Effect of a Multicomponent Intervention with Tele-Rehabilitation and the Vivifrail© Exercise Programme on Functional Capacity after Hip Fracture: Study Protocol for the ActiveFLS Randomized Controlled Trial
Abstract
:1. Background and Rationale
2. Objectives
Hypothesis
3. Methods and Analysis
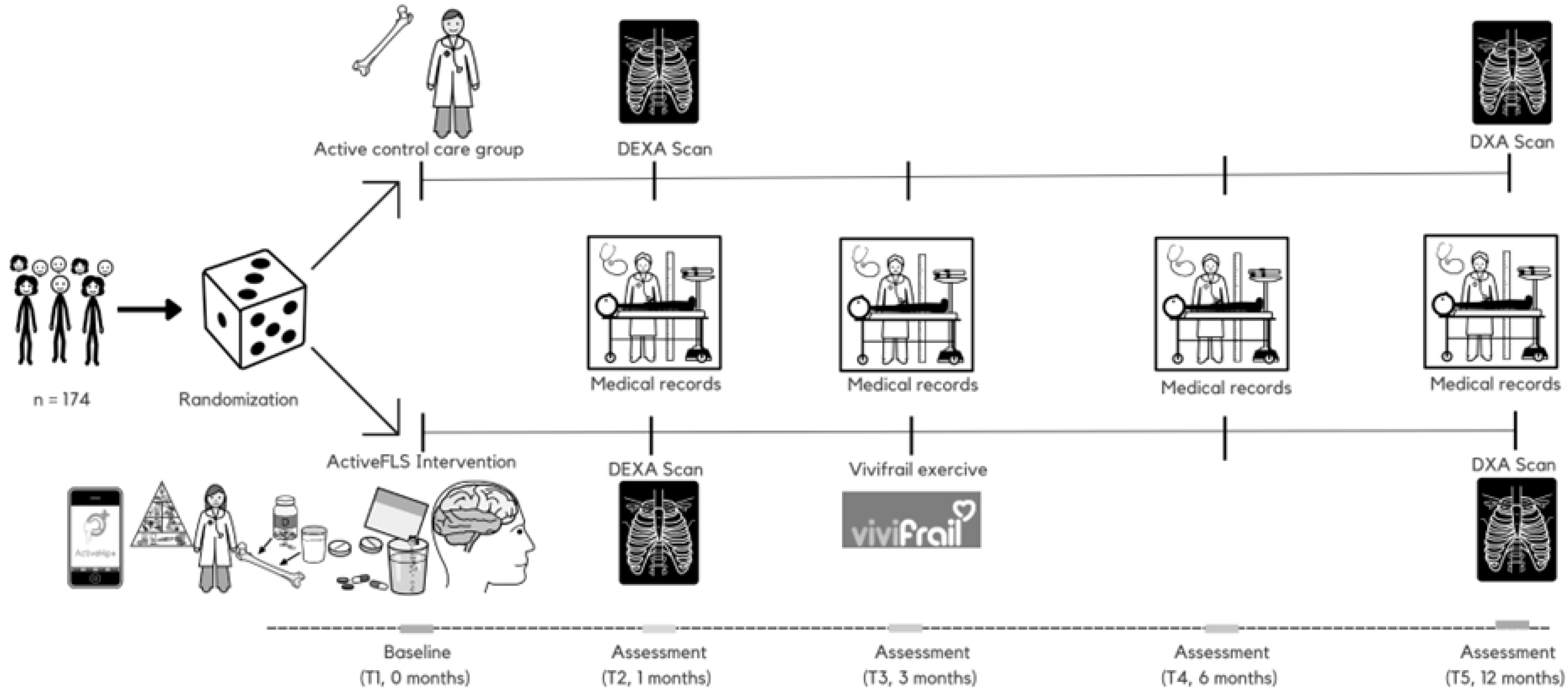
3.1. Trial Design
3.2. Study Setting
3.3. Eligibility Criteria and Recruitment
3.4. Who Will Take Informed Consent?
3.5. Additional Consent Provisions for Collection and Use of Participant Data and Biological Specimens
3.6. Explanation of the Choice of Comparators
3.6.1. Interventions
3.6.2. Intervention Description
3.7. Participant Timeline
3.8. Criteria for Discontinuing or Modifying Allocated Interventions
Strategies to Improve Adherence to Interventions
3.9. Relevant Concomitant Care Permitted or Prohibited during the Trial
3.10. Provisions for Post-Trial Care
4. Outcomes
4.1. Primary Outcome
4.2. Secondary Outcomes
- −
- −
- Cognitive status [35]: The GDS, outlining seven distinct stages ranging from normal cognitive function to severe Alzheimer’s dementia, and the 16-item Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), with scoring for each question from 1 (significantly improved) to 5 (significantly worse), will be employed. An average score of 3.31/3.38 serves as the cut-off point, offering an equilibrium in detecting sensitivity and specificity of cognitive impairment [36]. Delirium assessment during hospitalization will be carried out with the Abbreviated Mental Test 4 (4AT) [37].
- −
- Mood status: Depression will be screened using the 15-item Yesavage Geriatric Depression Scale (scale: 0, best; 15, worst), which is independently associated with hip fracture [38]. Additionally, the level of fear regarding falls will be evaluated using the Falls Efficacy Scale International (FES-I), with validated thresholds for low concern (16–19 points), moderate concern (20–27 points) and high concern (28–64 points) [39].
- −
