The Risk of Venous Thromboembolism and Ischemic Stroke Stratified by VTE Risk Following Multiple Myeloma: A Korean Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Population
3.2. Cumulative Incidence of VTE and IS
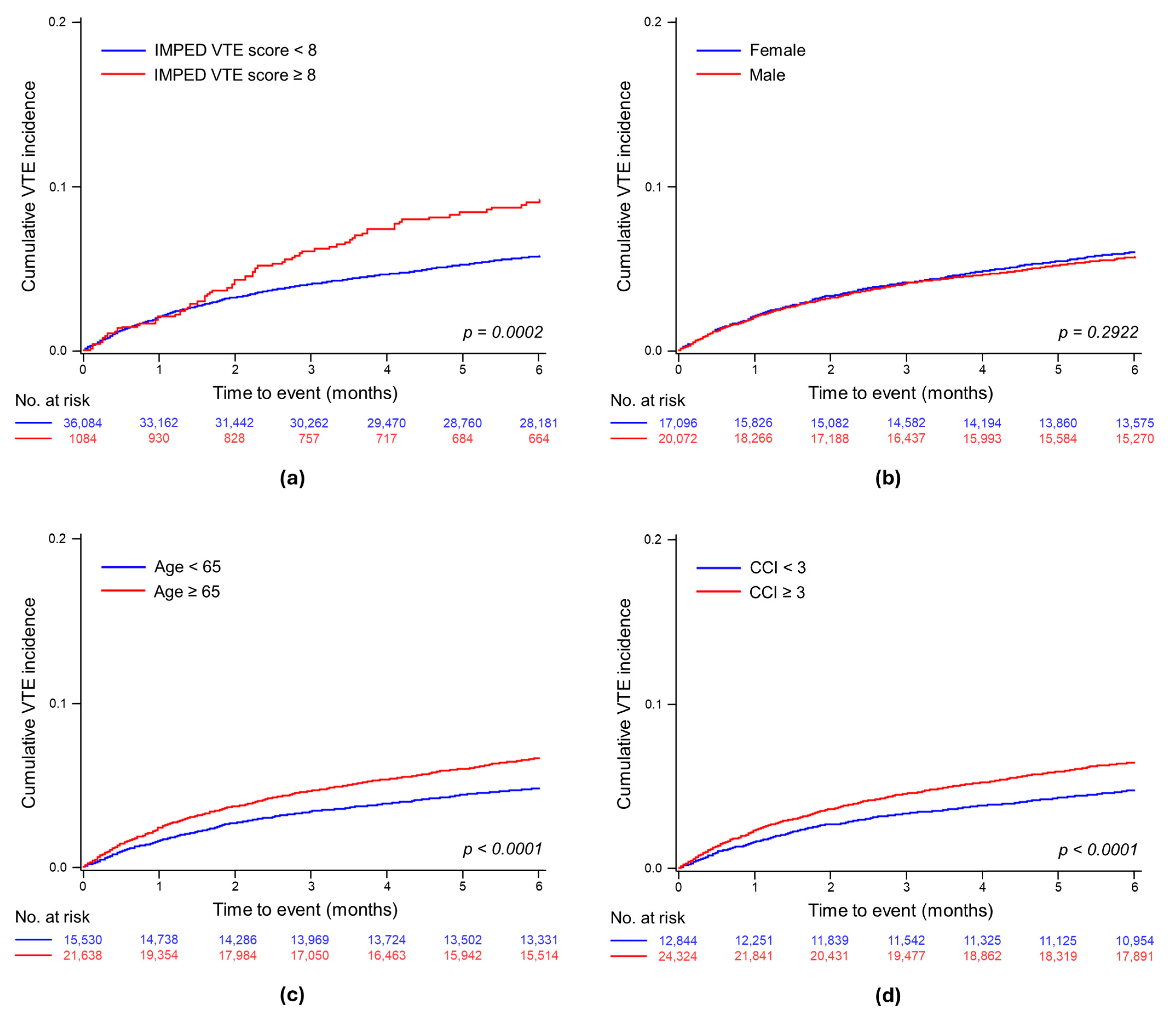
3.2.1. Cumulative Incidence of VTE
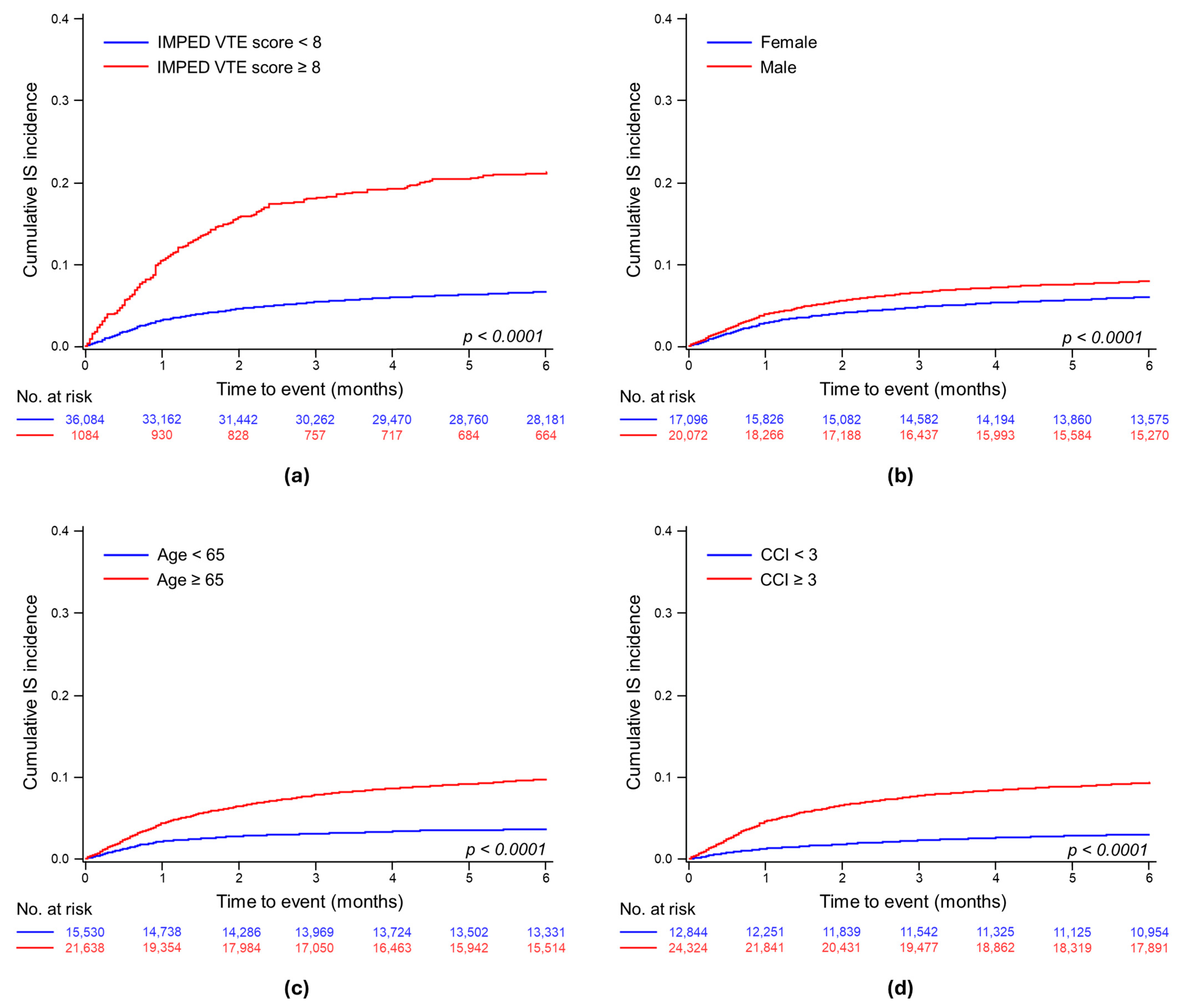
3.2.2. Cumulative Incidence of IS
3.3. Stratified Risks of VTE and IS Using IMPEDE VTE Scores
3.3.1. Assessing the Impact of the IMPEDE VTE Score on the Venous Thromboembolism Risk
3.3.2. Assessing the Impact of the IMPEDE VTE Score on the Risk of Ischemic Stroke
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludwig, H.; Novis Durie, S.; Meckl, A.; Hinke, A.; Durie, B. Multiple myeloma incidence and mortality around the globe; interrelations between health access and quality, economic resources, and patient empowerment. Oncologist 2020, 25, e1406–e1413. [Google Scholar] [CrossRef] [PubMed]
- Leebeek, F.W.; Kruip, M.J.; Sonneveld, P. Risk and management of thrombosis in multiple myeloma. Thromb. Res. 2012, 129, S88–S92. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, S.Y.; Pfeiffer, R.M.; Björkholm, M.; Goldin, L.R.; Schulman, S.; Blimark, C.; Mellqvist, U.-H.; Wahlin, A.; Turesson, I.; Landgren, O. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: A population-based study. Blood J. Am. Soc. Hematol. 2010, 115, 4991–4998. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, S.Y.; Pfeiffer, R.M.; Björkholm, M.; Schulman, S.; Landgren, O. Thrombosis is associated with inferior survival in multiple myeloma. Haematologica 2012, 97, 1603. [Google Scholar] [CrossRef] [PubMed]
- Streiff, M.B.; Holmstrom, B.; Angelini, D.; Ashrani, A.; Elshoury, A.; Fanikos, J.; Fertrin, K.Y.; Fogerty, A.E.; Gao, S.; Goldhaber, S.Z. Cancer-associated venous thromboembolic disease, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1181–1201. [Google Scholar]
- Covut, F.; Ahmed, R.; Chawla, S.; Ricaurte, F.; Samaras, C.J.; Anwer, F.; Garcia, A.V.M.; Angelini, D.E.; Mazzoni, S.; Faiman, B.; et al. Validation of the IMPEDE VTE score for prediction of venous thromboembolism in multiple myeloma: A retrospective cohort study. Br. J. Haematol. 