Musculoskeletal Rehabilitation: New Perspectives in Postoperative Care Following Total Knee Arthroplasty Using an External Motion Sensor and a Smartphone Application for Remote Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hospital Information System
2.2. Electronic Application System (App)
2.3. External Motion Sensor
2.4. Patient-Reported Outcome Measures (PROMS)
2.5. Data Platform (Pheno4U)
2.6. Data Analysis
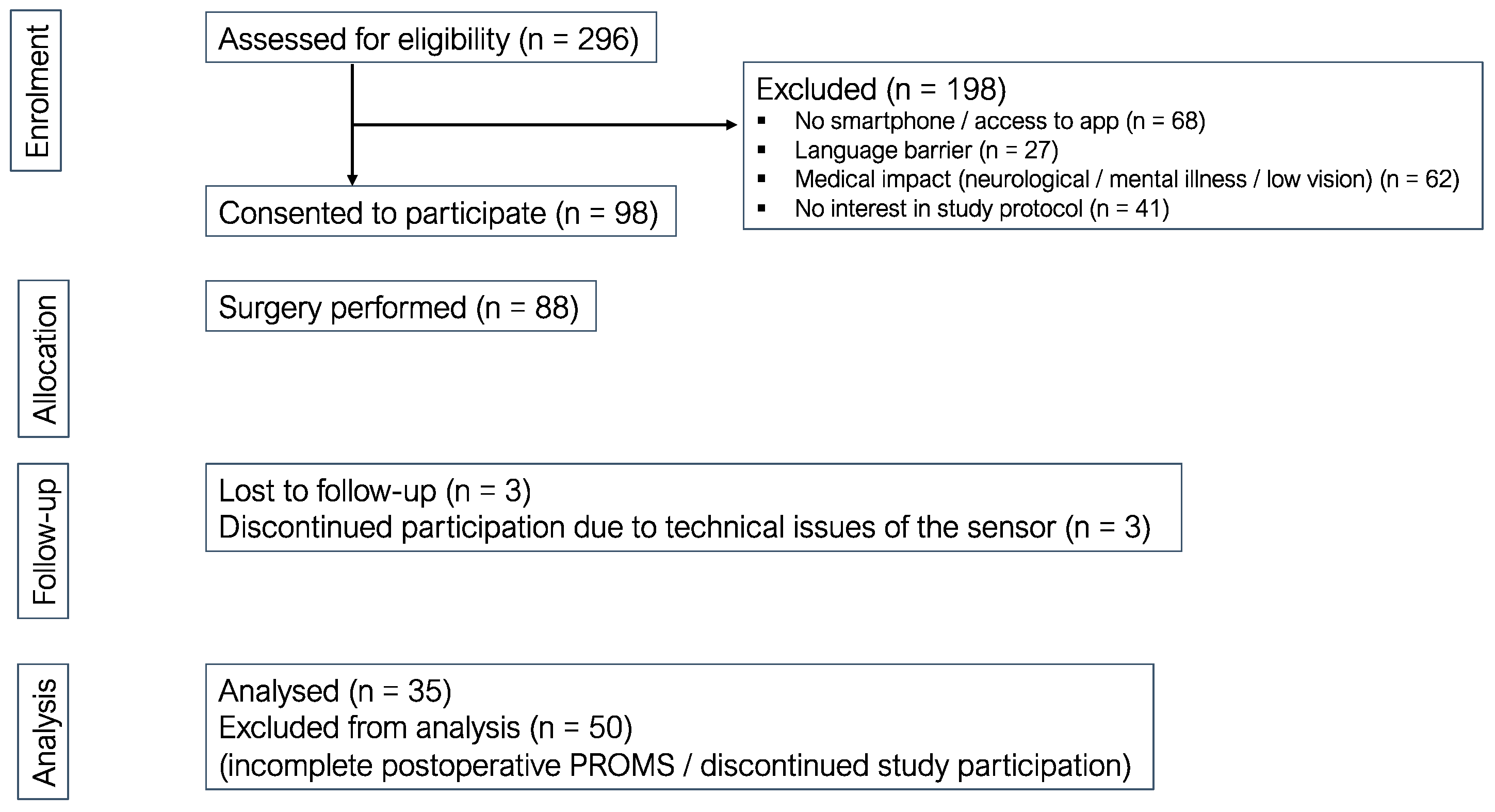
3. Results
3.1. Functional Results
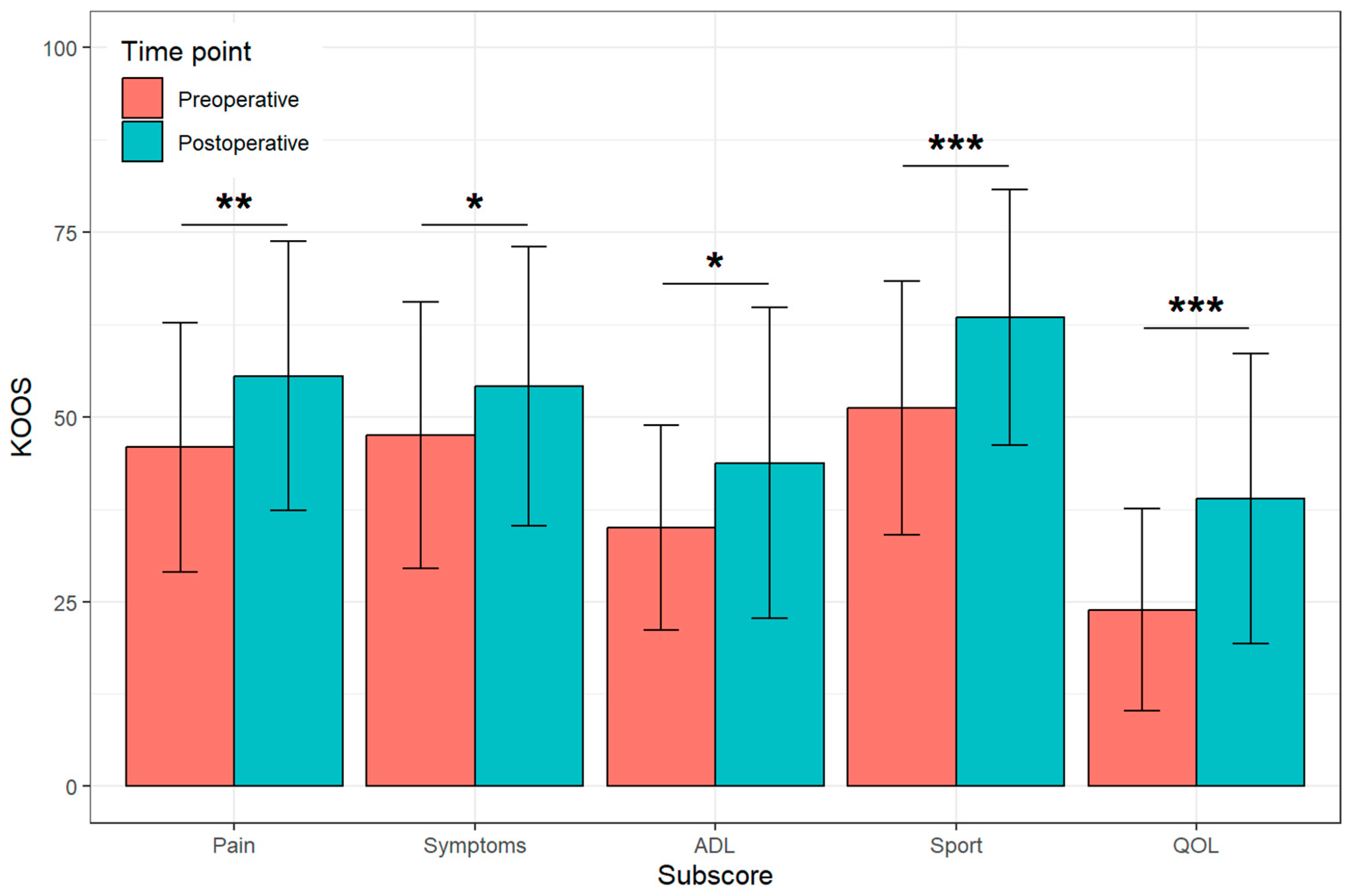
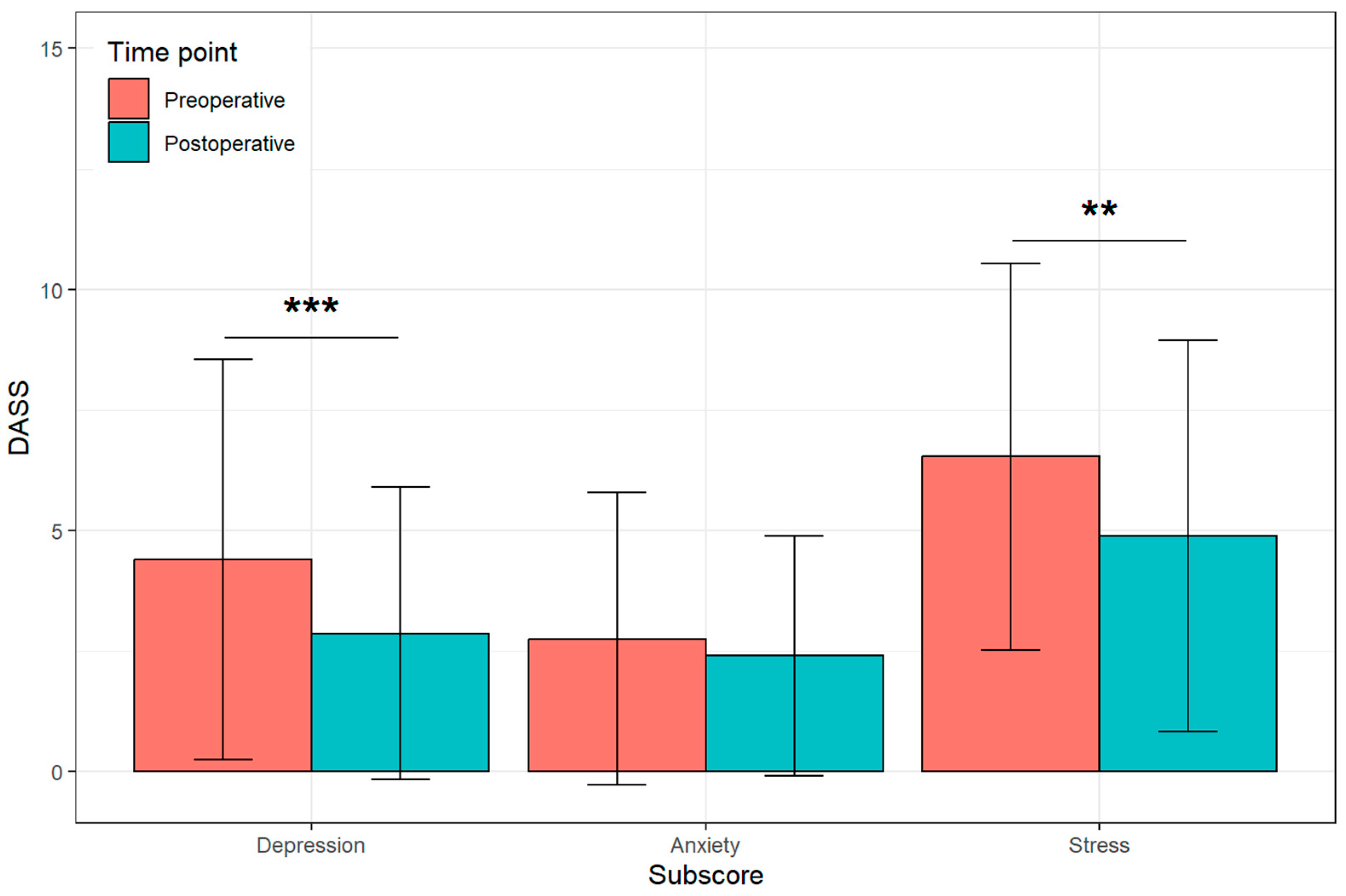
3.2. Patient-Reported Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Osteoarthritis|BMUS: The Burden of Musculoskeletal Diseases in the United States. Available online: https://www.boneandjointburden.org/fourth-edition/iiib10/osteoarthritis (accessed on 10 August 2023).