- Frailty and sarcopenia: The presence of frailty will be initially screened using the FRAIL questionnaire and further confirmed by the adapted criteria of Fried’s frailty [40]. Sarcopenia will be determined by: (i) handgrip strength < 16 kg for women or < 27 kg for men; and (ii) appendicular skeletal muscle mass (ASMM)/ height2 < 7.0 kg/m2 for men or < 5.5 kg/m2 for women [41]. Handgrip strength will be measured using the Groningen Elderly Test with a Smedley hand dynamometer [42]. We will record the best of three attempts (with a 30 s rest in between). Severe sarcopenia will be defined as gait speed ≤ 0.8 m/s or SPPB ≤ 8 points.
- −
- Quality of life: The EuroQol-5D and the Sarcopenia and Quality of Life (SarQoL) scales will be used to measure the quality of life: the former assesses five dimensions of health status and is a valid instrument for hip fracture patients [43], and the latter is a novel validated instrument for measuring the quality of life in sarcopenia patients [44].
- −
- Other clinical assessments: A comprehensive geriatric assessment will be conducted to evaluate geriatric syndromes [45], including falls (defined as an unplanned and involuntary loss of stability resulting in the individual unintentionally coming into contact with the ground), polypharmacy (defined as five or more medications) [46] and pain (Visual Analogue Scale: 0, best; 10, worst). Digital stadiometer will be used for height measured. The evaluation of nutrition will be conducted through the calculation of body mass index (BMI), determined by weight divided by height squared, and by administering the Mini-Nutritional Assessment (MNA) instrument [47]. Comorbidities will be evaluated with the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) [48], ranging from 0 (best) to 56 (worst). Osteoporosis risk assessment is evaluated using the FRAX and QFracture tools [49], and pain is evaluated using the Visual Analogue Scale (VAS).
- −
- Adverse events: As per the International Conference on Harmonization Guidelines, a serious adverse event will be classified as any occurrence that leads to death, poses a threat to life, necessitates hospital admission or extends current hospitalization, causes lasting or substantial disability or is a congenital anomaly or birth defect [50].
- −
- Use of health sources: This will include hospital admissions, nursing home admissions, visits to primary care physicians and visits to the emergency department.
