Evaluation and Rehabilitation after Adult Lumbar Spine Surgery
Abstract
1. Introduction
2. Historical Review of Rehabilitation (Table 1)
| Year | Author | Rehabilitation Method |
|---|---|---|
| 1937 | Williams [18] | Lumbar flexion exercises |
| 1955 | Kelly [19] | Hanging exercises |
| 1962 | Pheasant [20] | Posture building |
| 1968 | Calliet [21] | Lumbar lateral flexion exercises |
| 1971 | Böhler [22] | Lumbar extension exercises |
| 1979 | McKenzie [23] | Lumbar extension exercises |
3. Various Kinds of Rehabilitation
4. Patients-Reported Outcome (PRO) Measures Used after Lumbar Surgery
4.1. Roland-Morris Disability Questionnaire (RMDQ) (Appendix A)
4.2. Oswestry Disability Index (ODI) (Appendix B)
4.3. Zurich Claudication Questionnaire (ZCQ)
4.4. Scoliosis Research Society 22-Item Questionnaire (SRS-22)
4.5. Lumbar Stiffness Disability Index (LSDI)
5. Physical Performance Tests
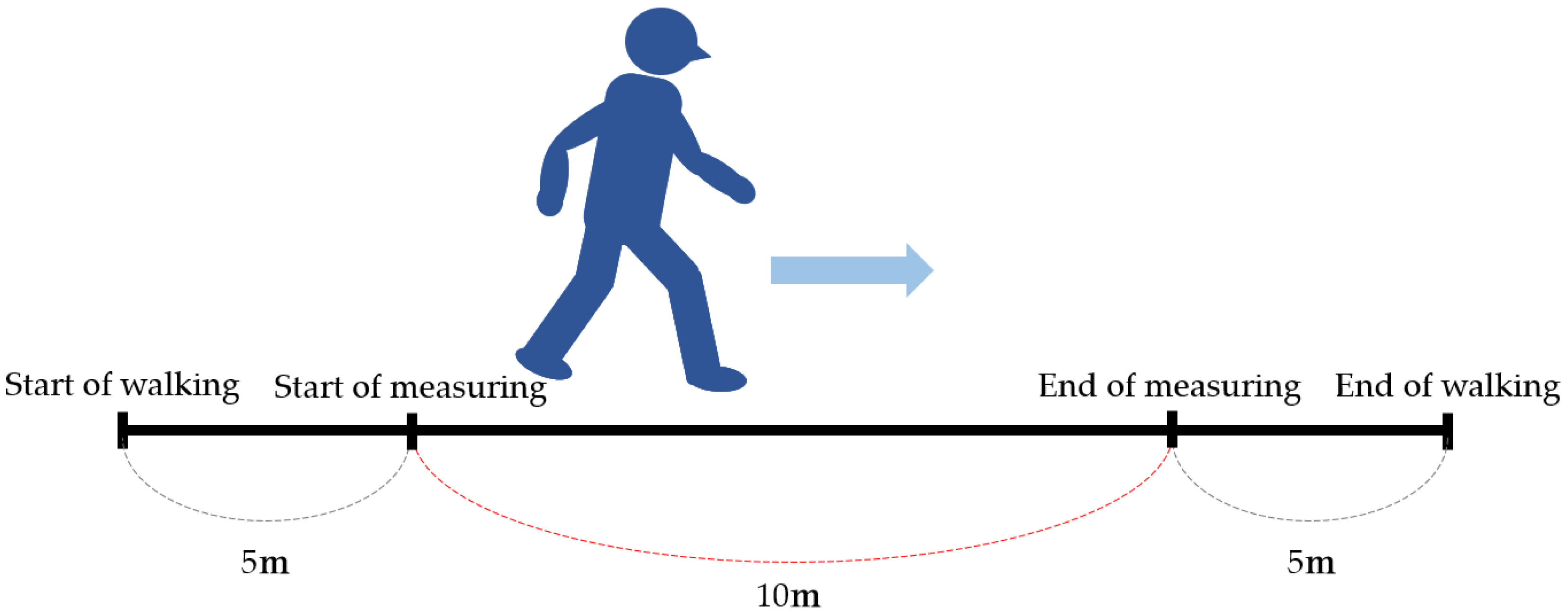
5.1. Walk Velocity (Figure 4)
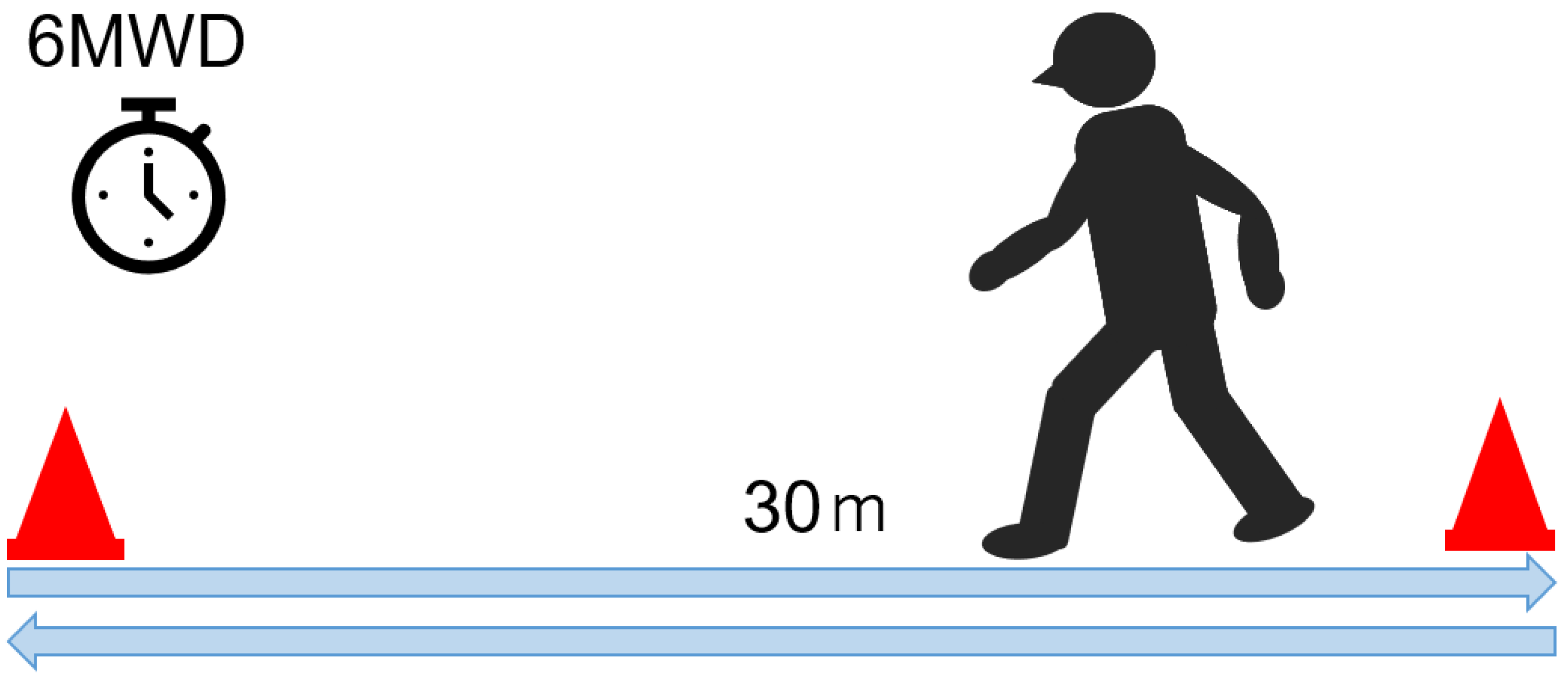
5.2. Six-Minute Walk Test (Figure 5)
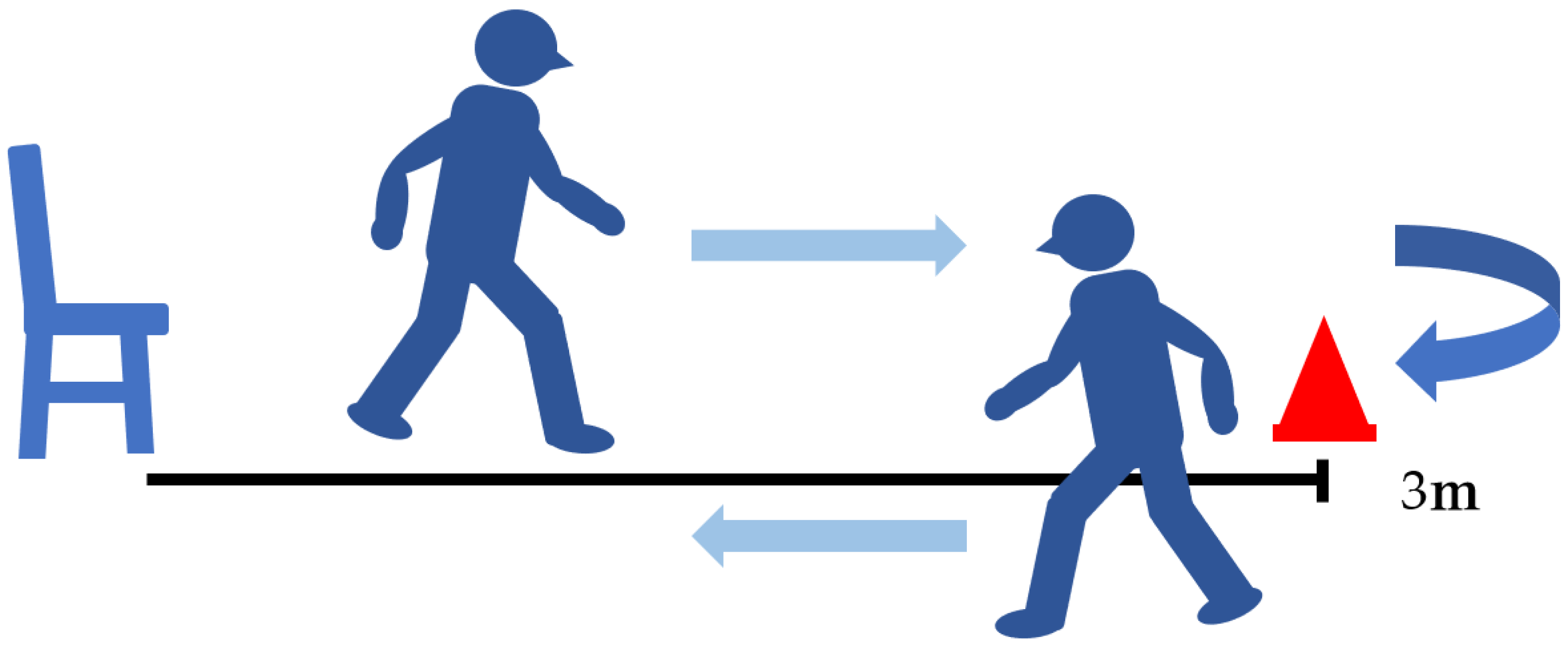
5.3. Timed up and Go Test (TUG) (Figure 6)
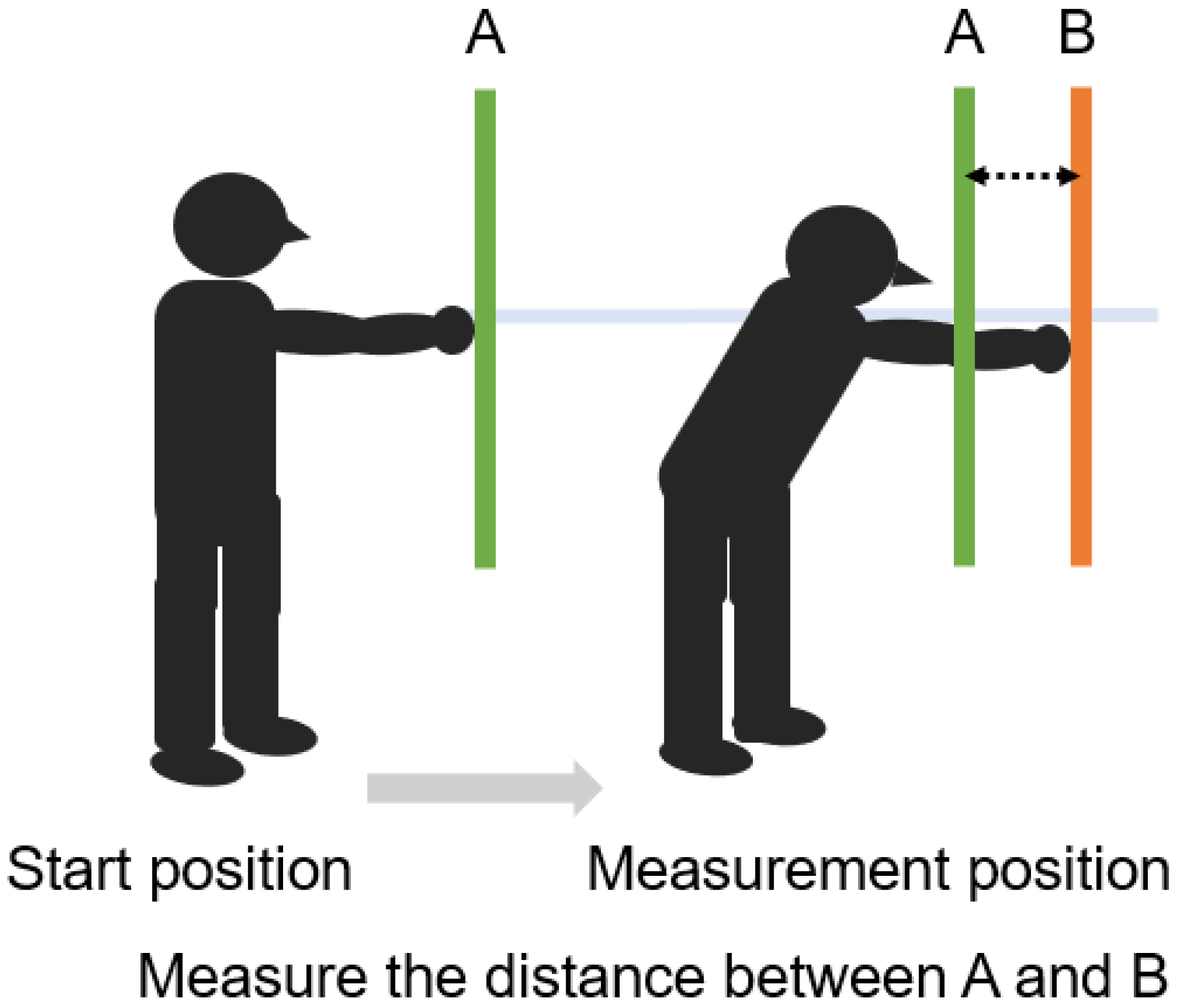
5.4. Functional Reach Test (FRT) (Figure 7)
5.5. The Balance Evaluation Systems Test (BESTest) (Table 4)
| I. Biomechanical Constraints | II. Stability Limits | III. Anticipatory Postural Adjustments | IV. Postural Responses | V. Sensory Orientation | VI. Stability in Gait |
|---|---|---|---|---|---|
| 1. Base of support | 6. Sitting verticality (left and right) and lateral lean | 9. Sit to stand | 14. In-place response, forward | 19. Sensory integration for balance, Stance on firm surface, | 21. Gait level surface |
| 2. CoM alignment | 7. Functional reach forward | 10. Rise to toes | 15. In-place response, backward | 20. Incline, EC | 22. Change in gait speed |
| 3. Ankle strength and ROM | 8. Functional reach lateral | 11. Stand on one leg | 16. Compensatory stepping correction, forward | 23. Walk with head turns, horizontal | |
| 4. Hip/trunk lateral strength | 12. Alternate stair touching | 17. Compensatory stepping correction, backward | 24. Walk with pivot turns | ||
| 5. Sit on floor and stand up | 13. Standing arm raise | 18. Compensatory stepping correction, lateral | 25. Step over obstacles | ||
| 26. Timed “Get Up and Go” Test | |||||
| 27. Timed “Get Up and Go” Test with dual task |
| Anticipatory Postural Adjustments | Postural Responses | Sensory Orientation | Dynamic Gait |
|---|---|---|---|
| 1. Sit to stand | 4. Compensatory stepping correction, forward | 7. Stance on firm surface, EO | 11. Change in gait speed |
| 2. Rise to toes | 5. Compensatory stepping correction, backward | 9. Stance on foam, EC | 12. Walk with head turns, horizontal |
| 3. Stand on one leg (left and right) | 6. Compensatory stepping correction, lateral (left and right) | 10. Incline, EC | 13. Walk with pivot turns |
| 12. Step over obstacles | |||
| 14. Cognitive Get up and Go |
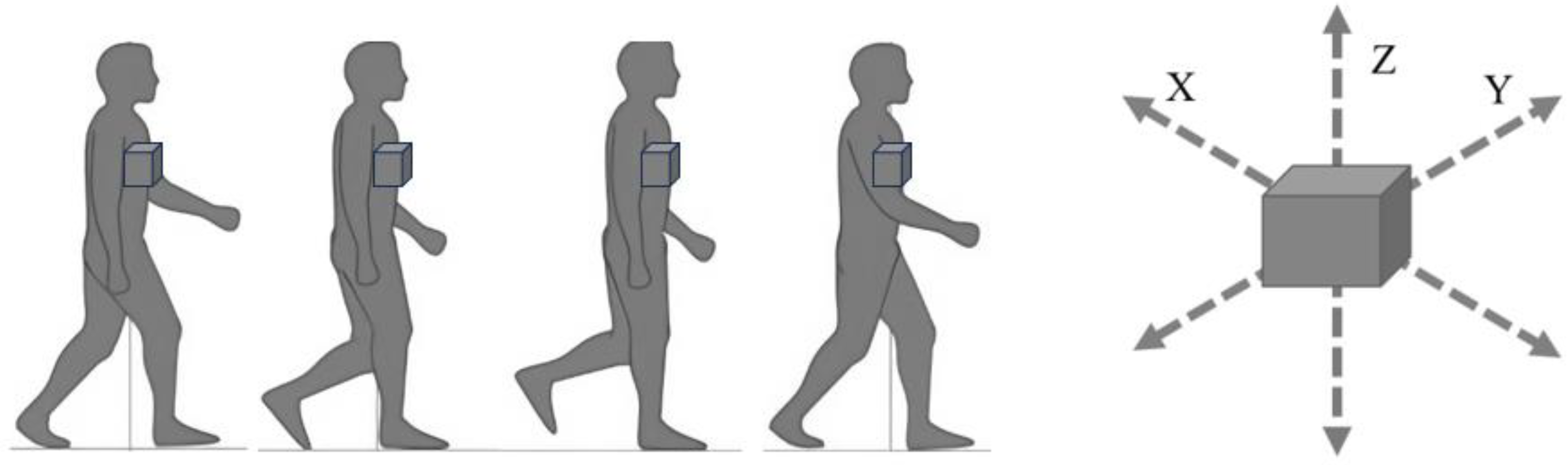
5.6. Three-Dimensional Motion Analyzers and Force Plate