- Gandhi, N.; Qadeer, A.S.; Meher, A.; Rachel, J.; Patra, A.; John, J.; Anilkumar, A.; Dutta, A.; Nanda, L.; Rout, S.K. Costs and models used in the economic analysis of Total Knee Replacement (TKR): A systematic review. PLoS ONE 2023, 18, e0280371. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzaman, H.; Kinghorn, P.; Oppong, R. Cost-effectiveness of surgical interventions for the management of osteoarthritis: A systematic review of the literature. BMC Musculoskelet. Disord. 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.B.; Chesworth, B.M.; Davis, A.M.; Mahomed, N.N.; Charron, K.D.J. Patient satisfaction after total knee arthroplasty: Who is satisfied and who is not? Clin. Orthop. 2010, 468, 57–63. [Google Scholar] [CrossRef]
- Scott, C.E.H.; Howie, C.R.; MacDonald, D.; Biant, L.C. Predicting dissatisfaction following total knee replacement: A prospective study of 1217 patients. J. Bone Joint Surg. Br. 2010, 92, 1253–1258. [Google Scholar] [CrossRef]
- Maccagnano, G.; Pesce, V.; Noia, G.; Coviello, M.; Vicenti, G.; Vitiello, R.; Ziranu, A.; Spinarelli, A.; Moretti, B. The effects of a new protocol on blood loss in total knee arthroplasty. Orthop. Rev. 2022, 14, 37625. [Google Scholar] [CrossRef]
- Scott, C.E.H.; Bugler, K.E.; Clement, N.D.; MacDonald, D.; Howie, C.R.; Biant, L.C. Patient expectations of arthroplasty of the hip and knee. J. Bone Joint Surg. Br. 2012, 94, 974–981. [Google Scholar] [CrossRef]
- Lützner, C.; Postler, A.; Beyer, F.; Kirschner, S.; Lützner, J. Fulfillment of expectations influence patient satisfaction 5 years after total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2019, 27, 2061–2070. [Google Scholar] [CrossRef]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Lond. Engl. 2021, 396, 2006–2017. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Tormene, P.; Bartolo, M.; De Nunzio, A.M.; Fecchio, F.; Quaglini, S.; Tassorelli, C.; Sandrini, G. Estimation of human trunk movements by wearable strain sensors and improvement of sensor’s placement on intelligent biomedical clothes. Biomed. Eng. Online 2012, 11, 95. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Tang, S.; Luo, H. Privacy Protection for E-Health Systems by Means of Dynamic Authentication and Three-Factor Key Agreement. IEEE Trans. Ind. Electron. 2018, 65, 2795–2805. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Drummond, C.; Craker, J.; Rowbottom, J.R.; Voos, J.E. Wearable Devices for Sports: New Integrated Technologies Allow Coaches, Physicians, and Trainers to Better Understand the Physical Demands of Athletes in Real time. IEEE Pulse 2017, 8, 38–43. [Google Scholar] [CrossRef]
- Municio, E.; Daneels, G.; De Brouwer, M.; Ongenae, F.; De Turck, F.; Braem, B.; Famaey, J.; Latre, S. Continuous Athlete Monitoring in Challenging Cycling Environments Using IoT Technologies. IEEE Internet Things J. 2019, 6, 10875–10887. [Google Scholar] [CrossRef]
- Rossi, M.; Rizzi, A.; Lorenzelli, L.; Brunelli, D. Remote rehabilitation monitoring with an IoT-enabled embedded system for precise progress tracking. In Proceedings of the 2016 IEEE International Conference on Electronics, Circuits and Systems (ICECS), Monte Carlo, Monaco, 11–14 December 2016; pp. 384–387. [Google Scholar] [CrossRef]
- Capecci, M.; Ceravolo, M.G.; Ferracuti, F.; Iarlori, S.; Monteriu, A.; Romeo, L.; Verdini, F. The KIMORE Dataset: Kinematic Assessment of Movement and Clinical Scores for Remote Monitoring of Physical Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 2019, 27, 1436–1448. [Google Scholar] [CrossRef]
- Marques, C.J.; Bauer, C.; Grimaldo, D.; Tabeling, S.; Weber, T.; Ehlert, A.; Mendes, A.H.; Lorenz, J.; Lampe, F. Sensor Positioning Influences the Accuracy of Knee Rom Data of an E-Rehabilitation System: A Preliminary Study with Healthy Subjects. Sensors 2020, 20, 2237. [Google Scholar] [CrossRef]