- −
- Biochemical analyses: Blood samples will be collected in Vacutainer tubes and centrifuged at 3300 rpm for 10 min at room temperature using a fixed-angle rotor. Following centrifugation, the serum from the top layer will be meticulously separated from the plasma in the lower layer, portioned into 100 μL aliquots and promptly preserved at −80 °C. Additionally, both plasma and buffy coat will be extracted and kept in polypropylene tubes at −80 °C until the time of analysis. Bone turnover markers (BTMs) will be measured at the Clinical Neuroproteomics Unit (Navarrabiomed), whereas other measurements will be performed at the Central Laboratory Unit of Navarra (LUNA). Biological samples will be collected following an overnight fast, between 8 and 10 am. Tests for 25-hydroxyvitamin D3 (vitamin D), calcium, phosphorus, alkaline phosphatase, parathyroid hormone (PTH), thyroid-stimulating hormone (TSH), creatinine and albumin will be conducted clinically right after the samples are delivered to the laboratory. Given the common occurrence of hypoalbuminemia in older adults, serum levels of albumin and calcium will be used to adjust the calcium value (corrected calcium value = Ca + 0.8 [40 − albumin]). This corrected calcium value will then be utilized in further analyses. Measurements of C-terminal cross-linked telopeptide of type I collagen (CTX), sclerostin (SCL), bone-specific alkaline phosphatase (B-ALP), procollagen type 1 N propeptide (P1NP) and osteocalcin (OC) will be carried out using enzyme-linked immunosorbent assays as per the manufacturer’s guidelines on the frozen samples [51].
- −
- Dual-energy X-ray absorptiometry (DXA): Bone mineral density (BMD) along with body composition, including fat and lean mass, will be evaluated using a Hologic DPX-IQ Discovery DXA device provided by GE Healthcare, located in Pollards Wood, UK. To minimize variability, all measurements will be performed by the same operator. The DXA machine will be calibrated daily. BMD will be gauged in grams per square centimetre at the non-dominant wrist, lumbar spine, and avaible proximal femur (encompassing the neck, trochanter, intertrochanteric area and Ward’s triangle) [52]. The L1 to L4 region will be included by positioning the patient in alignment with the table’s axis during examination. For BMD measurements in the proximal femur, the patient’s legs will be rotated 15–30° to subtly reveal the smaller trochanter of the femur. Z-scores and T-scores will be calculated at both sites, with a coefficient of variation set at 1.14%. Osteopenia and osteoporosis are defined according the World Health Organization standard criteria, which classify a BMD T-score between −1.0 SD and −2.49 SD below the young adult mean as osteopenia, and a score below −2.5 SD as osteoporosis [53]. Lean mass will be quantified as appendicular skeletal muscle mass (ASM), normalized either for height squared (resulting in the appendicular skeletal muscle mass index, ASMI) or for body mass index (ASM/BMI) [41].
4.3. Sample Size
4.4. Assignment of Interventions: Allocation
4.4.1. Sequence Generation
4.4.2. Concealment Mechanism
4.4.3. Implementation
4.5. Assignment of Interventions: Blinding
4.5.1. Who Will Be Blinded
4.5.2. Procedure for Unblinding if Needed
4.6. Data Collection Methods (Plans for Assessment and Plans to Complete Follow-Up) and Data Outcome Management
4.7. Confidentiality
5. Statistical Methods
5.1. Statistical Methods for Primary and Secondary Outcomes
5.2. Interim Analyses
5.3. Methods for Additional Analyses (e.g., Subgroup Analyses)
5.4. Oversight and Monitoring
5.4.1. Composition of the Coordinating Centre and Trial Steering Committee
5.4.2. Composition, Role and Reporting Structure of the Data Monitoring Committee
5.4.3. Adverse Event Reporting and Harms
5.4.4. Frequency and Plans for Auditing Trial Conduct
5.4.5. Plans for Communicating Important Protocol Amendments to Relevant Parties (e.g., Trial Participants, Ethical Committees)
5.4.6. Dissemination Plans
6. Discussion
6.1. Contribution to the Field
6.2. Trial Status
7. Ethics and Dissemination
7.1. Ethics Statement
7.2. Availability of Data and Materials