2021, 193, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Fang, L.J.; Xiao, M.Y.; Lu, M.Q.; Chu, B.; Shi, L.; Gao, S.; Xiang, Q.Q.; Wang, Y.T.; Liu, X.; et al. Validation of the IMPEDE VTE score for prediction of venous thromboembolism in Chinese patients with multiple myeloma: A single-center retrospective cohort study. Thromb. Res. 2024, 236, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Association, A.S. Stroke Risk Assessment. Available online: https://www.stroke.org/en/about-stroke/stroke-risk-factors/stroke-risk-assessment (accessed on 2 April 2024).
- Corley, A.M.; Sullivan, M.J.; Friedman, S.E.; O’Rourke, D.J.; Palac, R.T.; Gemignani, A.S. Relation of venous thromboembolism risk to ischemic stroke risk in hospitalized patients with cancer. Am. J. Cardiol. 2019, 123, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Yoon, S.; Kim, L.-Y.; Kim, D.-S. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: Strengths, limitations, applications, and strategies for optimal use of HIRA data. J. Korean Med. Sci. 2017, 32, 718. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, K.M.; Luo, S.; Wang, T.F.; Fiala, M.; Schoen, M.; Wildes, T.M.; Mikhael, J.; Kuderer, N.M.; Calverley, D.C.; Keller, J. Predicting venous thromboembolism in multiple myeloma: Development and validation of the IMPEDE VTE score. Am. J. Hematol. 2019, 94, 1176–1184. [Google Scholar] [CrossRef]
- Chalayer, E.; Talbot, A.; Frenzel, L.; Karlin, L.; Collet, P.; Guyotat, D.; Attal, M.; Leleu, X.; Tardy, B. Prediction of venous thromboembolism in patients with multiple myeloma treated with lenalidomide, bortezomib, dexamethasone, and transplantation: Lessons from the substudy of IFM/DFCI 2009 cohort. J. Thromb. Haemost. 2022, 20, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Ko, J.H.; Son, E.S.; Yu, Y.M. Stratification of Venous Thromboembolism Risk in Multiple Myeloma and Analysis of the Use of Antithrombotic Agents. J. Korean Soc. Health-Syst. Pharm. 2023, 40, 158–170. [Google Scholar] [CrossRef]
- Li, A.; Wu, Q.; Luo, S.; Warnick, G.S.; Zakai, N.A.; Libby, E.N.; Gage, B.F.; Garcia, D.A.; Lyman, G.H.; Sanfilippo, K.M. Derivation and Validation of a Risk Assessment Model for Immunomodulatory Drug-Associated Thrombosis Among Patients with Multiple Myeloma. J. Natl. Compr. Canc Netw. 2019, 17, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, X.; Fang, B.; Leng, Y.; Sun, F.; Wang, Y.; Wang, Q.; Jin, J.; Yang, M.; Xu, B.; et al. Development and validation of a new risk assessment model for immunomodulatory drug-associated venous thrombosis among Chinese patients with multiple myeloma. Thromb. J. 2023, 21, 105. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-T.; Hsieh, C.-Y.; Tsai, T.-T.; Wang, Y.-C.; Sung, S.-F. Performance of ICD-10-CM diagnosis codes for identifying acute ischemic stroke in a national health insurance claims database. Clin. Epidemiol. 2020, 12, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.A.; West, J.; Stephansson, O.; Grainge, M.J.; Tata, L.J.; Fleming, K.M.; Humes, D.; Ludvigsson, J.F. Defining venous thromboembolism and measuring its incidence using Swedish health registries: A nationwide pregnancy cohort study. BMJ Open 2015, 5, e008864. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, F.; Bermejo, J.M.B.; Mateos, M.-V.; Louzada, M. Thromboprophylaxis in multiple myeloma patients treated with lenalidomide—A systematic review. Thromb. Res. 2016, 141, 84–90. [Google Scholar] [CrossRef]
- Baz, R.; Li, L.; Kottke-Marchant, K.; Srkalovic, G.; McGowan, B.; Yiannaki, E.; Karam, M.A.; Faiman, B.; Jawde, R.A.; Andresen, S.; et al. The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracycline-based chemotherapy for multiple myeloma. Mayo Clin. Proc. 2005, 80, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Marchetti, M.; Russo, L. Venous thromboembolism in the hematologic malignancies. Curr. Opin. Oncol. 2012, 24, 702–710. [Google Scholar] [CrossRef]
- Schoen, M.W.; Carson, K.R.; Luo, S.; Gage, B.F.; Li, A.; Afzal, A.; Sanfilippo, K.M. Venous thromboembolism in multiple myeloma is associated with increased mortality. Res. Pract. Thromb. Haemost. 2020, 4, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, C.A.; Craig, Z.; Cook, G.; Pawlyn, C.; Cairns, D.A.; Hockaday, A.; Paterson, A.; Jenner, M.W.; Jones, J.R.; Drayson, M.T.; et al. Thrombosis in patients with myeloma treated in the Myeloma IX and Myeloma XI phase 3 randomized controlled trials. Blood 2020, 136, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, J.J.; Kim, K.; Min, C.K.; Lee, J.O.; Suh, C.; Kim, J.S.; Lee, Y.J.; Yoon, S.S.; Jo, J.C.; et al. Venous thromboembolism in relapsed or refractory multiple myeloma patients treated with lenalidomide plus dexamethasone. Int. J. Hematol. 2019, 109, 79–90. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Zhang, L.; Gu, L.; Tang, T.; Zhou, H.; Tian, G. Prognostic significance of thromboembolism in multiple myeloma: A systematic review and meta-analysis. Transl. Cancer Res. 2023, 12, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Covut, F.; Sanfilippo, K.M. Mitigating the risk of venous thromboembolism in patients with multiple myeloma receiving immunomodulatory-based therapy. Hematol. Am. Soc. Hematol. Educ. Program. 2022, 2022, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Chalayer, E.; Teste, A.; Guyotat, D.; Elalamy, I.; Leleu, X.; Tardy, B. Predicting the risk of venous thromboembolism in newly diagnosed myeloma with immunomodulatory drugs: External validation of the IMPEDE VTE score. Am. J. Hematol. 2020, 95, E18–E20. [Google Scholar] [CrossRef] [PubMed]
- da Costa, I.H.F.; de Pádua, C.A.M.; de Miranda Drummond, P.L.; Silveira, L.P.; Malta, J.S.; Dos Santos, R.M.M.; Reis, A.M.M. Comparison of three risk assessment models for thromboembolism in multiple myeloma patients receiving immunomodulators: A Brazilian historical cohort. J. Thromb. Thrombolysis 2023, 56, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Lee, Y.T.; Yeh, C.M.; Hsu, P.; Lin, T.W.; Gau, J.P.; Yu, Y.B.; Hsiao, L.T.; Tzeng, C.H.; Chiou, T.J. Risk of stroke in patients with newly diagnosed multiple myeloma: A retrospective cohort study. Hematol. Oncol. 2017, 35, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Antipova, D.; Eadie, L.; Macaden, A.; Wilson, P. Diagnostic accuracy of clinical tools for assessment of acute stroke: A systematic review. BMC Emerg. Med. 2019, 19, 49. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total | Event-Free | VTE | IS | p-Value |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| No. of patients | 37,168 | 32,900 (88.5) | 1910 (5.1) | 2358 (6.3) | |
| Sex | |||||
| Male | 20,072 (54.0) | 17,648 (53.6) | 997 (52.2) | 1427 (60.5) | <0.0001 |
| Female | 17,096 (46.0) | 15,252 (46.4) | 913 (47.8) | 931 (39.5) | |
| Age at diagnosis, mean (SD) | 65.9 (13.1) | 65.4 (13.2) | 68.0 (11.7) | 71.5 (11.1) | <0.0001 |
| <20 | 52 (0.1) | 51 (0.1) | 1 (0.1) | 0 (0.0) | <0.0001 |
| 20–29 | 394 (1.1) | 381 (1.1) | 7 (0.4) | 6 (0.3) | |
| 30–39 | 949 (2.6) | 900 (2.6) | 26 (1.4) | 23 (1.0) | |
| 40–49 | 2725 (7.3) | 2544 (7.3) | 100 (5.2) | 81 (3.4) | |
| 50–59 | 6650 (17.9) | 6162 (17.9) | 275 (14.4) | 213 (9.0) | |
| 60–69 | 10,057 (27.1) | 8976 (27.1) | 561 (29.4) | 520 (22.1) | |
| 70–79 | 11,053 (29.7) | 9469 (29.7) | 633 (33.1) | 951 (23.9) | |