- Roos, E.M.; Lohmander, L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): From joint injury to osteoarthritis. Health Qual. Life Outcomes 2003, 1, 64. [Google Scholar] [CrossRef]
- Mancuso, C.A.; Sculco, T.P.; Wickiewicz, T.L.; Jones, E.C.; Robbins, L.; Warren, R.F.; Williams-Russo, P. Patients’ expectations of knee surgery. J. Bone Joint Surg. Am. 2001, 83, 1005–1012. [Google Scholar] [CrossRef]
- Mancuso, C.A.; Graziano, S.; Briskie, L.M.; Peterson, M.G.E.; Pellicci, P.M.; Salvati, E.A.; Sculco, T.P. Randomized trials to modify patients’ preoperative expectations of hip and knee arthroplasties. Clin. Orthop. 2008, 466, 424–431. [Google Scholar] [CrossRef]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef]
- Osman, A.; Wong, J.L.; Bagge, C.L.; Freedenthal, S.; Gutierrez, P.M.; Lozano, G. The Depression Anxiety Stress Scales—21 (DASS-21): Further Examination of Dimensions, Scale Reliability, and Correlates. J. Clin. Psychol. 2012, 68, 1322–1338. [Google Scholar] [CrossRef] [PubMed]
- Reichheld, F.F. The one number you need to grow. Harv. Bus. Rev. 2003, 81, 46–54. [Google Scholar] [PubMed]
- Mohamed Refai, M.I.; van Beijnum, B.J.F.; Buurke, J.H.; Veltink, P.H. Gait and Dynamic Balance Sensing Using Wearable Foot Sensors. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 2019, 27, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Ngamsuriyaroj, S.; Chira-Adisai, W.; Somnuk, S.; Leksunthorn, C.; Saiphim, K. Walking Gait Measurement and Analysis via Knee Angle Movement and Foot Plantar Pressures. In Proceedings of the 15th International Joint Conference on Computer Science and Software Engineering, JCSSE 2018, Nakhonpathom, Thailand, 11–13 July 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Eguchi, R.; Takahashi, M. Insole-Based Estimation of Vertical Ground Reaction Force Using One-Step Learning With Probabilistic Regression and Data Augmentation. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 2019, 27, 1217–1225. [Google Scholar] [CrossRef]
- Cui, C.; Bian, G.-B.; Hou, Z.-G.; Zhao, J.; Su, G.; Zhou, H.; Peng, L.; Wang, W. Simultaneous Recognition and Assessment of Post-Stroke Hemiparetic Gait by Fusing Kinematic, Kinetic, and Electrophysiological Data. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 2018, 26, 856–864. [Google Scholar] [CrossRef]
- Pereira, A.; Folgado, D.; Nunes, F.; Almeida, J.; Sousa, I. Using Inertial Sensors to Evaluate Exercise Correctness in Electromyography-based Home Rehabilitation Systems. In Proceedings of the 2019 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Istanbul, Turkey, 26–28 June 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Lu, Z.; Cao, S.; Wu, D.; Zhang, X. Development of an EMG-ACC-Based Upper Limb Rehabilitation Training System. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 2017, 25, 244–253. [Google Scholar] [CrossRef]
- Todd, C.J.; Hubner, P.P.; Hubner, P.; Schubert, M.C.; Migliaccio, A.A. StableEyes-A Portable Vestibular Rehabilitation Device. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 2018, 26, 1223–1232. [Google Scholar] [CrossRef]
- Tannous, H.; Dao, T.T.; Istrate, D. Serious Game for Functional Rehabilitation. In Proceedings of the 2015 International Conference on Advances in Biomedical Engineering (ICABME), Beirut, Lebanon, 16–18 September 2015. [Google Scholar] [CrossRef]
- do Nascimento, L.M.S.; Bonfati, L.V.; Freitas, M.L.B.; Mendes Junior, J.J.A.; Siqueira, H.V.; Stevan, S.L. Sensors and Systems for Physical Rehabilitation and Health Monitoring-A Review. Sensors 2020, 20, 4063. [Google Scholar] [CrossRef]
- mHealth Market Size, Share, Growth & Trends|Global Report. 2030. Available online: https://www.fortunebusinessinsights.com/industry-reports/mhealth-market-100266 (accessed on 16 September 2023).