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility fractures in Europe: Burden, management and opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Bartra, A.; Caeiro, J.R.; Mesa-Ramos, M.; Etxebarría-Foronda, I.; Montejo, J.; Carpintero, P.; Sorio-Vilela, F.; Gatell, S.; Canals, L. Cost of osteoporotic hip fracture in Spain per Autonomous Region. Rev. Esp. Cir. Ortop. Traumatol. 2019, 63, 56–68. [Google Scholar] [CrossRef]
- Li, N.; Hiligsmann, M.; Boonen, A.; van Oostwaard, M.M.; de Bot, R.T.A.L.; Wyers, C.E.; Bours, S.P.G.; van den Bergh, J.P. The impact of fracture liaison services on subsequent fractures and mortality: A systematic literature review and meta-analysis. Osteoporos. Int. 2021, 32, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, K.J.; Williamson, L.; Alexander, J.; Filliter, C.; Sobolev, B.; Guy, P.; Bearne, L.M.; Sackley, C. Prognostic factors of functional outcome after hip fracture surgery: A systematic review. Age Ageing 2018, 47, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Nuotio, M.; Luukkaala, T. Factors associated with changes in mobility and living arrangements in a comprehensive geriatric outpatient assessment after hip fracture. Disabil. Rehabil. 2016, 38, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, P.S.; Kristensson, J.; McGuigan, F.E.; Akesson, K.E. Frailty and prediction of recurrent falls over 10 years in a community cohort of 75-year-old women. Aging Clin. Exp. Res. 2020, 32, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef]
- Lee, K.-J.; Um, S.-H.; Kim, Y.-H. Postoperative Rehabilitation after Hip Fracture: A Literature Review. Hip Pelvis 2020, 32, 125. [Google Scholar] [CrossRef]
- Fairhall, N.J.; Dyer, S.M.; Mak, J.C.; Diong, J.; Kwok, W.S.; Sherrington, C. Interventions for improving mobility after hip fracture surgery in adults. Cochrane Database Syst. Rev. 2022, 2022, CD001704. [Google Scholar] [CrossRef]
- Min, K.; Beom, J.; Kim, B.R.; Lee, S.Y.; Lee, G.J.; Lee, J.H.; Lee, S.Y.; Won, S.J.; Ahn, S.; Bang, H.J.; et al. Clinical Practice Guideline for Postoperative Rehabilitation in Older Patients With Hip Fractures. Ann. Rehabil. Med. 2021, 45, 225–259. [Google Scholar] [CrossRef] [PubMed]
- Araiza-Nava, B.; Méndez-Sánchez, L.; Clark, P.; Peralta-Pedrero, M.L.; Javaid, M.K.; Calo, M.; Martínez-Hernández, B.M.; Guzmán-Jiménez, F. Short- and long-term prognostic factors associated with functional recovery in elderly patients with hip fracture: A systematic review. Osteoporos. Int. 2022, 33, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
- Câmara, S.M.A.; Falvey, J.R.; Orwig, D.; Gruber-Baldini, A.L.; Auais, M.; Feng, Z.; Guralnik, J.; Magaziner, J. Associations between living alone, social interactions, and physical performance differ by sex: Results from the Baltimore Hip Studies. J. Am. Geriatr. Soc. 2023, 71, 2788–2797. [Google Scholar] [CrossRef] [PubMed]
- Brehon, K.; Carriere, J.; Churchill, K.; Loyola-Sanchez, A.; O’Connell, P.; Papathanasoglou, E.; MacIsaac, R.; Tavakoli, M.; Ho, C.; Manhas, K.P. Evaluating the impact of a novel telerehabilitation service to address neurological, musculoskeletal, or coronavirus disease 2019 rehabilitation concerns during the coronavirus disease 2019 pandemic. Digit. Health 2022, 8, 205520762211016. [Google Scholar] [CrossRef]
- Werneke, M.W.; Deutscher, D.; Grigsby, D.; Tucker, C.A.; Mioduski, J.E.; Hayes, D. Telerehabilitation During the COVID-19 Pandemic in Outpatient Rehabilitation Settings: A Descriptive Study. Phys. Ther. 2021, 101, pzab110. [Google Scholar] [CrossRef]
- Ortiz-Piña, M.; Salas-Fariña, Z.; Mora-Traverso, M.; Martín-Martín, L.; Galiano-Castillo, N.; García-Montes, I.; Cantarero-Villanueva, I.; Fernández-Lao, C.; Arroyo-Morales, M.; Mesa-Ruíz, A.; et al. A home-based tele-rehabilitation protocol for patients with hip fracture called @ctivehip. Res. Nurs. Health 2019, 42, 29–38. [Google Scholar] [CrossRef]
- Ortiz-Piña, M.; Molina-Garcia, P.; Femia, P.; Ashe, M.C.; Martín-Martín, L.; Salazar-Graván, S.; Salas-Fariña, Z.; Prieto-Moreno, R.; Castellote-Caballero, Y.; Estevez-Lopez, F.; et al. Effects of tele-rehabilitation compared with home-based in-person rehabilitation for older adult’s function after hip fracture. Int. J. Environ. Res. Public Health 2021, 18, 5493. [Google Scholar] [CrossRef]
- Izquierdo, M.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Martínez-Velilla, N.; Alonso-Bouzon, C.; Rodriguez-Mñas, L. Multicomponent Physical Exercise Program VIVIFRAIL. Available online: http://www.vivifrail.com/resources/ (accessed on 1 December 2023).
