, F.; Suatoni, P.; Morelli, D.; Pastorino, U. C-reactive protein level predicts mortality in COPD: A systematic review and meta-analysis. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2017, 26, 160070. [Google Scholar] [CrossRef] [PubMed]
- Perusina Lanfranca, M.; Lin, Y.; Fang, J.; Zou, W.; Frankel, T. Biological and pathological activities of interleukin-22. J. Mol. Med. 2016, 94, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.F.; Zhu, M.; Wu, H.X.; Fan, L.L.; Cheng, D.Y. Immunophenotype in acute exacerbation of chronic obstructive pulmonary disease: A cross-sectional study. Respir. Res. 2022, 23, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, Z.; Liu, W.; Wu, K. Expression of interleukin (IL)-10, IL-17A and IL-22 in serum and sputum of stable chronic obstructive pulmonary disease patients. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.; Greening, N. Airway inflammation in COPD: Progress to precision medicine. Eur. Respir. J. 2019, 54, 1900651. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Wood, J.G.; Gonzalez, N.C. Alveolar hypoxia, alveolar macrophages, and systemic inflammation. Respir. Res. 2009, 10, 54. [Google Scholar] [CrossRef]
- Modi, P.; Cascella, M. Diffusing Capacity of The Lungs for Carbon Monoxide; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lee, H.; Um, S.J.; Kim, Y.S.; Kim, D.K.; Jang, A.S.; Choi, H.S.; Kim, Y.H.; Kim, T.E.; Yoo, K.H.; Jung, K.S. Association of the Neutrophil-to-Lymphocyte Ratio with Lung Function and Exacerbations in Patients with Chronic Obstructive Pulmonary Disease. PLoS ONE 2016, 11, e0156511. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Zhang, Q.; Yang, X.; Shan, H.; Ming, Z.; Chen, H.; Liu, Y.; Yin, J.; Li, Y. D-dimer as a potential biomarker for the progression of COPD. Clin. Chim. Acta; Int. J. Clin. Chem. 2016, 455, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Bozkanat, E.; Tozkoparan, E.; Baysan, O.; Deniz, O.; Ciftci, F.; Yokusoglu, M. The significance of elevated brain natriuretic peptide levels in chronic obstructive pulmonary disease. J. Int. Med. Res. 2005, 33, 537–544. [Google Scholar] [CrossRef]
- Sunil Kumar Gothwal, V.P.; Barjatiya, H.C.; Banseria, R.; Sharma, P.; Goyal, P.K.; Ramaswamy, V.M.C.; Singh, Y.; Gupt, G. Study of lung function test in association with laboratory findings of serum iron in patients with chronic obstructive pulmonary disease. Clin. Epidemiol. Glob. Health 2022, 16, 101091. [Google Scholar] [CrossRef]
- Kato, M.; Kitada, S.; Kawada, Y.; Nakasuka, K.; Kikuchi, S.; Seo, Y.; Ohte, N. Left Ventricular End-Systolic Volume Is a Reliable Predictor of New-Onset Heart Failure with Preserved Left Ventricular Ejection Fraction. Cardiol. Res. Pract. 2020, 2020, 3106012. [Google Scholar] [CrossRef] [PubMed]