| ≥80 | 5288 (14.2) | 4417 (14.2) | 307 (16.1) | 564 (77.4) | |
| Age ≥ 65, n (%) | 21,638 (58.2) | 18,592 (56.5) | 1120 (63.9) | 1826 (77.4) | |
| CCI, mean (SD) | 4.2 (3.1) | 4.1 (3.1) | 4.8 (3.4) | 5.5 (3.1) | <0.0001 |
| 0 | 2734 (7.4) | 2598 (7.9) | 110 (5.8) | 26 (1.1) | <0.0001 |
| 1 | 4656 (12.5) | 4354 (13.2) | 194 (10.2) | 108 (4.6) | |
| 2 | 5454 (14.7) | 4967 (15.1) | 262 (13.7) | 225 (9.5) | |
| ≥3 | 24,324 (65.4) | 20,981 (63.8) | 1344 (70.4) | 1999 (84.8) | |
| Comorbidities | |||||
| Cerebrovascular disease | 6991 (18.8) | 4679 (14.2) | 385 (20.2) | 1927 (81.7) | <0.0001 |
| Myocardial infarction | 944 (2.5) | 777 (2.4) | 55 (2.9) | 112 (4.7) | <0.0001 |
| Congestive heart failure | 5586 (15.0) | 4518 (13.7) | 432 (22.6) | 636 (27.0) | <0.0001 |
| Peripheral vascular disease | 2485 (6.7) | 2091 (6.4) | 170 (8.9) | 224 (9.5) | <0.0001 |
| Paralysis | 969 (2.6) | 525 (1.6) | 74 (2.9) | 370 (15.7) | <0.0001 |
| Diabetes Mellitus | 17,228 (46.4) | 14,715 (44.7) | 960 (50.3) | 1553 (65.9) | <0.0001 |
| Renal disease | 7363 (19.8) | 6308 (19.2) | 388 (20.3) | 667 (28.3) | <0.0001 |
| Liver disease | 563 (1.5) | 495 (1.5) | 23 (1.2) | 45 (1.9) | 0.1567 |
| Cardiac arrhythmias | 3742 (10.1) | 2908 (8.8) | 293 (15.3) | 541 (22.9) | <0.0001 |
| Hypertension | 23,221 (62.5) | 19,902 (60.5) | 1310 (68.6) | 2009 (85.2) | <0.0001 |
| Coagulopathy | 1853 (5.0) | 1522 (4.6) | 165 (8.6) | 166 (7.0) | <0.0001 |
| Anemia | 2725 (7.3) | 2383 (7.2) | 145 (7.6) | 197 (8.4) | 0.1224 |
| IMPEDE VTE | |||||
| Score, mean (SD) | 1.8 (2.8) | 1.7 (2.7) | 1.8 (3.3) | 2.7 (3.8) | <0.0001 |
| (95% CI) | (1.75–1.81) | (1.67–1.74) | (1.66–1.96) | (2.54–2.85) | |
| IMPEDE score ≥ 8 | 1084 (2.9) | 815 (2.5) | 76 (4.0) | 193 (8.2) | <0.0001 |
| Body mass index ≥ 25 kg/m2 | 35 (0.1) | 31 (0.1) | 2 (0.1) | 2 (0.1) | <0.0001 |
| Pathologic fracture pelvis/hip/femur | 210 (0.6) | 184 (0.6) | 16 (0.8) | 10 (0.4) | <0.0001 |
| VTE history | 2438 (6.6) | 1022 (3.1) | 228 (11.9) | 1188 (50.4) | <0.0001 |
| Tunneled line/CVC | 3150 (8.5) | 2767 (8.4) | 216 (11.8) | 167 (7.1) | <0.0001 |
| Immunomodulatory drug | 5768 (15.5) | 5108 (15.5) | 486 (25.4) | 174 (7.4) | <0.0001 |
| Erythropoiesis-stimulating agent | 3412 (9.2) | 2919 (8.9) | 265 (13.9) | 228 (9.7) | <0.0001 |
| Dexamethasone (high dose) | 7853 (21.1) | 6967 (21.2) | 511 (26.8) | 375 (15.9) | <0.0001 |