- Rolland, Y.; Cesar, M.; Fielding, R.A.; Reginster, J.Y.; Vellas, B.; Cruz-Jentoft, A.J. Osteoporosis in Frail Older Adults: Recommendations for Research from the ICFSR Task Force 2020. J. Frailty Aging 2021, 10, 168–175. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Takahashi, A.; Naruse, H.; Kitade, I.; Shimada, S.; Tsubokawa, M.; Kokubo, Y.; Matsumine, A. Functional outcomes after the treatment of hip fracture. PLoS ONE 2020, 15, e0236652. [Google Scholar] [CrossRef]
- Ebeling, P.R.; Nguyen, H.H.; Aleksova, J.; Vincent, A.J.; Wong, P.; Milat, F. Secondary Osteoporosis. Endocr. Rev. 2022, 43, 240–313. [Google Scholar] [CrossRef] [PubMed]
- Mora-Traverso, M.; Molina-Garcia, P.; Prieto-Moreno, R.; Borges-Cosic, M.; Cruz Guisado, V.; del Pino Algarrada, R.; Moreno-Ramírez, P.; Gomez-Jurado, G.; Gomez Tarrias, C.; Hidalgo Isla, M.; et al. An m-Health telerehabilitation and health education program on physical performance in patients with hip fracture and their family caregivers: Study protocol for the ActiveHip+ randomized controlled trial. Res. Nurs. Health 2022, 45, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.Y.; Yan, S.; Low, L.L.; Vasanwala, F.F.; Low, S.G. Predictors of poor functional outcomes and mortality in patients with hip fracture: A systematic review. BMC Musculoskelet. Disord. 2019, 20, 568. [Google Scholar] [CrossRef] [PubMed]
- Malafarina, V.; Uriz-Otano, F.; Malafarina, C.; Martinez, J.A.; Zulet, M.A. Effectiveness of Nutritional Supplementation on Sarcopenia and Recovery in Hip Fracture Patients. A Multi-Centre Randomized Trial. Maturitas 2017, 101, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Riancho, J.; Peris, P.; González-Macías, J.; Pérez-Castrillón, J. Guías de práctica clínica en la osteoporosis postmenopáusica, glucocorticoidea y del varón (actualización 2022). Rev. Osteoporos. Metab. Miner. 2022, 14, 13–33. [Google Scholar] [CrossRef]
- Narayanasamy, M.; Bishop, S.; Sahota, O.; Paskins, Z.; Gittoes, N.; Langley, T. Acceptability and engagement amongst patients on oral and intravenous bisphosphonates for the treatment of osteoporosis in older adults. Age Ageing 2022, 51, afac255. [Google Scholar] [CrossRef]
- Gallo, C.; Vilosio, J.; Saimovici, J. Actualización de los criterios STOPP-START: Una herramienta para la detección de medicación potencialmente inadecuada en ancianos New version of STOPP-START criteria: Tools for the detection of potentially inappropriate medications in the elderly. Actual en la Práctica Ambulatoria 2015, 18, 6. [Google Scholar]
- Coronado-Zarco, R.; Olascoaga-Gómez de León, A.; García-Lara, A.; Quinzaños-Fresnedo, J.; Nava-Bringas, T.I.; Macías-Hernández, S.I. Nonpharmacological interventions for osteoporosis treatment: Systematic review of clinical practice guidelines. Osteoporos. Sarcopenia 2019, 5, 69–77. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Pfeiffer, K.; Kampe, K.; Klenk, J.; Rapp, K.; Kohler, M.; Albrecht, D.; Büchele, G.; Hautzinger, M.; Taraldsen, K.; Becker, C. Effects of an intervention to reduce fear of falling and increase physical activity during hip and pelvic fracture rehabilitation. Age Ageing 2020, 49, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mosk, C.A.; Mus, M.; Vroemen, J.P.A.M.; Van Der Ploeg, T.; Vos, D.I.; Elmans, L.H.G.J.; Van Der Laan, L. Dementia and delirium, the outcomes in elderly hip fracture patients. Clin. Interv. Aging 2017, 12, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.K.; Stott, D.J.; McShane, R.; Noel-Storr, A.H.; Swann-Price, R.S.; Quinn, T.J. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the early detection of dementia across a variety of healthcare settings. Cochrane Database Syst. Rev. 2021, 2021, CD011333. [Google Scholar] [CrossRef]