5.7. Triaxial Accelerometer (Figure 8)
6. Physical Therapy after Lumbar Spine Surgery
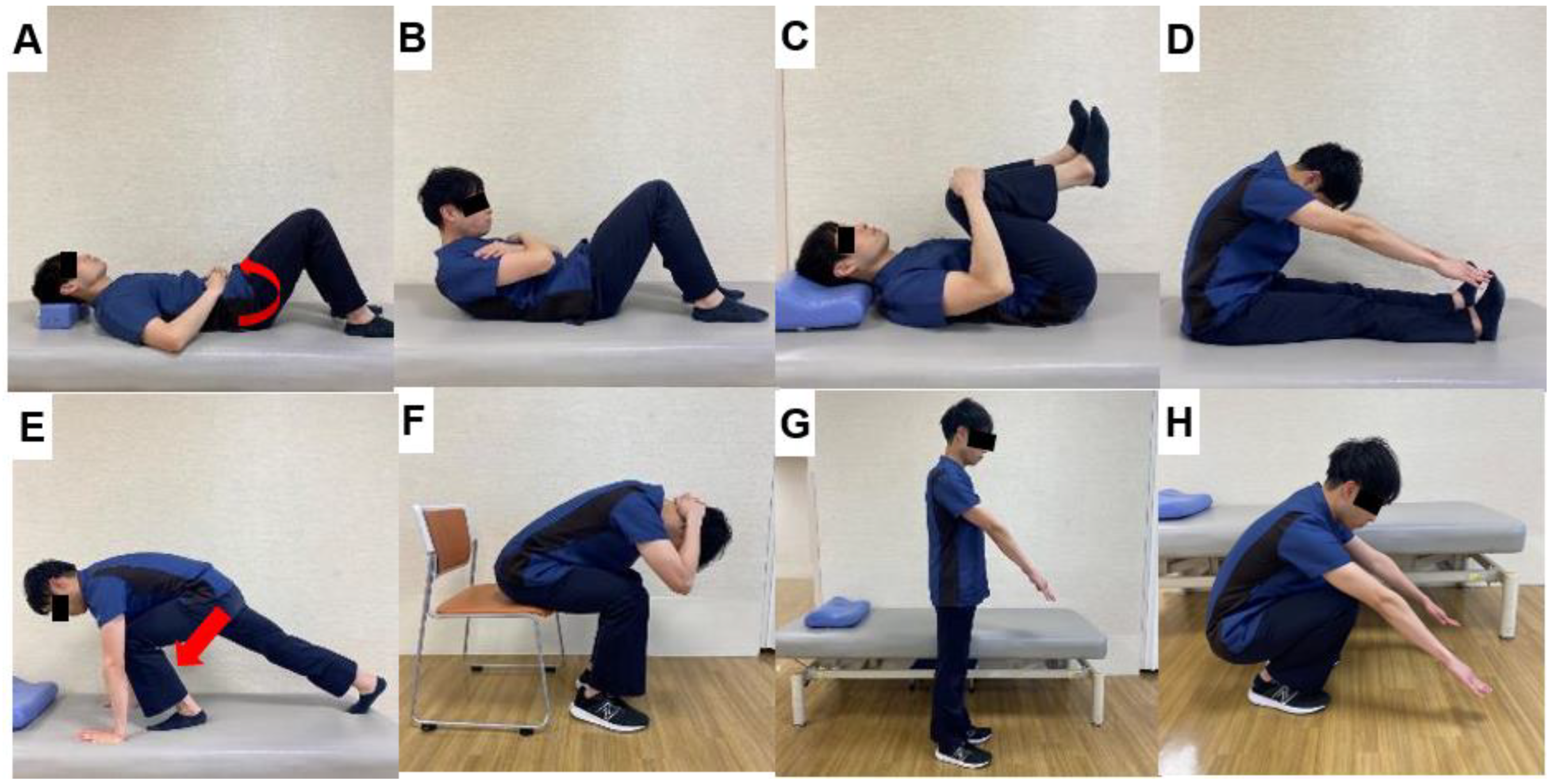
6.1. Trunk Muscle Strengthening (Figure 11)
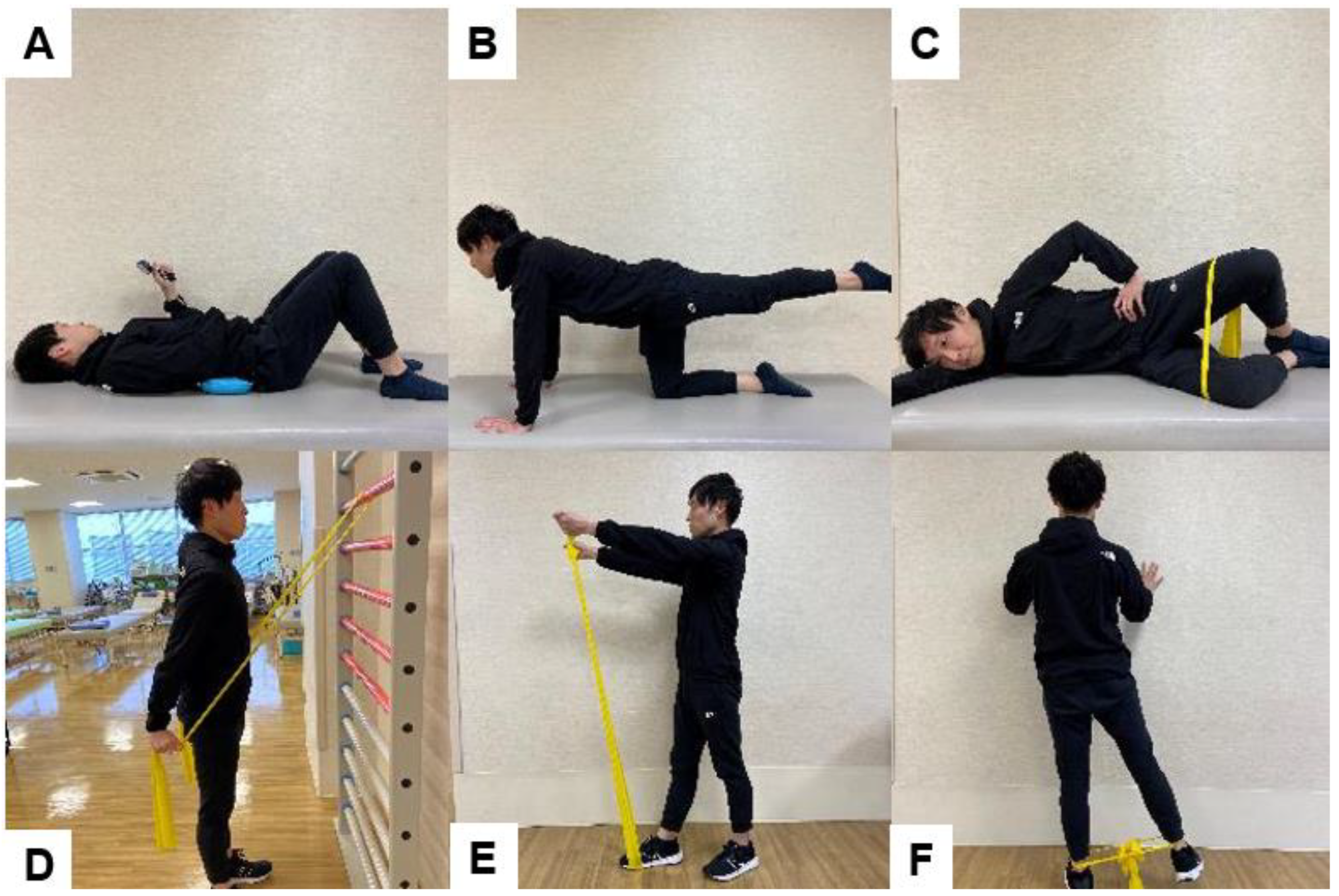
6.2. Psoas Muscle Strengthening
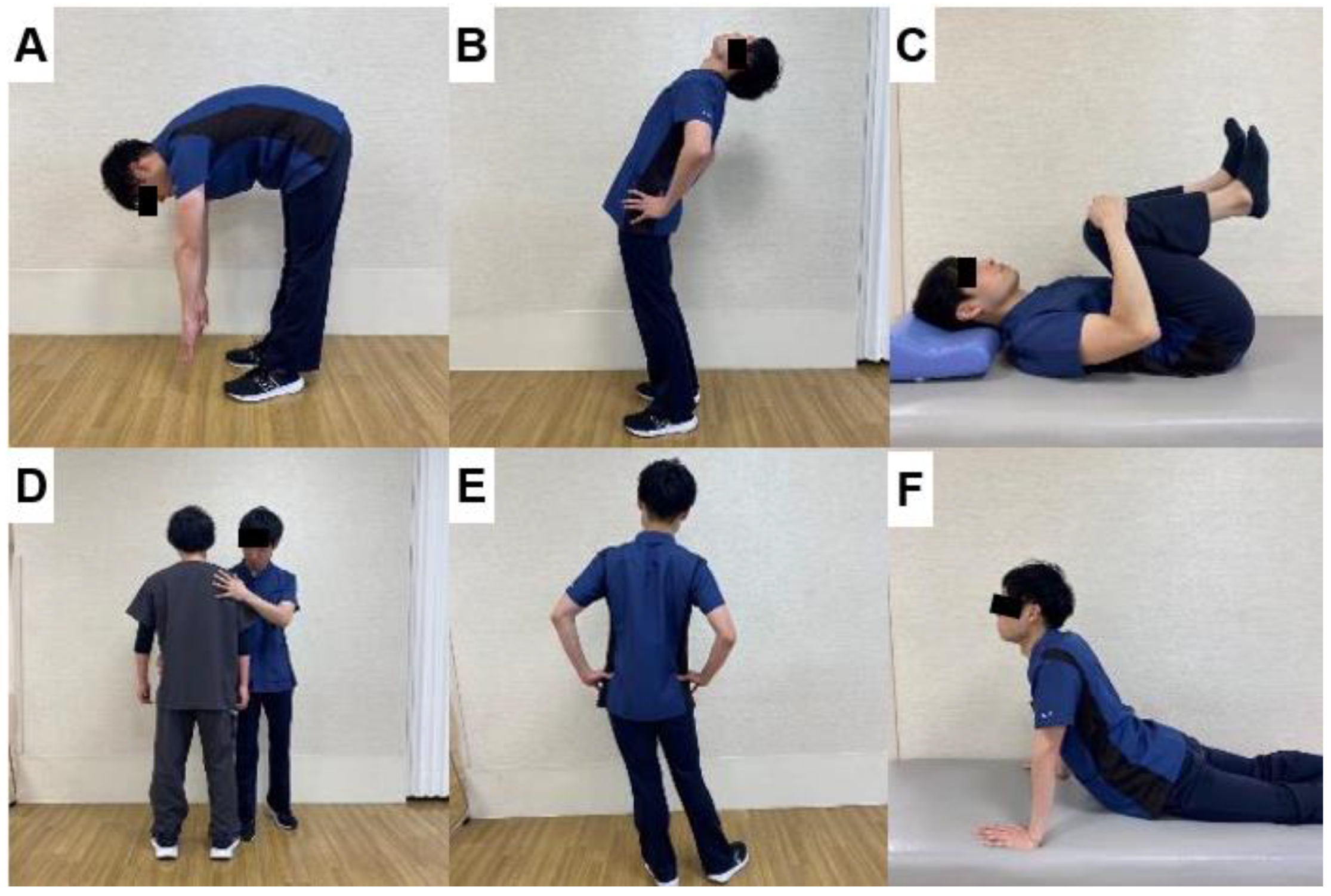
6.3. Exercises to Improve Balance after Spinal Fusion Surgery
6.4. Guidance on ADL after Spinal Fusion Surgery
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B

References
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar] [PubMed]
- Manchikanti, L.; Singh, V.; Falco, F.J.; Benyamin, R.M.; Hirsch, J.A. Epidemiology of low back pain in adults. Neuromodulation Technol. Neural Interface 2014, 17, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Tetreault, L.; Nater, A.; Choma, T.; Harrop, J.; Mroz, T.; Santaguida, C.; Smith, J.S. The aging of the global population: The changing epidemiology of disease and spinal disorders. Neurosurgery 2015, 77, S1–S5. [Google Scholar] [CrossRef]
- Sivasubramaniam, V.; Patel, H.C.; Ozdemir, B.A.; Papadopoulos, M.C. Trends in hospital admissions and surgical procedures for degenerative lumbar spine disease in England: A 15-year time-series study. BMJ Open 2015, 5, e009011. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Sato, K.; Kato, F.; Kanemura, T.; Yoshihara, H.; Sakai, Y.; Shinjo, R.; Ohara, T.; Yagi, H.; Matsubara, Y.; et al. Trends in the numbers of spine surgeries and spine surgeons over the past 15 years. Nagoya J. Med. Sci. 2022, 84, 155–162. [Google Scholar] [PubMed]
- Cancelliere, C.; Wong, J.J.; Yu, H.; Nordin, M.; Mior, S.; Pereira, P.; Brunton, G.; Shearer, H.; Connell, G.; Verville, L.; et al. Postsurgical rehabilitation for adults with low back pain with or without radiculopathy who were treated surgically: Protocol for a mixed studies systematic review. BMJ Open 2020, 10, e036817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ebenbichler, G.R.; Inschlag, S.; Pflüger, V.; Stemberger, R.; Wiesinger, G.; Novak, K.; Christoph, K.; Resch, K.L. Twelve-year follow-up of a randomized controlled trial of comprehensive physiotherapy following disc herniation operation. Clin. Rehabil. 2015, 29, 548–560, Erratum in Clin. Rehabil. 2016, 30, 623. [Google Scholar] [CrossRef] [PubMed]
- Archer, K.R.; Devin, C.J.; Vanston, S.W.; Koyama, T.; Phillips, S.E.; Mathis, S.L.; George, S.Z.; McGirt, M.J.; Spengler, D.M.; Aaronson, O.S.; et al. Cognitive-Behavioral-Based Physical Therapy for Patients With Chronic Pain Undergoing Lumbar Spine Surgery: A Randomized Controlled Trial. J. Pain 2016, 17, 76–89, Erratum in J. Pain 2017, 18, 477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindgreen, P.; Rolving, N.; Nielsen, C.V.; Lomborg, K. Interdisciplinary Cognitive-Behavioral Therapy as Part of Lumbar Spinal Fusion Surgery Rehabilitation: Experience of Patients With Chronic Low Back Pain. Orthop. Nurs. 2016, 35, 238–247. [Google Scholar] [CrossRef]