| Dexamethasone (low dose) | 13,445 (36.2) | 11,919 (36.2) | 795 (41.6) | 731 (31.0) | <0.0001 |
| Doxorubicin | 779 (2.1) | 717 (2.2) | 47 (2.5) | 15 (0.6) | <0.0001 |
| Existing therapeutic warfarin or LMWH use | 1637 (4.4) | 1023 (3.1) | 411 (21.5) | 203 (8.6) | <0.0001 |
| Existing prophylactic aspirin or LMWH use | 11,973 (32.2) | 9933 (30.2) | 970 (50.8) | 1070 (45.4) | <0.0001 |
| Variables | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| VTE IMPEDE score ≥ 8 | 1.54 (1.23–1.94) | 0.0002 | 1.54 (1.23–1.94) | 0.0002 | 1.62 (1.29–2.04) | <0.0001 | 1.59 (1.26–2.00) | <0.0001 |
| Male | 0.95 (0.87–1.04) | 0.2816 | 0.96 (0.87–1.05) | 0.3223 | 0.95 (0.86–1.03) | 0.2219 | ||
| Age ≥ 65 | 1.40 (1.27–1.53) | <0.0001 | 1.34 (1.22–1.47) | <0.0001 | ||||
| CCI ≥ 3 | 1.29 (1.17–1.42) | <0.0001 | ||||||
| Variables | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| VTE IMPEDE score ≥ 8 | 3.23 (2.79–3.75) | <0.0001 | 3.22 (2.78–3.73) | <0.0001 | 3.72 (3.21–4.31) | <0.0001 | 3.47 (2.99–4.02) | |
| Male | 1.33 (1.22–1.44) | <0.0001 | 1.35 (1.24–1.46) | <0.0001 | 1.30 (1.20–1.41) | <0.0001 | ||
| Age ≥ 65 | 2.80 (2.51–3.05) | <0.0001 | 2.42 (2.42–2.20) | <0.0001 | ||||
| CCI ≥ 3 | 2.65 (2.37–2.97) | <0.0001 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.J.; Kim, M.; Lee, J.; Suh, H.S. The Risk of Venous Thromboembolism and Ischemic Stroke Stratified by VTE Risk Following Multiple Myeloma: A Korean Population-Based Cohort Study. J. Clin. Med. 2024, 13, 2829. https://doi.org/10.3390/jcm13102829
Han HJ, Kim M, Lee J, Suh HS. The Risk of Venous Thromboembolism and Ischemic Stroke Stratified by VTE Risk Following Multiple Myeloma: A Korean Population-Based Cohort Study. Journal of Clinical Medicine. 2024; 13(10):2829. https://doi.org/10.3390/jcm13102829
Chicago/Turabian StyleHan, Hyun Jin, Miryoung Kim, Jiyeon Lee, and Hae Sun Suh. 2024. "The Risk of Venous Thromboembolism and Ischemic Stroke Stratified by VTE Risk Following Multiple Myeloma: A Korean Population-Based Cohort Study" Journal of Clinical Medicine 13, no. 10: 2829. https://doi.org/10.3390/jcm13102829
APA StyleHan, H. J., Kim, M., Lee, J., & Suh, H. S. (2024). The Risk of Venous Thromboembolism and Ischemic Stroke Stratified by VTE Risk Following Multiple Myeloma: A Korean Population-Based Cohort Study. Journal of Clinical Medicine, 13(10), 2829. https://doi.org/10.3390/jcm13102829