- Tesic, M.; Seferovic, J.; Trifunovic, D.; Djordjevic-Dikic, A.; Giga, V.; Jovanovic, I.; Petrovic, O.; Marinkovic, J.; Stankovic, S.; Stepanovic, J.; et al. N-terminal pro-brain natriuretic peptide is related with coronary flow velocity reserve and diastolic dysfunction in patients with asymmetric hypertrophic cardiomyopathy. J. Cardiol. 2017, 70, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.W.; Lam, W.W.; Chiu, C.S.; Chau, A.K.; Cheung, S.C.; Cheung, Y.F. Plasma brain natriuretic peptide levels, right ventricular volume overload and exercise capacity in adolescents after surgical repair of tetralogy of Fallot. Int. J. Cardiol. 2007, 121, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M.; Shah, P.S.; Shah, A.D.; Francis, S.A.; Patel, N.V.; Kothari, K.K. Chronic obstructive pulmonary disease and cardiac comorbidities: A cross-sectional study. Lung India Off. Organ Indian Chest Soc. 2016, 33, 404–409. [Google Scholar] [CrossRef]
- Nicolae, B.; Ecaterina, L. Natriuretic peptides in elderly patients with chronic obstructive pulmonary disease. Egypt. J. Bronchol. 2022, 16, 26. [Google Scholar] [CrossRef]
- Kyuma, M.; Nakata, T.; Hashimoto, A.; Nagao, K.; Sasao, H.; Takahashi, T.; Tsuchihashi, K.; Shimamoto, K. Incremental prognostic implications of brain natriuretic peptide, cardiac sympathetic nerve innervation, and noncardiac disorders in patients with heart failure. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2004, 45, 155–163. [Google Scholar]
- Matsuo, S.; Nakamura, Y.; Matsumoto, T.; Nakae, I.; Murata, K.; Horie, M. Prognostic value of iodine-123 metaiodobenzylguanidine imaging in patients with heart failure. Exp. Clin. Cardiol. 2003, 8, 95–98. [Google Scholar]
- Gong, Y.; Lv, Y.; Liu, H.; Zheng, Q.; Li, L. Quantitative analysis of efficacy and safety of LABA/LAMA fixed-dose combinations in the treatment of stable COPD. Ther. Adv. Respir. Dis. 2022, 16, 66068. [Google Scholar] [CrossRef]
- Singh, D.; Ferguson, G.T.; Bolitschek, J.; Grönke, L.; Hallmann, C.; Bennett, N.; Abrahams, R.; Schmidt, O.; Bjermer, L. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir. Med. 2015, 109, 1312–1319. [Google Scholar] [CrossRef]
- Watz, H.; Troosters, T.; Beeh, K.M.; Garcia-Aymerich, J.; Paggiaro, P.; Molins, E.; Notari, M.; Zapata, A.; Jarreta, D.; Garcia Gil, E. ACTIVATE: The effect of aclidinium/formoterol on hyperinflation, exercise capacity, and physical activity in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2545–2558. [Google Scholar] [CrossRef] [PubMed]
- Maltais, F.; Singh, S.; Donald, A.C.; Crater, G.; Church, A.; Goh, A.H.; Riley, J.H. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: Two randomized, double-blind clinical trials. Ther. Adv. Respir. Dis. 2014, 8, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Salomon, J.; Stolz, D.; Domenighetti, G.; Frey, J.G.; Turk, A.J.; Azzola, A.; Sigrist, T.; Fitting, J.W.; Schmidt, U.; Geiser, T.; et al. Indacaterol and glycopyrronium versus indacaterol on body plethysmography measurements in COPD-a randomised controlled study. Respir. Res. 2017, 18, 13. [Google Scholar] [CrossRef]
- Beeh, K.M.; Westerman, J.; Kirsten, A.M.; Hébert, J.; Grönke, L.; Hamilton, A.; Tetzlaff, K.; Derom, E. The 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2015, 32, 53–59. [Google Scholar] [CrossRef]