- Lisk, R.; Yeong, K.; Enwere, P.; Jenkinson, J.; Robin, J.; Irvin-Sellers, M.; Fluck, D.; Osmani, A.; Sharmin, R.; Sharma, P.; et al. Associations of 4AT with mobility, length of stay and mortality in hospital and discharge destination among patients admitted with hip fractures. Age Ageing 2020, 49, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.T.; Min, M.; Zhang, Y.; Sun, C.Y.; Liang, M.M.; Sun, Y.H. Depression and risk of hip fracture: A systematic review and meta-analysis of cohort studies. Osteoporos. Int. 2019, 30, 1157–1165. [Google Scholar] [CrossRef]
- Delbaere, K.; Close, J.C.T.; Mikolaizak, A.S.; Sachdev, P.S.; Brodaty, H.; Lord, S.R. The Falls Efficacy Scale International (FES-I). A comprehensive longitudinal validation study. Age Ageing 2010, 39, 210–216. [Google Scholar] [CrossRef]
- Cesari, M.; Calvani, R.; Marzetti, E. Frailty in Older Persons. Clin. Geriatr. Med. 2017, 33, 293–303. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Soer, R.; van der Schans, C.P.; Geertzen, J.H.; Groothoff, J.W.; Brouwer, S.; Dijkstra, P.U.; Reneman, M.F. Normative Values for a Functional Capacity Evaluation. Arch. Phys. Med. Rehabil. 2009, 90, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Amarilla-Donoso, F.J.; Roncero-Martin, R.; Lavado-Garcia, J.M.; Toribio-Felipe, R.; Moran-Garcia, J.M.; Lopez-Espuela, F. Quality of life after hip fracture: A 12-month prospective study. PeerJ 2020, 2020, e9215. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Biver, E.; Reginster, J.Y.; Rizzoli, R.; Rolland, Y.; Bautmans, I.; Petermans, J.; Gillain, S.; Buckinx, F.; Dardenne, N.; et al. Validation of the SarQoL®, a specific health-related quality of life questionnaire for Sarcopenia. J. Cachexia Sarcopenia Muscle 2017, 8, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Merel, S.E.; Yukawa, M. Geriatric Syndromes and Geriatric Assessment for the Generalist. Med. Clin. N. Am. 2015, 99, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.; Paauw, D.S. Appropriate Prescribing and Important Drug Interactions in Older Adults. Med. Clin. N. Am. 2015, 99, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E. Mini nutritional assessment. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Conwell, Y.; Forbes, N.T.; Cox, C.; Caine, E.D. Validation of a Measure of Physical Illness Burden at Autopsy: The Cumulative Illness Rating Scale. J. Am. Geriatr. Soc. 1993, 41, 38–41. [Google Scholar] [CrossRef]
- Kanis, J.A.; Harvey, N.C.; Johansson, H.; Odén, A.; McCloskey, E.V.; Leslie, W.D. Overview of Fracture Prediction Tools. J. Clin. Densitom. 2017, 20, 444–450. [Google Scholar] [CrossRef]
- European Medicines Agency. Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. (CPMP/ICH/377/95). 1995. Available online: http://www.ema.europa.eu/docs/enGB/DocumentLibrary/Scientificguideline/2009/09/WC500002749.pdf (accessed on 13 March 2021).