- Rushton, A.; Eveleigh, G.; Petherick, E.J.; Heneghan, N.; Bennett, R.; James, G.; Wright, C. Physiotherapy rehabilitation following lumbar spinal fusion: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2012, 2, e000829. [Google Scholar] [CrossRef]
- Wibault, J.; Öberg, B.; Dedering, Å.; Löfgren, H.; Zsigmond, P.; Peolsson, A. Structured postoperative physiotherapy in patients with cervical radiculopathy: 6-month outcomes of a randomized clinical trial. J. Neurosurg. Spine 2017, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, L.; Thys, T.; Depreitere, B.; Dankaerts, W.; Amerijckx, C.; Van Wambeke, P.; Jacobs, K.; Boonen, H.; Brumagne, S.; Moke, L.; et al. Rehabilitation to improve outcomes of lumbar fusion surgery: A systematic review with meta-analysis. Eur. Spine J. 2022, 31, 1525–1545. [Google Scholar] [CrossRef] [PubMed]
- Manni, T.; Ferri, N.; Vanti, C.; Ferrari, S.; Cuoghi, I.; Gaeta, C.; Sgaravatti, I.; Pillastrini, P. Rehabilitation after lumbar spine surgery in adults: A systematic review with meta-analysis. Arch. Physiother. 2023, 13, 21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ditunno, J.F. Linking spinal cord injury rehabilitation between the World Wars: The R. Tait McKenzie legacy. J. Spinal Cord Med. 2017, 40, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Shrosbree, R.D. Spinal cord injuries as a result of motorcycle accidents. Paraplegia 1978, 16, 102–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bolton, D. Looking forward to a decade of the biopsychosocial model. BJPsych Bull. 2022, 46, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Ríos, J.R.; Nava-Bringas, T.I. Lumbar stabilization exercises. Cir. Cir. 2014, 82, 306–313. [Google Scholar]
- Dydyk, A.M.; Sapra, A. Williams Back Exercises; Stat Pearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551558/ (accessed on 4 April 2024).
- Kelly, R.P.; Johnson, J.T. Acute low back pain. J. Am. Med. Assoc. 1955, 158, 1520–1521. [Google Scholar] [CrossRef]
- Pheasant, H.C. Practical posture building. Clin. Orthop. 1962, 25, 83–91. [Google Scholar] [PubMed]
- Cailliet, R. Low Back Pain Syndrome; Daves Company: Philadelphia, PA, USA, 1998; pp. 156–178. [Google Scholar]
- Böhler, L. Ubungsbehandlung von Wirbelbrüchen [Exercise therapy in vertebral fractures]. Hefte Unfallheilkd. 1971, 108, 60–63. [Google Scholar]
- McKenzie, R. Acute low back ache and exercises. N. Z. Med. J. 1994, 107, 318. [Google Scholar] [PubMed]
- Niederer, D.; Mueller, J. Sustainability effects of motor control stabilisation exercises on pain and function in chronic nonspecific low back pain patients: A systematic review with meta-analysis and meta-regression. PLoS ONE 2020, 15, e0227423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO Guideline for Non-Surgical Management of Chronic Primary Low Back Pain in Adults. Available online: https://www.who.int/publications/i/item/9789240081789 (accessed on 4 April 2024).
- The World Health Organization World Report on Disability: Chapter 4 Rehabilitation. Available online: https://www.spine.org/Documents/ResearchClinicalCare/Guidelines/LumbarDiscHerniation.pdf (accessed on 4 April 2024).