- Smith, B.M.; Kawut, S.M.; Bluemke, D.A.; Basner, R.C.; Gomes, A.S.; Hoffman, E.; Kalhan, R.; Lima, J.A.; Liu, C.Y.; Michos, E.D.; et al. Pulmonary hyperinflation and left ventricular mass: The Multi-Ethnic Study of Atherosclerosis COPD Study. Circulation 2013, 127, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Pelà, G.; Li Calzi, M.; Pinelli, S.; Andreoli, R.; Sverzellati, N.; Bertorelli, G.; Goldoni, M.; Chetta, A. Left ventricular structure and remodeling in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1015–1022. [Google Scholar] [CrossRef]
- Jörgensen, K.; Müller, M.F.; Nel, J.; Upton, R.N.; Houltz, E.; Ricksten, S.E. Reduced intrathoracic blood volume and left and right ventricular dimensions in patients with severe emphysema: An MRI study. Chest 2007, 131, 1050–1057. [Google Scholar] [CrossRef]
- Macnee, W.; Maclay, J.; McAllister, D. Cardiovascular injury and repair in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2008, 5, 824–833. [Google Scholar] [CrossRef]
- MacNee, W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part Two. Am. J. Respir. Crit. Care Med. 1994, 150, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- D’Urzo, A.D.; Rennard, S.I.; Kerwin, E.M.; Mergel, V.; Leselbaum, A.R.; Caracta, C.F. Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: The 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir. Res. 2014, 15, 123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, H.; Wang, J.; Ruan, Z.; Dai, Y.; Xia, Z.; Lv, Q. Indacaterol/glycopyrronium affects lung function and cardiovascular events in patients with chronic obstructive pulmonary diseases: A meta-analysis. Heart Lung J. Crit. Care 2021, 50, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cheng, W.; Guo, J.; Guan, W. Relationship of inhaled long-acting bronchodilators with cardiovascular outcomes among patients with stable COPD: A meta-analysis and systematic review of 43 randomized trials. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Donohue, J.F.; Niewoehner, D.; Brooks, J.; O’Dell, D.; Church, A. Safety and tolerability of once-daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: Results from a 52-week, randomized, double-blind, placebo-controlled study. Respir. Res. 2014, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.T.; Buhl, R.; Bothner, U.; Hoz, A.; Voß, F.; Anzueto, A.; Calverley, P.M. Safety of tiotropium/olodaterol in chronic obstructive pulmonary disease: Pooled analysis of three large, 52-week, randomized clinical trials. Respir. Med. 2018, 143, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Requena, G.; Dedman, D.; Quint, J.K.; Ghosh, R.E.; Williams, R.; Pimenta, J.M. The Utilization and Safety of Umeclidinium and Umeclidinium/Vilanterol in UK Primary Care: A Retrospective Cohort Study. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Tamaki, N.; Nakata, T.; Yamashina, S.; Yamazaki, J. Determination of the survival rate in patients with congestive heart failure stratified by 123I-MIBG imaging: A meta-analysis from the studies performed in Japan. Ann. Nucl. Med. 2011, 25, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Bax, J.J.; Kraft, O.; Buxton, A.E.; Fjeld, J.G.; Parízek, P.; Agostini, D.; Knuuti, J.; Flotats, A.; Arrighi, J.; Muxi, A.; et al. 123 I-mIBG scintigraphy to predict inducibility of ventricular arrhythmias on cardiac electrophysiology testing: A prospective multicenter pilot study. Circ. Cardiovasc. Imaging 2008, 1, 131–140. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Nakata, T.; Hashimoto, A.; Yuda, S.; Tsuchihashi, K.; Travin, M.I.; Shimamoto, K. Assessment of underlying etiology and cardiac sympathetic innervation to identify patients at high risk of cardiac death. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2001, 42, 1757–1767. [Google Scholar]
| Characteristic | Value |
|---|---|
| Age (years), median (IQR) | 60 (10) |
| BMI (kg/m2), median (IQR) | 26.5 (6.6) |
| Smoking history (pack years), median (IQR) | 40 (30) |
Gender, n (%)
|
|
Severity of COPD (airflow limitation), n (%)
|
|
| Emphysema, n (%) | 23 (79.3) |
Concomitant diseases, n (%)
|
|
| Pulmonary Function Parameter (N = 29) | Baseline Median (IQR) |
|---|---|
| FEV1, % pred. FEV1, L | 53 (20) 1.83 (0.79) |
| FVC, % pred. FVC, L | 80 (19.5) 3.4 (0.92) |
| FRC, % pred. FRC, L | 123 (35) 4.2 (1.37) |
| ResV, % pred. ResV, L | 145 (40) 3.6 (1.05) |
| ResV/TLC, % pred. ResV/TLC, % | 130 (17) 51.8 (7.87) |
| FRC/TLC, % pred. FRC/TLC, % | 118 (22) 61.08 (9.58) |
| TLC, % pred. TLC, L | 100 (20) 6.97 (1.69) |
| DLCOc, % pred. DLCOc, mmol/min/kPa | 76 (23) 6.66 (3.14) |
| Blood Biomarker (N = 29) | Baseline Median (IQR) |
|---|---|
| BNP (ng/L) | 14.4 (15.1) |
| LDH (U/L) | 224 (46) |
| Ferritin (μg/L) | 87 (111.8) |
| D-dimer (mg/L) | 0.65 (0.45) |
| Hs-CRP (mg/L) | 1.77 (2.69) |
| NLR | 2.14 (1.01) |
| IL-22 (N = 19) (pg/mL) | 158.24 (37.06) |
| Cardiac MRI Parameters (N = 28) | Baseline Median (IQR) |
|---|---|
| LV-EDVi (mL/m2) | 72.19 (30.04) |
| RV-EDVi (mL/m2) | 71.5 (22.64) |
| LV-ESVi (mL/m2) | 30.5 (15.05) |
| RV-ESVi (mL/m2) | 29 (21.43) |
| LV-EF (%) | 59.5 (11.5) |
| RV-EF (%) | 59 (10.5) |
| CI (L/min/m2) | 2.89 (0.99) |
| LV-SVi (mL/m2) | 44.06 (17.75) |
| RV-SVi (mL/m2) | 42.92 (15) |
| LV-CMi (g/m2) | 70 (19.5) |
| Cardiac MRI | Pulmonary Function Tests | Correlation Coefficient (r) | p-Value * |
|---|---|---|---|
| LV-EF, % | FRC, % pred. | −0.378 | 0.047 |
| FRC, L | −0.413 | 0.029 | |
| ResV, % pred. | −0.36 | 0.06 | |
| LV-EDVi, ml/m2 | ResV/TLC, % | −0.406 | 0.032 |
| FEV1, L | 0.371 | 0.052 | |
| LV-ESVi, ml/m2 | FVC, L | 0.416 | 0.028 |
| LV-SVi, ml/m2 | ResV/TLC, % | −0.442 | 0.018 |
| CI, L/min/m2 | ResV/TLC, % | −0.586 | 0.001 |
| FVC, L | 0.388 | 0.041 | |
| FEV1, L | 0.354 | 0.064 |
| Cardiac MRI | Pulmonary Function Tests | Correlation Coefficient (r) | p-Value * |
|---|---|---|---|
| RV-EDVi, mL/m2 | ResV/TLC, % | −0.394 | 0.038 |
| FEV1, L | 0.416 | 0.028 | |
| FVC, L | 0.388 | 0.041 | |
| RV-SVi, mL/m2 | FEV1, L | 0.413 | 0.029 |
| RV-EF, % | FEV1, % pred. | 0.370 | 0.052 |