- Johansson, H.; Odén, A.; Kanis, J.A.; McCloskey, E.V.; Morris, H.A.; Cooper, C.; Vasikaran, S. A meta-analysis of reference markers of bone turnover for prediction of fracture. Calcif. Tissue Int. 2014, 94, 560–567. [Google Scholar] [CrossRef]
- Lupsa, B.C.; Insogna, K. Bone Health and Osteoporosis. Endocrinol. Metab. Clin. N. Am. 2015, 44, 517–530. [Google Scholar] [CrossRef]
- World Health Organization. Assessment of Osteoporosis at the Primary Health Care Level. Summary Report of a WHO Scientific Group. WHO, Geneva. 2007. Available online: www.who.int/chp/topics/rheumatic/en/index.html. (accessed on 20 December 2023).
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. N. Engl. J. Med. 1995, 332, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef] [PubMed]
- Åkesson, K.E.; Ganda, K.; Deignan, C.; Oates, M.K.; Volpert, A.; Brooks, K.; Lee, D.; Dirschl, D.R.; Singer, A.J. Post-fracture care programs for prevention of subsequent fragility fractures: A literature assessment of current trends. Osteoporos. Int. 2022, 33, 1659–1676. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Greggi, C.; Visconti, V.V.; Cariati, I.; Bonanni, R.; Gasperini, B.; Iundusi, R.; Gasbarra, E.; Tranquilli Leali, P.; Brandi, M.L. Fracture liaison service model: Project design and accreditation. Osteoporos. Int. 2023, 34, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Astrone, P.; Perracini, M.R.; Martin, F.C.; Marsh, D.R.; Cesari, M. The potential of assessment based on the WHO framework of intrinsic capacity in fragility fracture prevention. Aging Clin. Exp. Res. 2022, 34, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, I.; Shojaa, M.; Kohl, M.; von Stengel, S.; Becker, C.; Gosch, M.; Jakob, F.; Kerschan-Schindl, K.; Kladny, B.; Clausen, J.; et al. Exercise reduces the number of overall and major osteoporotic fractures in adults. Does supervision make a difference? Systematic review and meta-analysis. J. Bone Miner. Res. 2022, 37, 2132–2148. [Google Scholar] [CrossRef]
- Casas-Herrero, Á.; Sáez de Asteasu, M.L.; Antón-Rodrigo, I.; Sánchez-Sánchez, J.L.; Montero-Odasso, M.; Marín-Epelde, I.; Ramón-Espinoza, F.; Zambom-Ferraresi, F.; Petidier-Torregrosa, R.; Elexpuru-Estomba, J.; et al. Effects of Vivifrail multicomponent intervention on functional capacity: A multicentre, randomized controlled trial. J. Cachexia Sarcopenia Muscle 2022, 13, 884–893. [Google Scholar] [CrossRef]
- Cations, M.; Laver, K.E.; Crotty, M.; Cameron, I.D. Rehabilitation in dementia care. Age Ageing 2018, 47, 171–174. [Google Scholar] [CrossRef]
- Avenell, A.; Smith, T.O.; Curtain, J.P.; Mak, J.C.; Myint, P.K. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst. Rev. 2016, 2016, CD001880. [Google Scholar] [CrossRef]

| Measure | Screening | T1 Baseline | T2 1 Month | T3 3 Months | T4 6 Months | T5 12 Months |
|---|---|---|---|---|---|---|
| Primary outcome | ||||||
| Short Physical Performance Battery (SPPB) | x | x | x | x | x | |
| Secondary outcomes | ||||||
| Barthel index | x | x | x | x | x | |
| Functional Ambulation Classification (FAC) | x | x | x | x | x | |
| Lawton’s Instrumental Activities of Daily Living (IADL) | x | x | x | x | x | |
| Global Deterioration Scale (GDS) | x | x | x | x | x | |