- Snowdon, M.; Peiris, C.L. Physiotherapy Commenced Within the First Four Weeks Post-Spinal Surgery Is Safe and Effective: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2016, 97, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Elsayyad, M.M.; Abdel-Aal, N.M.; Helal, M.E. Effect of Adding Neural Mobilization Versus Myofascial Release to Stabilization Exercises after Lumbar Spine Fusion: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2021, 102, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Daniels, C.J.; Cupler, Z.A.; Gliedt, J.A.; Walters, S.; Schielke, A.L.; Hinkeldey, N.A.; Golley, D.J.; Hawk, C. Manipulative and manual therapies in the management of patients with prior lumbar surgery: A systematic review. Complement. Ther. Clin. Pract. 2021, 42, 101261. [Google Scholar] [CrossRef] [PubMed]
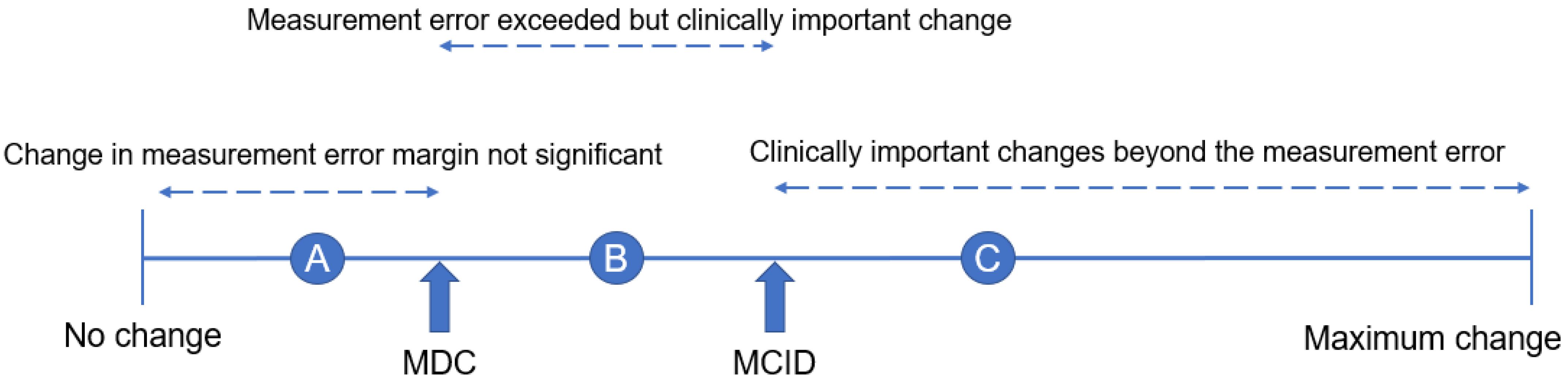
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef] [PubMed]
- van Hooff, M.L.; Mannion, A.F.; Staub, L.P.; Ostelo, R.W.; Fairbank, J.C. Determination of the Oswestry Disability Index score equivalent to a “satisfactory symptom state” in patients undergoing surgery for degenerative disorders of the lumbar spine—A Spine Tango registry-based study. Spine J. 2016, 16, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Issa, T.Z.; Lee, Y.; Henry, T.W.; Trenchfield, D.; Schroeder, G.D.; Vaccaro, A.R.; Kepler, C.K. Values derived from patient reported outcomes in spine surgery: A systematic review of the minimal clinically important difference, substantial clinical benefit, and patient acceptable symptom state. Eur. Spine J. 2023, 32, 3333–3351. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.L.; Adogwa, O.; Paul, A.R.; Anderson, W.N.; Aaronson, O.; Cheng, J.S.; McGirt, M.J. Utility of minimum clinically important difference in assessing pain, disability, and health state after transforaminal lumbar interbody fusion for degenerative lumbar spondylolisthesis. J. Neurosurg. Spine 2011, 14, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.L.; Adogwa, O.; Mendenhall, S.K.; Shau, D.N.; Anderson, W.N.; Cheng, J.S.; Devin, C.J.; McGirt, M.J. Determination of minimum clinically important difference (MCID) in pain, disability, and quality of life after revision fusion for symptomatic pseudoarthrosis. Spine J. 2012, 12, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, L.G.; Hellum, C.; Nygaard, O.P.; Storheim, K.; Brox, J.I.; Rossvoll, I.; Leivseth, G.; Grotle, M. Comparison of the SF6D, the EQ5D, and the oswestry disability index in patients with chronic low back pain and degenerative disc disease. BMC Musculoskelet. Disord. 2013, 14, 148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Solberg, T.; Johnsen, L.G.; Nygaard, Ø.P.; Grotle, M. Can we define success criteria for lumbar disc surgery?: Estimates for a substantial amount of improvement in core outcome measures. Acta Orthop. 2013, 84, 196–201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoshida, G.; Hasegawa, T.; Yamato, Y.; Kobayashi, S.; Shin, O.; Banno, T.; Mihara, Y.; Arima, H.; Ushirozako, H.; Yasuda, T.; et al. Minimum Clinically Important Differences in Oswestry Disability Index Domains and Their Impact on Adult Spinal Deformity Surgery. Asian Spine J. 2019, 13, 35–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukushima, M.; Oka, H.; Oshima, Y.; Yuzawa, Y.; Matsudaira, K.; Tanaka, S.; Inanami, H. Evaluation of the Minimum Clinically Important Differences of the Zurich Claudication Questionnaire in Patients With Lumbar Spinal Stenosis. Clin. Spine Surg. 2020, 33, E499–E503. [Google Scholar] [CrossRef] [PubMed]
- Roland, M.; Morris, R. A study of the natural history of back pain. Part I: Development of a reliable and sensitive measure of disability in low-back pain. Spine 1983, 8, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Kobayashi, N.; Saiki, K.; Kitagawa, T.; Tamai, K.; Saotome, K. Association of the Japanese Orthopaedic Association score with the Oswestry Disability Index, Roland-Morris Disability Questionnaire, and short-form 36. Spine 2003, 28, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Kersten, R.F.M.R.; Fikkers, J.; Wolterbeek, N.; Öner, F.C.; van Gaalen, S.M. Are the Roland Morris Disability Questionnaire and Oswestry Disability Index interchangeable in patients after lumbar spinal fusion? J. Back Musculoskelet. Rehabil. 2021, 34, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry Disability Index. Spine 2000, 25, 2940–2952; discussion 2952. [Google Scholar] [CrossRef] [PubMed]
- Vianin, M. Psychometric properties and clinical usefulness of the Oswestry Disability Index. J. Chiropr. Med. 2008, 7, 161–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haro, H.; Maekawa, S.; Hamada, Y. Prospective analysis of clinical evaluation and self-assessment by patients after decompression surgery for degenerative lumbar canal stenosis. Spine J. 2008, 8, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Carreon, L.Y.; Berven, S.H.; Djurasovic, M.; Bratcher, K.R.; Glassman, S.D. The discriminative properties of the SF-6D compared with the SF-36 and ODI. Spine 2013, 38, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, K.; Widbom-Kolhanen, S.; Pernaa, K.; Arokoski, J.; Saltychev, M. Reliability and validity of Oswestry Disability Index among patients undergoing lumbar spinal surgery. BMC Surg. 2024, 24, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stucki, G.; Daltroy, L.; Liang, M.H.; Lipson, S.J.; Fossel, A.H.; Katz, J.N. Measurement properties of a self-administered outcome measure in lumbar spinal stenosis. Spine 1996, 21, 796–803. [Google Scholar] [CrossRef] [PubMed]
- North American Spine Society (NASS). Clinical Guidelines for Multidisciplinary Spine Care Diagnosis and Treatment of Degenerative Lumbar Spinal Stenosis; North American Spine Society (NASS): Burr Ridge, IL, USA, 2007. [Google Scholar]