- Rodgers, M.M.; Alon, G.; Pai, V.M.; Conroy, R.S. Wearable technologies for active living and rehabilitation: Current research challenges and future opportunities. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668319839607. [Google Scholar] [CrossRef]


| Female: 43 (46.7%) | Male: 49 (53.3%) | Total | |
|---|---|---|---|
| Age (at surgery) [years] | 61.7 ± 7.5 (45–77) (n = 36) | 63.8 ± 7.8 (41–80) (n = 48) | 62.9 ± 7.7 (41–80) (n = 84) |
| Risk factors | |||
| None | 12 (33.3%) (n = 36) | 16 (33.3%) (n = 48) | 28 (33.3%) (n = 84) |
| BMI [kg/m²] | 30.4 ± 6.3 (19–42) (n = 36) | 30.0 ± 5.1 (21–46) (n = 48) | 30.1 ± 5.6 (19–46) (n = 84) |
| Smoking | 5 (13.9%) (n = 36) | 6 (12.5%) (n = 48) | 11 (13.1%) (n = 84) |
| Diabetes | 4 (11.1%) (n = 36) | 6 (12.5%) (n = 48) | 10 (11.9%) (n = 84) |
| Hypertension | 20 (55.6%) (n = 36) | 27 (56.3%) (n = 48) | 47 (56.0%) (n = 84) |
| Total reviewed | n = 36 | n = 48 | n = 84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann-Langen, M.V.; Ochs, B.G.; Lützner, J.; Postler, A.; Kirschberg, J.; Sehat, K.; Selig, M.; Grupp, T.M. Musculoskeletal Rehabilitation: New Perspectives in Postoperative Care Following Total Knee Arthroplasty Using an External Motion Sensor and a Smartphone Application for Remote Monitoring. J. Clin. Med. 2023, 12, 7163. https://doi.org/10.3390/jcm12227163
Neumann-Langen MV, Ochs BG, Lützner J, Postler A, Kirschberg J, Sehat K, Selig M, Grupp TM. Musculoskeletal Rehabilitation: New Perspectives in Postoperative Care Following Total Knee Arthroplasty Using an External Motion Sensor and a Smartphone Application for Remote Monitoring. Journal of Clinical Medicine. 2023; 12(22):7163. https://doi.org/10.3390/jcm12227163
Chicago/Turabian StyleNeumann-Langen, Mirjam Victoria, Björn Gunnar Ochs, Jörg Lützner, Anne Postler, Julia Kirschberg, Khosrow Sehat, Marius Selig, and Thomas M. Grupp. 2023. "Musculoskeletal Rehabilitation: New Perspectives in Postoperative Care Following Total Knee Arthroplasty Using an External Motion Sensor and a Smartphone Application for Remote Monitoring" Journal of Clinical Medicine 12, no. 22: 7163. https://doi.org/10.3390/jcm12227163
APA StyleNeumann-Langen, M. V., Ochs, B. G., Lützner, J., Postler, A., Kirschberg, J., Sehat, K., Selig, M., & Grupp, T. M. (2023). Musculoskeletal Rehabilitation: New Perspectives in Postoperative Care Following Total Knee Arthroplasty Using an External Motion Sensor and a Smartphone Application for Remote Monitoring. Journal of Clinical Medicine, 12(22), 7163. https://doi.org/10.3390/jcm12227163