| Cardiac 123I-MIBG Parameter (N = 26) | Baseline Median (IQR) |
|---|---|
| HMR (early) | 1.89 (0.45) |
| HMR (late) | 1.89 (0.30) |
| WR (%) | 31.65 (30.3) |
| LV TDS (early) | 11 (13) |
| LV TDS (late) | 20.5 (12) |
| N = 22 | Baseline Median (IQR) | 12 Weeks Follow-Up Median (IQR) | p-Value * |
|---|---|---|---|
| FEV1, % pred. FEV1, L | 56 (20.5) 1.84 (1.01) | 62.5 (23) 2.15 (1) | 0.000124 0.000139 |
| FVC, % pred. FVC, L | 77.5 (21.75) 3.39 (1.14) | 77 (24.25) 3.41 (0.91) | 0.356 0.179 |
| FRC, % | 117.5 (46) | 113.5 (40) | 0.149 |
| FRC, L | 4.14 (1.84) | 4.05 (1.08) | 0.179 |
| ResV, % pred. ResV, L | 152 (55) 3.62 (1.25) | 136 (37.25) 3.38 (0.96) | 0.093 0.276 |
| ResV/TLC, % pred. ResV/TLC, % | 130.5 (28) 50.33 (11.08) | 119.5 (18.25) 46 (9.81) | 0.012 0.017 |
| FRC/TLC, % pred. FRC/TLC, % | 116.5 (23) 60.03 (9.89) | 113 (17) 57.79 (10.28) | 0.047 0.079 |
| TLC, % pred. TLC, L | 100 (29) 6.95 (1.99) | 100 (17) 7.02 (1.42) | 0.981 0.88 |
| DLCOc, % pred. DLCOc, mmol/min/kPa | 77 (21.25) 6.85 (3.17) | 80 (24.5) 7.35 (3.79) | 0.511 0.452 |
| N = 16 | Baseline Median (IQR) | 12 Weeks Follow-Up Median (IQR) | p-Value * |
|---|---|---|---|
| HMR (early) | 1.86 (0.43) | 2.03 (0.43) | p = 0.252 |
| HMR (late) | 1.88 (0.37) | 2 (0.41) | p = 0.026 |
| WR (%) | 31.65 (30.8) | 19.5 (21.85) | p = 0.464 |
| LV TDS (early) | 7 (14) | 8 (11.25) | p = 0.72 |
| LV TDS (late) | 21 (16) | 14.5 (15.75) | p = 0.502 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimiene, I.; Hoppenot, D.; Vajauskas, D.; Padervinskiene, L.; Rimkunas, A.; Zemaitis, M.; Barkauskiene, D.; Lapinskas, T.; Ereminiene, E.; Miliauskas, S. Systemic Manifestations of COPD and the Impact of Dual Bronchodilation with Tiotropium/Olodaterol on Cardiac Function and Autonomic Integrity. J. Clin. Med. 2024, 13, 2937. https://doi.org/10.3390/jcm13102937
Dimiene I, Hoppenot D, Vajauskas D, Padervinskiene L, Rimkunas A, Zemaitis M, Barkauskiene D, Lapinskas T, Ereminiene E, Miliauskas S. Systemic Manifestations of COPD and the Impact of Dual Bronchodilation with Tiotropium/Olodaterol on Cardiac Function and Autonomic Integrity. Journal of Clinical Medicine. 2024; 13(10):2937. https://doi.org/10.3390/jcm13102937
Chicago/Turabian StyleDimiene, Ieva, Deimante Hoppenot, Donatas Vajauskas, Lina Padervinskiene, Airidas Rimkunas, Marius Zemaitis, Diana Barkauskiene, Tomas Lapinskas, Egle Ereminiene, and Skaidrius Miliauskas. 2024. "Systemic Manifestations of COPD and the Impact of Dual Bronchodilation with Tiotropium/Olodaterol on Cardiac Function and Autonomic Integrity" Journal of Clinical Medicine 13, no. 10: 2937. https://doi.org/10.3390/jcm13102937
APA StyleDimiene, I., Hoppenot, D., Vajauskas, D., Padervinskiene, L., Rimkunas, A., Zemaitis, M., Barkauskiene, D., Lapinskas, T., Ereminiene, E., & Miliauskas, S. (2024). Systemic Manifestations of COPD and the Impact of Dual Bronchodilation with Tiotropium/Olodaterol on Cardiac Function and Autonomic Integrity. Journal of Clinical Medicine, 13(10), 2937. https://doi.org/10.3390/jcm13102937