- Hara, N.; Matsudaira, K.; Masuda, K.; Tohnosu, J.; Takeshita, K.; Kobayashi, A.; Murakami, M.; Kawamura, N.; Yamakawa, K.; Terayama, S.; et al. Psychometric Assessment of the Japanese Version of the Zurich Claudication Questionnaire (ZCQ): Reliability and Validity. PLoS ONE 2016, 11, e0160183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bae, J.; Theologis, A.A.; Strom, R.; Tay, B.; Burch, S.; Berven, S.; Mummaneni, P.V.; Chou, D.; Ames, C.P.; Deviren, V. Comparative analysis of 3 surgical strategies for adult spinal deformity with mild to moderate sagittal imbalance. J. Neurosurg. Spine 2018, 28, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Lonner, B.; Yoo, A.; Terran, J.S.; Sponseller, P.; Samdani, A.; Betz, R.; Shuffelbarger, H.; Shah, S.A.; Newton, P. Effect of spinal deformity on adolescent quality of life: Comparison of operative scheuermann kyphosis, adolescent idiopathic scoliosis, and normal controls. Spine 2013, 38, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Haher, T.R.; Gorup, J.M.; Shin, T.M.; Homel, P.; Merola, A.A.; Grogan, D.P.; Pugh, L.; Lowe, T.G.; Murray, M. Results of the Scoliosis Research Society instrument for evaluation of surgical outcome in adolescent idiopathic scoliosis. A multicenter study of 244 patients. Spine 1999, 24, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Berven, S.; Deviren, V.; Demir-Deviren, S.; Hu, S.S.; Bradford, D.S. Studies in the modified Scoliosis Research Society Outcomes Instrument in adults: Validation, reliability, and discriminatory capacity. Spine 2003, 28, 2164–2169; discussion 2169. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, H.; Hasegawa, K.; Okamoto, M.; Hatsushikano, S.; Watanabe, K. Relationship between sagittal radiographic parameters and disability in patients with spinal disease using 3D standing analysis. Orthop. Traumatol. Surg. Res. 2018, 104, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Klineberg, E.; Schwab, F.; Shaffrey, C.I.; Moal, B.; Ames, C.P.; Hostin, R.; Fu, K.M.; Burton, D.; Akbarnia, B.; et al. Change in classification grade by the SRS-Schwab Adult Spinal Deformity Classification predicts impact on health-related quality of life measures: Prospective analysis of operative and nonoperative treatment. Spine 2013, 38, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Inoue, H.; Arai, Y.; Shirado, O.; Doi, T.; Yamazaki, K.; Uno, K.; Yanagida, H.; Takeshita, K. Reliability and validity of a novel quality of life questionnaire for female patients with adolescent idiopathic scoliosis: Scoliosis Japanese Questionnaire-27: A multicenter, cross-sectional study. BMC Musculoskelet. Disord. 2018, 19, 99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
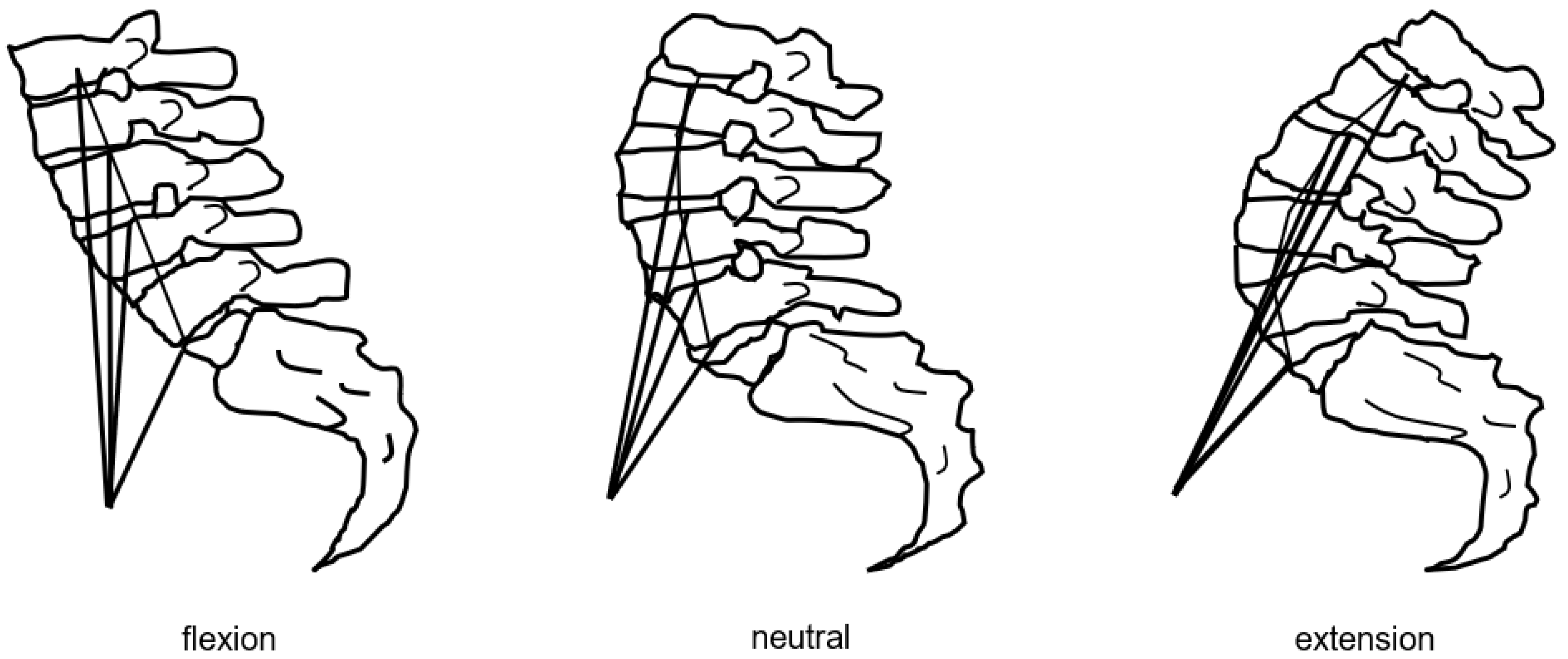
- Hart, R.A.; Pro, S.L.; Gundle, K.R.; Marshall, L.M. Lumbar stiffness as a collateral outcome of spinal arthrodesis: A preliminary clinical study. Spine J. 2013, 13, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.A.; Gundle, K.R.; Pro, S.L.; Marshall, L.M. Lumbar Stiffness Disability Index: Pilot testing of consistency, reliability, and validity. Spine J. 2013, 13, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Furuya, H.; Ito, T.; Hirohata, K.; Mitomo, S.; Yamasaki, K.; Igarashi, H.; Omori, K.; Hoshino, M.; Hart, R.A. Construct Validity and Reliability of the Japanese Version of the Lumbar Stiffness Disability Index. Spine 2021, 46, E333–E337. [Google Scholar] [CrossRef] [PubMed]
- Durand, W.M.; Daniels, A.H.; Hamilton, D.K.; Passias, P.G.; Kim, H.J.; Protopsaltis, T.; Lafage, V.; Smith, J.S.; Shaffrey, C.; Gupta, M.; et al. Younger Patients Are Differentially Affected by Stiffness-Related Disability Following Adult Spinal Deformity Surgery. World Neurosurg. 2019, 132, e297–e304. [Google Scholar] [CrossRef] [PubMed]
- Daniels, A.H.; Reid, D.; Durand, W.; Disilvestro, K.; Hamilton, D.K.; Passias, P.; Kim, H.J.; Protopsaltis, T.; LaFage, V.; Smith, J.S.; et al. Sexual Dysfunction Secondary to Lumbar Stiffness in Adult Spinal Deformity Patients Before and After Long-Segment Spinal Fusion. World Neurosurg. 2020, 139, e474–e479. [Google Scholar] [CrossRef] [PubMed]
- Watters, W.C., 3rd; Baisden, J.; Gilbert, T.J.; Kreiner, S.; Resnick, D.K.; Bono, C.M.; Ghiselli, G.; Heggeness, M.H.; Mazanec, D.J.; O’Neill, C.; et al. Degenerative lumbar spinal stenosis: An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J. 2008, 8, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, Y.; Yoshimura, N.; Muraki, S.; Yamada, H.; Nagata, K.; Hashizume, H.; Takiguchi, N.; Minamide, A.; Oka, H.; Kawaguchi, H.; et al. Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: The Wakayama Spine Study. Osteoarthr. Cartil. 2012, 20, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Mirza, S.K.; Martin, B.I.; Kreuter, W.; Goodman, D.C.; Jarvik, J.G. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 2010, 303, 1259–1265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagai, S.; Kawabata, S.; Michikawa, T.; Ito, K.; Takeda, H.; Ikeda, D.; Kaneko, S.; Fujita, N. Association between frailty and locomotive syndrome in elderly patients with lumbar spinal stenosis: A retrospective longitudinal analysis. Geriatr. Gerontol. Int. 2024, 24, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Demura, S.; Kabata, T.; Matsubara, H.; Kurokawa, Y.; Okamoto, Y.; Kuroda, K.; Kajino, Y.; Yokogawa, N.; Inoue, D.; et al. Risk factors that hinder locomotive syndrome improvement following surgery for musculoskeletal diseases in older patients: A multicentre prospective study. Mod. Rheumatol. 2023, 33, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Morimoto, T.; Otani, K.; Mawatari, M. Locomotive Syndrome and Lumbar Spine Disease: A Systematic Review. J. Clin. Med. 2022, 11, 1304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31, Erratum in Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wada, T.; Tanishima, S.; Kitsuda, Y.; Osaki, M.; Nagashima, H.; Noma, H.; Hagino, H. Walking speed is associated with postoperative pain catastrophizing in patients with lumbar spinal stenosis: A prospective observational study. BMC Musculoskelet. Disord. 2022, 23, 1108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakaguchi, T.; Meena, U.; Tanaka, M.; Xiang, H.; Fujiwara, Y.; Arataki, S.; Taoka, T.; Takamatsu, K.; Yasuda, Y.; Nakagawa, M.; et al. Minimal Clinically Important Differences in Gait and Balance Ability in Patients Who Underwent Corrective Long Spinal Fusion for Adult Spinal Deformity. J. Clin. Med. 2023, 12, 6500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, S.J.; Puhan, M.A.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, H.; Kamiya, M.; Sugiura, H.; Nishihama, K.; Ito, A.; Suzuki, J.; Hanamura, S. Responsiveness and Minimal Clinically Important Difference of the 6-minute Walk Distance in Patients Undergoing Lumbar Spinal Canal Stenosis Surgery. Clin. Spine Surg. 2022, 35, E345–E350. [Google Scholar] [CrossRef] [PubMed]