| Mini-Mental State Examination (MMSE) | x | x | x | x | x | |
| Abbreviated Mental Test 4 (4AT) | x | x | x | x | x | |
| Yesavage Geriatric Depression Scale | x | x | x | x | x | |
| Falls Efficacy Scale International (FES-I) | x | x | x | |||
| Frailty | x | x | x | x | x | |
| Handgrip | x | x | x | x | x | |
| Quality of Life (EuroQol-5D) | x | x | x | x | x | |
| Sarcopenia and Quality of Life (SarQoL) | x | x | x | |||
| FRAX, QFracture | x | x | ||||
| Urinary incontinence | x | x | x | x | x | |
| Fecal incontinence | x | x | x | x | x | |
| Pressure ulcers | x | x | x | x | x | |
| Constipation | x | x | x | x | x | |
| Polypharmacy | x | x | x | x | x | |
| Rate and risk of falls | x | x | x | x | x | |
| Visual Analogue Scale for Pain (VAS) | x | x | x | x | x | |
| Cumulative Illness Rating Scale for Geriatrics (CIRS-G) | x | |||||
| Mini-Nutritional Assessment (MNA) | x | x | x | x | x | |
| Adverse effects | x | x | x | x | ||
| Mortality | x | x | x | x | ||
| Admission and readmission to the hospital | x | x | x | x | ||
| Institutionalization | x | x | x | x | x | |
| Blood test | x | x | x | x | x | |
| Bone turnover markers (BTMs) | x | x | ||||
| Dual-energy X-ray absorptiometry (DXA) | x | x | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedeno-Veloz, B.A.; Casadamon-Munarriz, I.; Rodríguez-García, A.; Lozano-Vicario, L.; Zambom-Ferraresi, F.; Gonzalo-Lázaro, M.; Hidalgo-Ovejero, Á.M.; Izquierdo, M.; Martínez-Velilla, N. Effect of a Multicomponent Intervention with Tele-Rehabilitation and the Vivifrail© Exercise Programme on Functional Capacity after Hip Fracture: Study Protocol for the ActiveFLS Randomized Controlled Trial. J. Clin. Med. 2024, 13, 97. https://doi.org/10.3390/jcm13010097
Cedeno-Veloz BA, Casadamon-Munarriz I, Rodríguez-García A, Lozano-Vicario L, Zambom-Ferraresi F, Gonzalo-Lázaro M, Hidalgo-Ovejero ÁM, Izquierdo M, Martínez-Velilla N. Effect of a Multicomponent Intervention with Tele-Rehabilitation and the Vivifrail© Exercise Programme on Functional Capacity after Hip Fracture: Study Protocol for the ActiveFLS Randomized Controlled Trial. Journal of Clinical Medicine. 2024; 13(1):97. https://doi.org/10.3390/jcm13010097
Chicago/Turabian StyleCedeno-Veloz, Bernardo Abel, Irache Casadamon-Munarriz, Alba Rodríguez-García, Lucia Lozano-Vicario, Fabricio Zambom-Ferraresi, María Gonzalo-Lázaro, Ángel María Hidalgo-Ovejero, Mikel Izquierdo, and Nicolás Martínez-Velilla. 2024. "Effect of a Multicomponent Intervention with Tele-Rehabilitation and the Vivifrail© Exercise Programme on Functional Capacity after Hip Fracture: Study Protocol for the ActiveFLS Randomized Controlled Trial" Journal of Clinical Medicine 13, no. 1: 97. https://doi.org/10.3390/jcm13010097
APA StyleCedeno-Veloz, B. A., Casadamon-Munarriz, I., Rodríguez-García, A., Lozano-Vicario, L., Zambom-Ferraresi, F., Gonzalo-Lázaro, M., Hidalgo-Ovejero, Á. M., Izquierdo, M., & Martínez-Velilla, N. (2024). Effect of a Multicomponent Intervention with Tele-Rehabilitation and the Vivifrail© Exercise Programme on Functional Capacity after Hip Fracture: Study Protocol for the ActiveFLS Randomized Controlled Trial. Journal of Clinical Medicine, 13(1), 97. https://doi.org/10.3390/jcm13010097