- Kondo, R.; Yamato, Y.; Nagafusa, T.; Mizushima, T.; Hasegawa, T.; Kobayashi, S.; Togawa, D.; Oe, S.; Kurosu, K.; Matsuyama, Y. Effect of corrective long spinal fusion to the ilium on physical function in patients with adult spinal deformity. Eur. Spine J. 2017, 26, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Tomkins-Lane, C.C.; Battié, M.C. Validity and reproducibility of self-report measures of walking capacity in lumbar spinal stenosis. Spine 2010, 35, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, H.; Kamiya, M.; Sugiura, H.; Nishihama, K.; Suzuki, J.; Hanamura, S. Minimal Clinically Important Difference of the 6-Minute Walk Distance in Patients Undergoing Lumbar Spinal Canal Stenosis Surgery: 12 Months Follow-Up. Spine 2023, 48, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Gautschi, O.P.; Smoll, N.R.; Corniola, M.V.; Joswig, H.; Chau, I.; Hildebrandt, G.; Schaller, K.; Stienen, M.N. Validity and Reliability of a Measurement of Objective Functional Impairment in Lumbar Degenerative Disc Disease: The Timed Up and Go (TUG) Test. Neurosurgery 2016, 79, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.I.; Lin, R.M. Disability and walking capacity in patients with lumbar spinal stenosis: Association with sensorimotor function, balance, and functional performance. J. Orthop. Sports Phys. Ther. 2005, 35, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Joswig, H.; Stienen, M.N.; Smoll, N.R.; Corniola, M.V.; Chau, I.; Schaller, K.; Hildebrandt, G.; Gautschi, O.P. Effects of Smoking on Subjective and Objective Measures of Pain Intensity, Functional Impairment, and Health-Related Quality of Life in Lumbar Degenerative Disk Disease. World Neurosurg. 2017, 99, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Stienen, M.N.; Joswig, H.; Smoll, N.R.; Corniola, M.V.; Schaller, K.; Hildebrandt, G.; Gautschi, O.P. Influence of Body Mass Index on Subjective and Objective Measures of Pain, Functional Impairment, and Health-Related Quality of Life in Lumbar Degenerative Disc Disease. World Neurosurg. 2016, 96, 570–577.e1. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Maldaner, N.; Sosnova, M.; Ziga, M.; Zeitlberger, A.M.; Bozinov, O.; Gautschi, O.P.; Weyerbrock, A.; Regli, L.; Stienen, M.N. External Validation of the Minimum Clinically Important Difference in the Timed-up-and-go Test After Surgery for Lumbar Degenerative Disc Disease. Spine 2022, 47, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional reach: A new clinical measure of balance. J. Gerontol. 1990, 45, M192–M197. [Google Scholar] [CrossRef] [PubMed]
- Schenkman, M.; Morey, M.; Kuchibhatla, M. Spinal flexibility and balance control among community-dwelling adults with and without Parkinson’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M441–M445. [Google Scholar] [CrossRef] [PubMed]
- de Waroquier-Leroy, L.; Bleuse, S.; Serafi, R.; Watelain, E.; Pardessus, V.; Tiffreau, A.V.; Thevenon, A. The Functional Reach Test: Strategies, performance and the influence of age. Ann. Phys. Rehabil. Med. 2014, 57, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, F. Physical measurements as risk indicators for low-back trouble over a one-year period. Spine 1984, 9, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Araghi, K.; Dupont, M.M.; Shahi, P.; Bovonratwet, P.; Shinn, D.; Dalal, S.S.; Melissaridou, D.; Virk, S.S.; Iyer, S.; et al. Association between muscle health and patient-reported outcomes after lumbar microdiscectomy: Early results. Spine J. 2022, 22, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Wrisley, D.M.; Frank, J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys. Ther. 2009, 89, 484–498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moke, L.; Severijns, P.; Schelfaut, S.; Van de Loock, K.; Hermans, L.; Molenaers, G.; Jonkers, I.; Scheys, L. Performance on Balance Evaluation Systems Test (BESTest) Impacts Health-Related Quality of Life in Adult Spinal Deformity Patients. Spine 2018, 43, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Severijns, P.; Overbergh, T.; Scheys, L.; Moke, L.; Desloovere, K. Reliability of the balance evaluation systems test and trunk control measurement scale in adult spinal deformity. PLoS ONE 2019, 14, e0221489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franchignoni, F.; Horak, F.; Godi, M.; Nardone, A.; Giordano, A. Using psychometric techniques to improve the Balance Evaluation Systems Test: The mini-BESTest. J. Rehabil. Med. 2010, 42, 323–331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miura, K.; Kadone, H.; Koda, M.; Abe, T.; Funayama, T.; Noguchi, H.; Mataki, K.; Nagashima, K.; Kumagai, H.; Shibao, Y.; et al. Thoracic kyphosis and pelvic anteversion in patients with adult spinal deformity increase while walking: Analyses of dynamic alignment change using a three-dimensional gait motion analysis system. Eur. Spine J. 2020, 29, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Asada, T.; Miura, K.; Koda, M.; Kadone, H.; Funayama, T.; Takahashi, H.; Noguchi, H.; Shibao, Y.; Sato, K.; Eto, F.; et al. Can Proximal Junctional Kyphosis after Surgery for Adult Spinal Deformity Be Predicted by Preoperative Dynamic Sagittal Alignment Change with 3D Gait Analysis? A Case-Control Study. J. Clin. Med. 2022, 11, 5871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haddas, R.; Wood, A.; Lieberman, I.; Derman, P.B. Assessing the cone of economy in patients with spinal disease using only a force plate: An observational retrospective cohort study. Eur. Spine J. 2021, 30, 2504–2513. [Google Scholar] [CrossRef] [PubMed]
- Godzik, J.; Frames, C.W.; Smith Hussain, V.; Olson, M.C.; Kakarla, U.K.; Uribe, J.S.; Lockhart, T.E.; Turner, J.D. Postural Stability and Dynamic Balance in Adult Spinal Deformity: Prospective Pilot Study. World Neurosurg. 2020, 141, e783–e791. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Ohne, H.; Konomi, T.; Fujiyoshi, K.; Kaneko, S.; Takemitsu, M.; Machida, M.; Yato, Y.; Asazuma, T. Walking balance and compensatory gait mechanisms in surgically treated patients with adult spinal deformity. Spine J. 2017, 17, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Huysmans, S.M.D.; Senden, R.; Jacobs, E.; Willems, P.J.B.; Marcellis, R.G.J.; Boogaart, M.V.D.; Meijer, K.; Willems, P.C. Gait alterations in patients with adult spinal deformity. N. Am. Spine Soc. J. 2023, 17, 100306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, R.; Rau, P.P. Are cost-effective technologies feasible to measure gait in older adults? A systematic review of evidence-based literature. Arch. Gerontol. Geriatr. 2020, 87, 103970. [Google Scholar] [CrossRef] [PubMed]
- Sekine, M.; Tamura, T.; Yoshida, M.; Suda, Y.; Kimura, Y.; Miyoshi, H.; Kijima, Y.; Higashi, Y.; Fujimoto, T. A gait abnormality measure based on root mean square of trunk acceleration. J. Neuroeng. Rehabil. 2013, 10, 118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakaguchi, T.; Sake, N.; Tanaka, M.; Fujiwara, Y.; Arataki, S.; Taoka, T.; Kodama, Y.; Takamatsu, K.; Yasuda, Y.; Nakagawa, M.; et al. Use of a Triaxial Accelerometer to Measure Changes in Gait Sway and Related Motor Function after Corrective Spinal Fusion Surgery for Adult Spinal Deformity. J. Clin. Med. 2024, 13, 1923. [Google Scholar] [CrossRef] [PubMed]
- Hulleck, A.A.; Menoth Mohan, D.; Abdallah, N.; El Rich, M.; Khalaf, K. Present and future of gait assessment in clinical practice: Towards the application of novel trends and technologies. Front. Med. Technol. 2022, 4, 901331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait Posture 2003, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Amundsen, T.; Weber, H.; Nordal, H.J.; Magnaes, B.; Abdelnoor, M.; Lilleâs, F. Lumbar spinal stenosis: Conservative or surgical management?: A prospective 10-year study. Spine 2000, 25, 1424–1435; discussion 1435–1436. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.F.; Liu, S.H.; Chen, Z.X.; Fei, Q.M. Decompression plus fusion versus decompression alone for degenerative lumbar spondylolisthesis: A systematic review and meta-analysis. Eur. Spine J. 2017, 26, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, P.; Feng, F.; Chhantyal, K.; Yang, Y.; Rong, L. Decompression Alone Versus Decompression and Fusion for Lumbar Degenerative Spondylolisthesis: A Meta-Analysis. World Neurosurg. 2018, 111, e165–e177. [Google Scholar] [CrossRef] [PubMed]
- Briggs, H.; Milligan, P.R. Chip fusion of the low back following exploration of the spinal canal. J. Bone Jt. Surg. 1944, 26, 125–130. [Google Scholar]
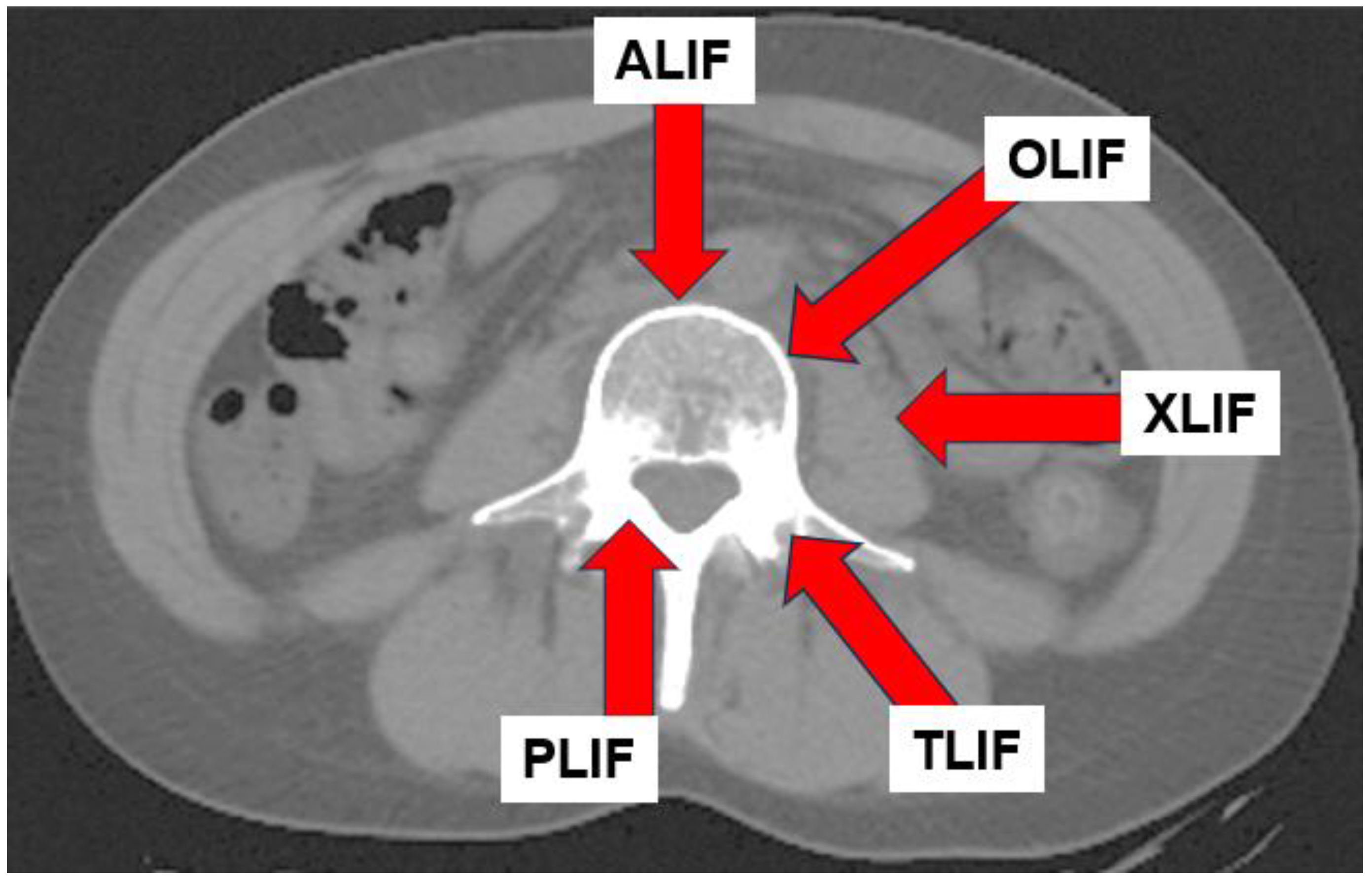
- Hammad, A.; Wirries, A.; Ardeshiri, A.; Nikiforov, O.; Geiger, F. Open versus minimally invasive TLIF: Literature review and meta-analysis. J. Orthop. Surg. Res. 2019, 14, 229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, M.W.P.; Sayampanathan, A.A.; Jiang, L.; Guo, C.M. Comparison of Outcomes Between Single-level Lateral Lumbar Interbody Fusion and Transforaminal Lumbar Interbody Fusion: A Meta-analysis and Systematic Review. Clin. Spine Surg. 2021, 34, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.C.; Koski, T.R.; O’Shaughnessy, B.A.; Sugrue, P.; Salehi, S.; Ondra, S.; Liu, J.C. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: Implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J. Neurosurg. Spine. 2007, 7, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Mobbs, R.J.; Phan, K.; Malham, G.; Seex, K.; Rao, P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015, 1, 2–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waschke, A.; Hartmann, C.; Walter, J.; Dünisch, P.; Wahnschaff, F.; Kalff, R.; Ewald, C. Denervation and atrophy of paraspinal muscles after open lumbar interbody fusion is associated with clinical outcome--electromyographic and CT-volumetric investigation of 30 patients. Acta Neurochir. 2014, 156, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Kim, S.H.; Ha, S.K.; Kim, S.D.; Lim, D.J.; Cha, J.; Kim, B.J. Paraspinal muscle changes after single-level posterior lumbar fusion: Volumetric analyses and literature review. BMC Musculoskelet. Disord. 2020, 21, 73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tarnanen, S.; Neva, M.H.; Kautiainen, H.; Ylinen, J.; Pekkanen, L.; Kaistila, T.; Vuorenmaa, M.; Häkkinen, A. The early changes in trunk muscle strength and disability following lumbar spine fusion. Disabil. Rehabil. 2013, 35, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Tarnanen, S.P.; Neva, M.H.; Häkkinen, K.; Kankaanpää, M.; Ylinen, J.; Kraemer, W.J.; Newton, R.U.; Häkkinen, A. Neutral spine control exercises in rehabilitation after lumbar spine fusion. J. Strength Cond. Res. 2014, 28, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Hagins, M.; Adler, K.; Cash, M.; Daugherty, J.; Mitrani, G. Effects of practice on the ability to perform lumbar stabilization exercises. J. Orthop. Sports Phys. Ther. 1999, 29, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Grooms, D.R.; Grindstaff, T.L.; Croy, T.; Hart, J.M.; Saliba, S.A. Clinimetric analysis of pressure biofeedback and transversus abdominis function in individuals with stabilization classification low back pain. J. Orthop. Sports Phys. Ther. 2013, 43, 184–193. [Google Scholar] [CrossRef] [PubMed]
- McGill, S.M.; Karpowicz, A.; Fenwick, C.M.; Brown, S.H. Exercises for the torso performed in a standing posture: Spine and hip motion and motor patterns and spine load. J. Strength. Cond. Res. 2009, 23, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Voight, M.L.; Hoogenboom, B.J.; Cook, G. The chop and lift reconsidered: Integrating neuromuscular principles into orthopedic and sports rehabilitation. N. Am. J. Sports Phys. Ther. 2008, 3, 151–159. [Google Scholar] [PubMed] [PubMed Central]
- Tarnanen, S.; Neva, M.H.; Dekker, J.; Häkkinen, K.; Vihtonen, K.; Pekkanen, L.; Häkkinen, A. Randomized controlled trial of postoperative exercise rehabilitation program after lumbar spine fusion: Study protocol. BMC Musculoskelet. Disord. 2012, 13, 123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
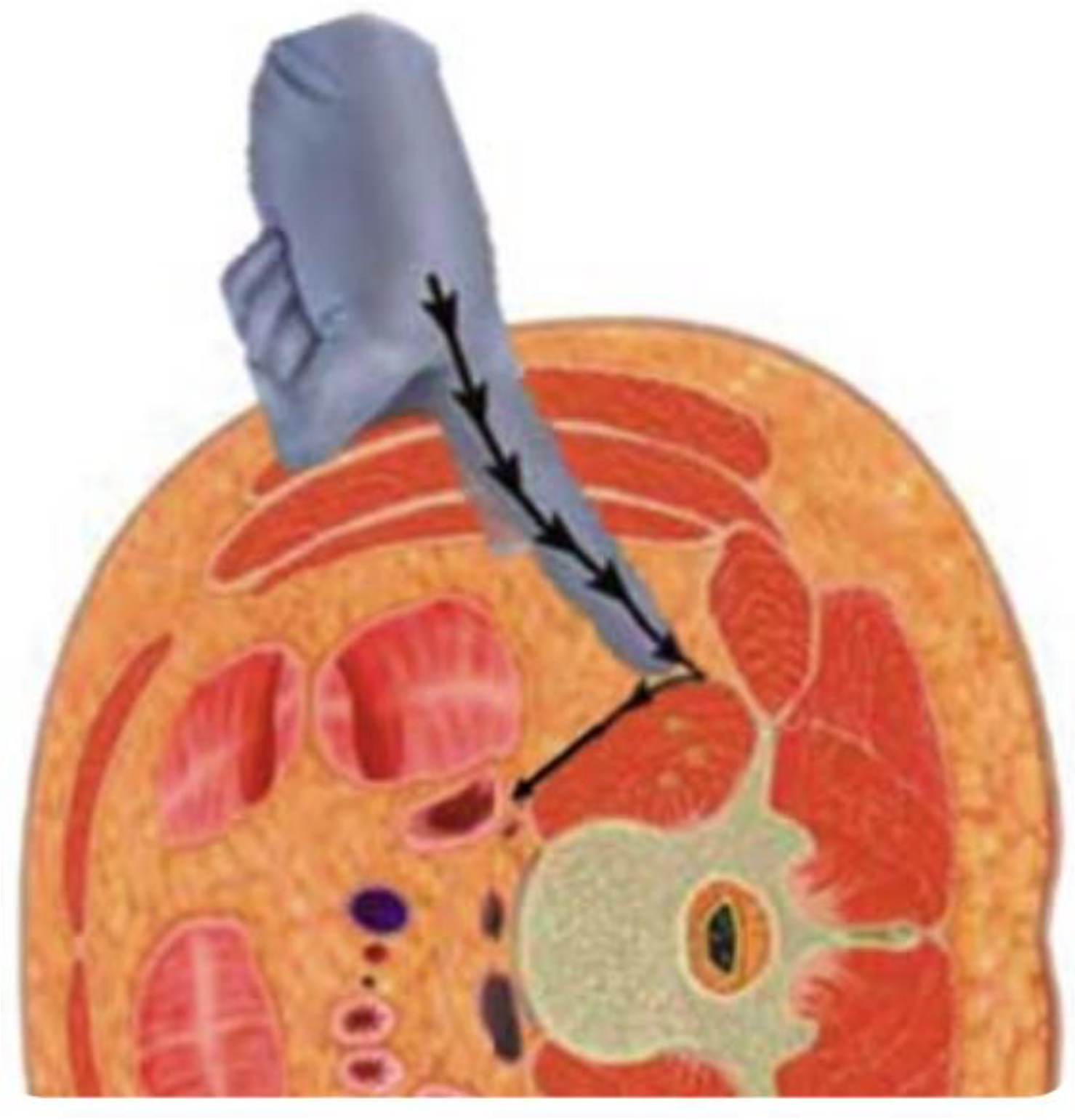
- Epstein, N.E. Review of Risks and Complications of Extreme Lateral Interbody Fusion (XLIF). Surg. Neurol. Int. 2019, 10, 237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.X.; Phan, K.; Mobbs, R. Oblique Lumbar Interbody Fusion: Technical Aspects, Operative Outcomes, and Complications. World Neurosurg. 2017, 98, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Fujita, N.; Hasegawa, T.; Inoue, G.; Kotani, Y.; Ohtori, S.; Orita, S.; Oshima, Y.; Sakai, D.; Sakai, T.; et al. Nationwide Survey of the Surgical Complications Associated with Lateral Lumbar Interbody Fusion in 2015-2020. Spine Surg. Relat. Res. 2022, 7, 249–256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, M.K.; Kim, S.B.; Park, C.K.; Malla, H.P.; Kim, S.M. Cross-Sectional Area of the Lumbar Spine Trunk Muscle and Posterior Lumbar Interbody Fusion Rate: A Retrospective Study. Clin. Spine Surg. 2017, 30, E798–E803. [Google Scholar] [CrossRef] [PubMed]
- Santaguida, P.L.; McGill, S.M. The psoas major muscle: A three-dimensional geometric study. J. Biomech. 1995, 28, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Blondel, B.; Schwab, F.; Ungar, B.; Smith, J.; Bridwell, K.; Glassman, S.; Shaffrey, C.; Farcy, J.P.; Lafage, V. Impact of magnitude and percentage of global sagittal plane correction on health-related quality of life at 2-years follow-up. Neurosurgery 2012, 71, 341–348; discussion 348. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Nazareth, A.; Hussain, A.K.; Dmytriw, A.A.; Nambiar, M.; Nguyen, D.; Kerferd, J.; Phan, S.; Sutterlin, C., 3rd; Cho, S.K.; et al. Relationship between sagittal balance and adjacent segment disease in surgical treatment of degenerative lumbar spine disease: Meta-analysis and implications for choice of fusion technique. Eur. Spine J. 2018, 27, 1981–1991, Erratum in Eur. Spine J. 2021, 30, 3774. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Chang, C.L.; Tai, T.W. Incidence and risk factors for hip fracture in elderly patients undergoing lumbar spine surgery: A nationwide database study with 11-year follow-up. Osteoporos. Int. 2018, 29, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Ohne, H.; Kaneko, S.; Machida, M.; Yato, Y.; Asazuma, T. Does corrective spine surgery improve the standing balance in patients with adult spinal deformity? Spine J. 2018, 18, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Tanaka, M.; Suthar, H.; Fujiwara, Y.; Uotani, K.; Arataki, S.; Yamauchi, T.; Sugyo, A.; Takamatsu, K.; Yasuda, Y.; et al. Chronological Evaluation of Gait Ability and Posture Balance after Adult Spinal Deformity Surgery. Appl. Sci. 2022, 12, 4285. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Tanaka, M.; Sake, N.; Latka, K.; Fujiwara, Y.; Arataki, S.; Yamauchi, T.; Takamatsu, K.; Yasuda, Y.; Nakagawa, M.; et al. The Most Significant Factor Affecting Gait and Postural Balance in Patients’ Activities of Daily Living Following Corrective Surgery for Deformity of the Adult Spine. Medicina 2022, 58, 1118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halvarsson, A.; Dohrn, I.M.; Ståhle, A. Taking balance training for older adults one step further: The rationale for and a description of a proven balance training programme. Clin. Rehabil. 2015, 29, 417–425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agmon, M.; Belza, B.; Nguyen, H.Q.; Logsdon, R.G.; Kelly, V.E. A systematic review of interventions conducted in clinical or community settings to improve dual-task postural control in older adults. Clin. Interv. Aging 2014, 9, 477–492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kimura, H.; Fujibayashi, S.; Otsuki, B.; Takahashi, Y.; Nakayama, T.; Matsuda, S. Effects of Lumbar Stiffness After Lumbar Fusion Surgery on Activities of Daily Living. Spine 2016, 41, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Togawa, D.; Hasegawa, T.; Yamato, Y.; Yoshida, G.; Kobayashi, S.; Yasuda, T.; Oe, S.; Banno, T.; Arima, H.; Mihara, Y.; et al. Postoperative Disability After Long Corrective Fusion to the Pelvis in Elderly Patients With Spinal Deformity. Spine 2018, 43, E804–E812. [Google Scholar] [CrossRef] [PubMed]
- Rohlmann, A.; Pohl, D.; Bender, A.; Graichen, F.; Dymke, J.; Schmidt, H.; Bergmann, G. Activities of everyday life with high spinal loads. PLoS ONE 2014, 9, e98510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rohlmann, A.; Schwachmeyer, V.; Graichen, F.; Bergmann, G. Spinal loads during post-operative physiotherapeutic exercises. PLoS ONE 2014, 9, e102005. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
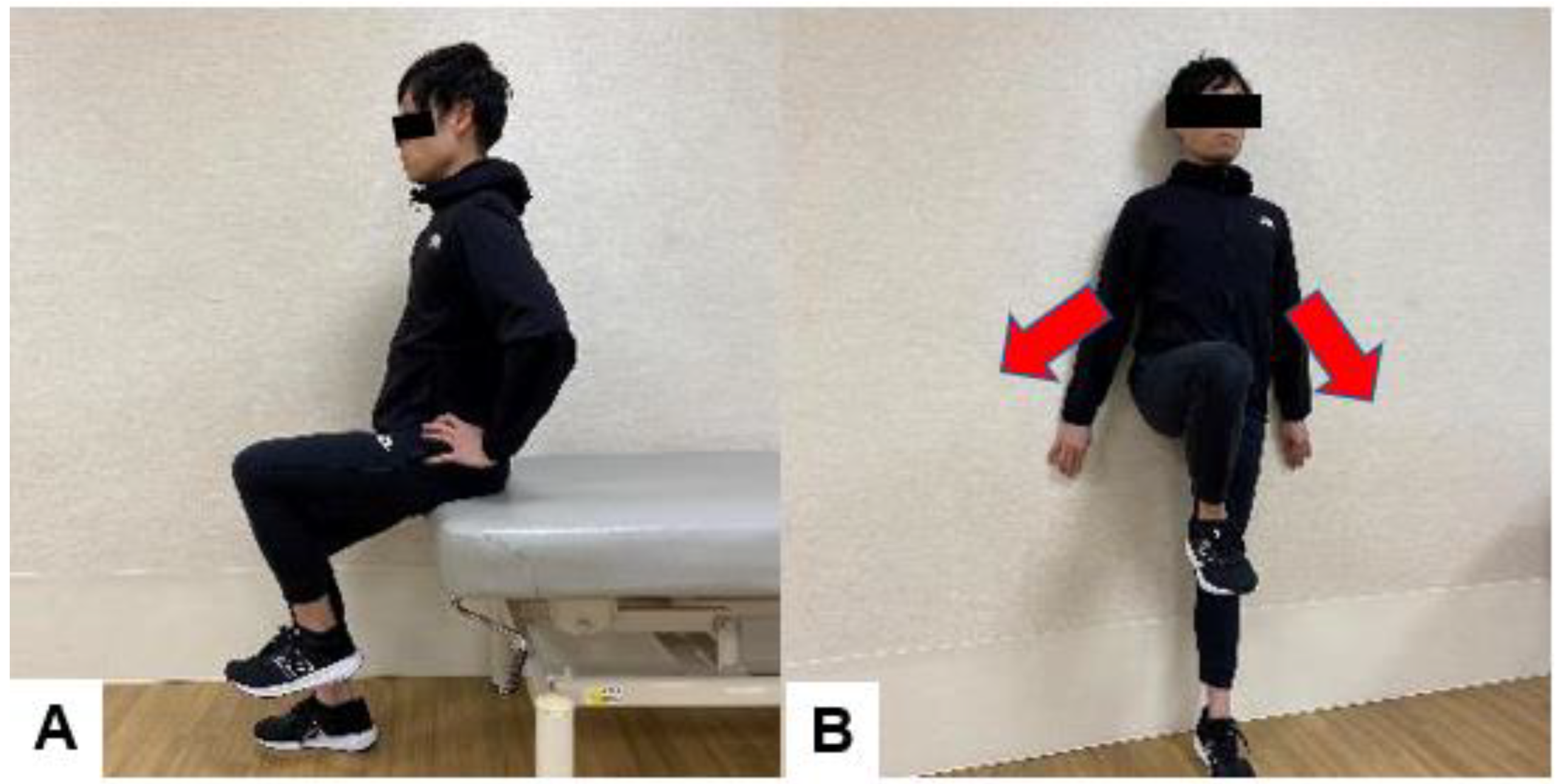
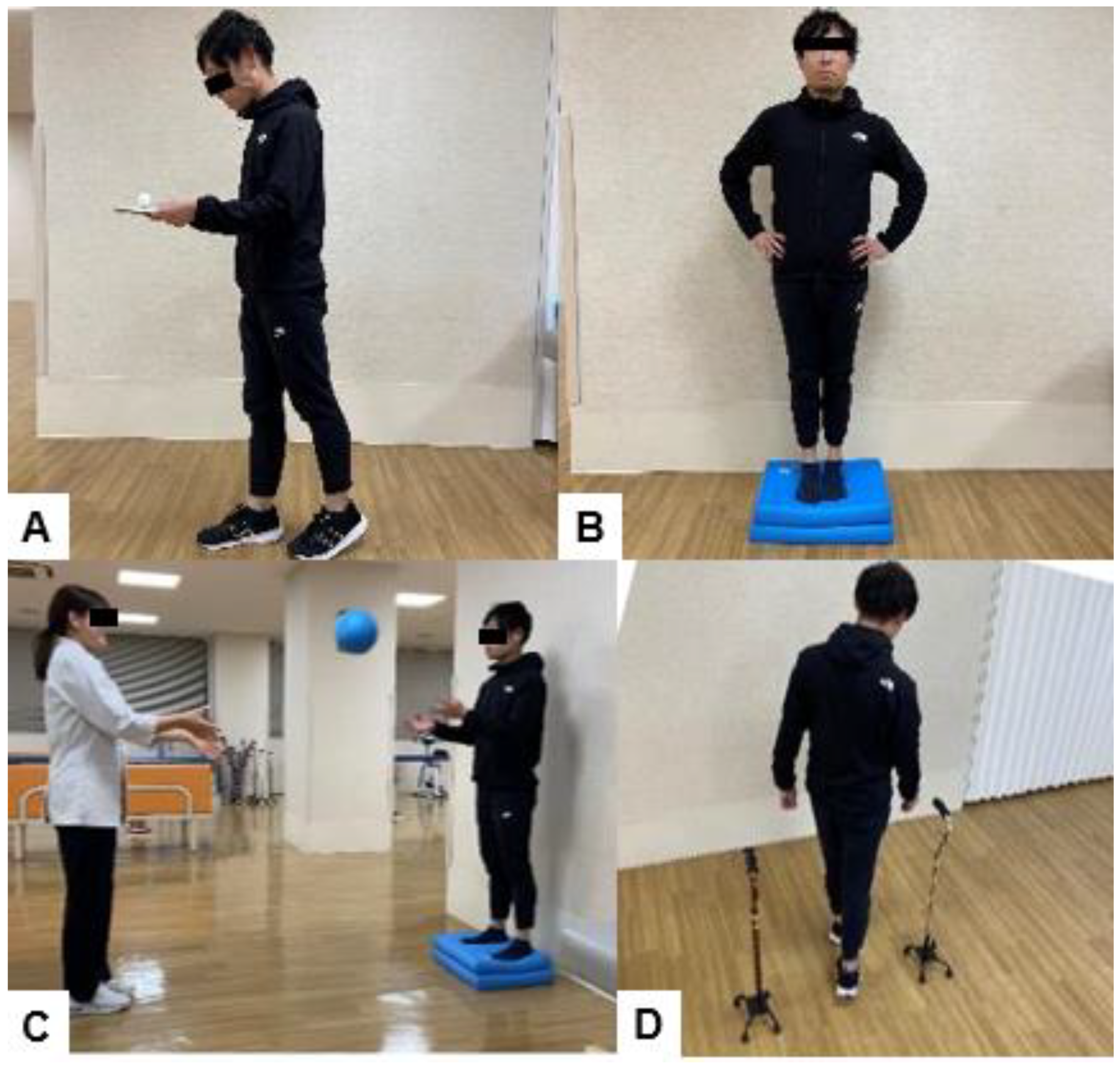
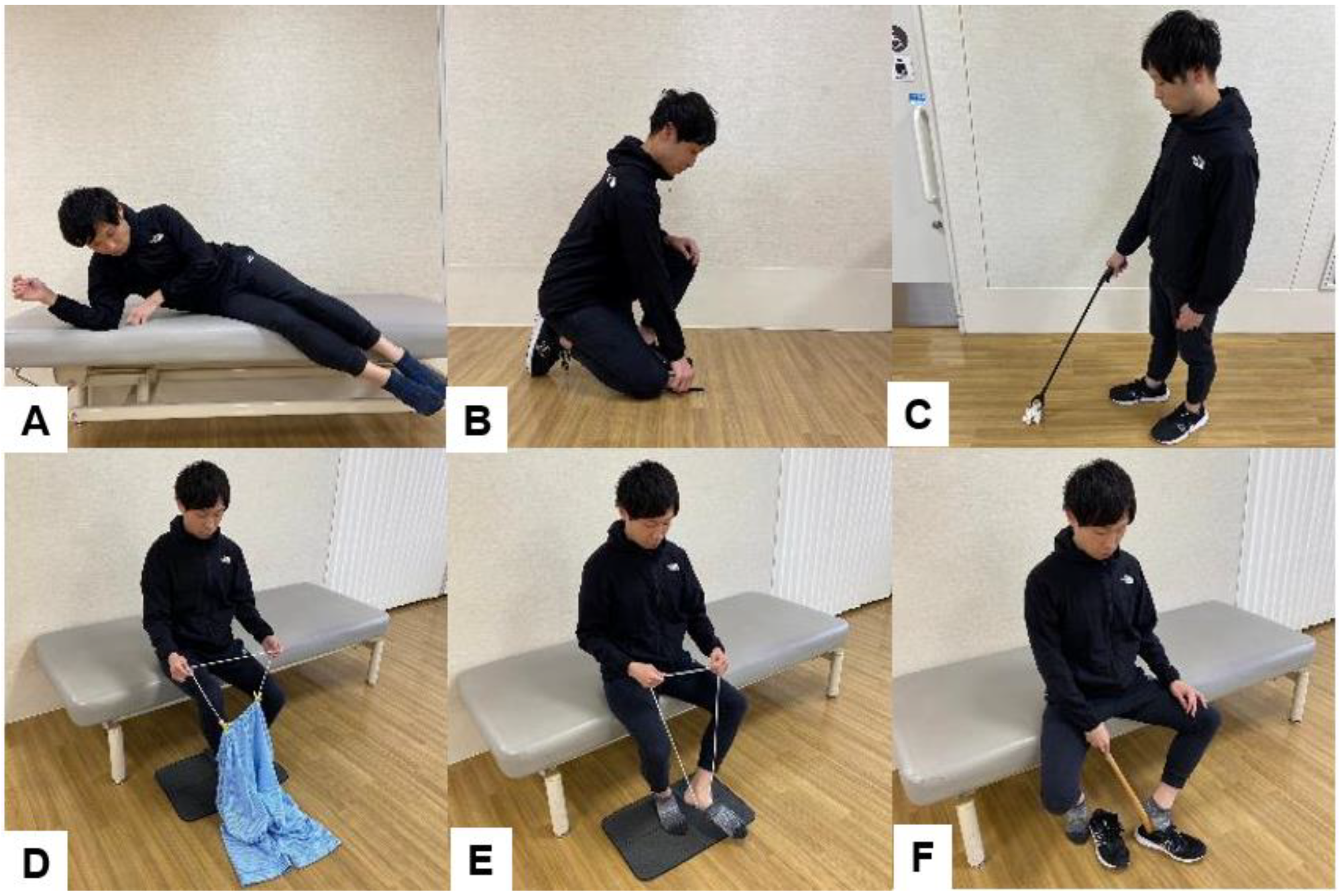
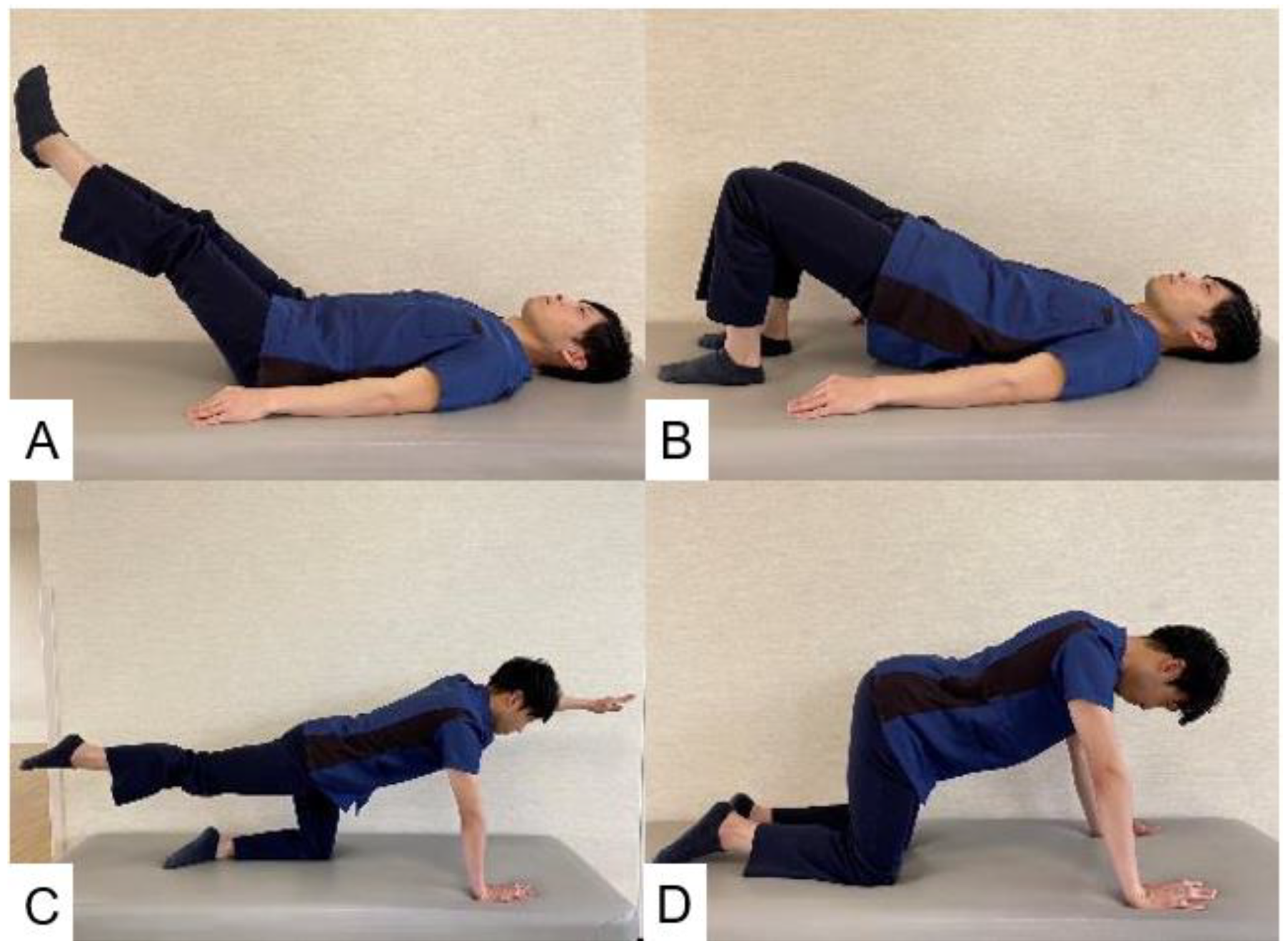
| Treatment Modality | Details | Example |
|---|---|---|
| Patient education and self-management [9] | Teaching patient’s skills that they can use to manage their health condition | How to deal with pain The importance of physical activity in pain reduction Restrictions and working posture post-operatively (ergonomics) Mitigate pain flare-ups Step-by-step rehabilitation methods for return to routine work |
| Early Mobilization [27] | A subcategory of supervised or unsupervised schematic and structured exercise program (e.g., by a healthcare professional) | Stretching, Muscle strengthening Endurance exercises Neuromuscular closed chain exercises Range of motion exercise |
| Manual therapies [28,29] | Myofascial release: Technique that applies low-impact, prolonged stretching to the fascial complex to alleviate pain and improve function. Neural mobilization: A technique that stretches damaged nerves and improves their glide and extensibility. Manipulation: techniques incorporating a high-velocity low-amplitude impulse or thrust applied at or near the end of a joint’s passive range of motion Mobilization: techniques incorporating a low-velocity and small or large amplitude oscillatory movement, within a joint’s passive range of motion | Myofascial release Neural mobilization Massage Lumbar manipulation, mobilization |
| Assistive technologies | Any modalities, used to, maintain, or improve the functional capability of the patient and reduce impairment. | Walking aids Socks aids Pants aids Shoehorn Reacher |
| Study | PRO | Recommended MCID | Procedure | Diagnosis |
|---|---|---|---|---|
| Parker [33] | ODI | 14.9 | TLIF | Lumber degenerative spondylolisthesis |
| VAS Back | 2.1 | |||
| VAS Leg | 2.8 | |||
| Parker [34] | ODI | 4 | Lumbar fusion | Pseudarthrosis |
| VAS Back | 3 | |||
| Johnsen [35] | ODI | 12.88 | Disk replacement | Degenerative disease |
| Solberg [36] | ODI | 20 | Discectomy | Lumbar disk herniation |
| NRS Back | 2.5 | |||
| NRS Leg | 3.5 | |||
| Yoshida [37] | ODI | 11 | Posterior corrective spinal fusion surgery | Adult spinal deformity |
| Fukushima [38] | ZCQ SSS | 1.0 | Microendoscopic laminectomy | Lumbar spinal stenosis |
| ZCQ PFS | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi, T.; Gunjotikar, S.; Tanaka, M.; Komatsubara, T.; Latka, K.; Ekade, S.J.; Prabhu, S.P.; Takamatsu, K.; Yasuda, Y.; Nakagawa, M. Evaluation and Rehabilitation after Adult Lumbar Spine Surgery. J. Clin. Med. 2024, 13, 2915. https://doi.org/10.3390/jcm13102915
Sakaguchi T, Gunjotikar S, Tanaka M, Komatsubara T, Latka K, Ekade SJ, Prabhu SP, Takamatsu K, Yasuda Y, Nakagawa M. Evaluation and Rehabilitation after Adult Lumbar Spine Surgery. Journal of Clinical Medicine. 2024; 13(10):2915. https://doi.org/10.3390/jcm13102915
Chicago/Turabian StyleSakaguchi, Tomoyoshi, Sharvari Gunjotikar, Masato Tanaka, Tadashi Komatsubara, Kajetan Latka, Shashank J. Ekade, Shrinivas P. Prabhu, Kazuhiko Takamatsu, Yosuke Yasuda, and Masami Nakagawa. 2024. "Evaluation and Rehabilitation after Adult Lumbar Spine Surgery" Journal of Clinical Medicine 13, no. 10: 2915. https://doi.org/10.3390/jcm13102915
APA StyleSakaguchi, T., Gunjotikar, S., Tanaka, M., Komatsubara, T., Latka, K., Ekade, S. J., Prabhu, S. P., Takamatsu, K., Yasuda, Y., & Nakagawa, M. (2024). Evaluation and Rehabilitation after Adult Lumbar Spine Surgery. Journal of Clinical Medicine, 13(10), 2915. https://doi.org/10.3390/jcm13102915








