A Retrospective Study of Trifluridine/Tipiracil with Fruquintinib in Patients with Chemorefractory Metastatic Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatments
2.3. Efficacy and Safety Assessments
2.4. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
3.2. Efficacy
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer. J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Wicherts, D.A.; Haas, R.J.; Ciacio, O.; Lévi, F.; Paule, B.; Ducreux, M.; Azoulay, D.; Bismuth, H.; Castaing, D. Patients with initially unresectable colorectal liver metastases: Is there a possibility of cure? J. Clin. Oncol. 2009, 7, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lin, Y.; Li, R.; Shen, X.; Xiang, M.; Xiong, G.; Zhang, K.; Xia, T.; Guo, J.; Miao, Z.; et al. Molecular targeted therapy for metastatic colorectal cancer: Current and evolving approaches. Front. Pharmacol. 2023, 14, 1165666. [Google Scholar] [CrossRef] [PubMed]
- Riedesser, J.E.; Ebert, M.P.; Betge, J. Precision medicine for metastatic colorectal cancer in clinical practice. Ther. Adv. Med. Oncol. 2022, 14, 17588359211072703. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Yuan, Y.; Wang, X.; Chen, G.; Wang, Y.; Sheng, W.; Li, X.; Zhou, A.; Zhang, Z.; Li, G.; et al. Updates in version 2020 of CSCO guidelines for colorectal cancer from version 2019. Chin. J. Cancer. Res. 2020, 32, 403–407. [Google Scholar] [CrossRef]
- Salvatore, L.; Rossini, D.; Moretto, R.; Cremolini, C.; Schirripa, M.; Antoniotti, C.; Marmorino, F.; Loupakis, F.; Falcone, A.; Masi, G. TAS-102 for the treatment of metastatic colorectal cancer. Expert Rev. Anticancer Ther. 2015, 15, 1283–1292. [Google Scholar] [CrossRef]
- Mayer, R.J.; Cutsem, E.V.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef]
- Xu, J.; Kim, T.W.; Shen, L.; Sriuranpong, V.; Pan, H.; Xu, R.; Guo, W.; Han, S.W.; Liu, T.; Park, Y.S.; et al. Results of a randomized, double-blind, placebo– controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: The TERRA study. J. Clin. Oncol. 2018, 36, 350–358. [Google Scholar] [CrossRef]
- Kuboki, Y.; Nishina, T.; Shinozaki, E.; Yamazaki, K.; Shitara, K.; Okamoto, W.; Kajiwara, T.; Matsumoto, T.; Tsushima, T.; Mochizuki, N.; et al. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): An investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol. 2017, 18, 1172–1181. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Yilmaz, M.; Möller, S.; Zitnjak, D.; Krogh, M.; Petersen, L.N.; Poulsen, L.Ø.; Winther, S.B.; Thomsen, K.G.; Qvortrup, C. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: An investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 412–420. [Google Scholar] [CrossRef]
- Tabernero, J.; Prager, G.W.; Fakih, M.; Ciardiello, F.; Cutsem, E.V.; Elez, E.; Cruz, F.M.; Wyrwicz, L.; Stroyakovskiy, D.; Papai, Z.; et al. Triuridine/tipiracil plus bevacizumab for third-line treatment of refractory metastatic colorectal cancer: The phase 3 randomized SUNLIGHT study. J. Clin. Oncol. 2023, 41, 4. [Google Scholar] [CrossRef]
- Fan, F.; Samuel, S.; Gaur, P.; Lu, J.; Dallas, N.A.; Xia, L.; Bose, D.; Ramachandran, V.; Ellis, L.M. Chronic exposure of colorectal cancer cells to bevacizumab promotes compensatory pathways that mediate tumor cell migration. Br. J. Cancer 2011, 104, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Itatani, Y.; Kawada, K.; Yamamoto, T.; Sakai, Y. Resistance to Anti-Angiogenic Therapy in Cancer—Alterations to Anti-VEGF Pathway. Int. J. Mol. Sci. 2018, 19, 1232. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, S.; Xu, R.; Shen, L.; Xu, J.; Bai, Y.; Yang, L.; Deng, Y.; Chen, Z.; Zhong, H.; et al. Effect of Fruquintinib vs. Placebo on Overall Survival in Patients with Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA 2018, 319, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Dasari1, N.A.; Lonardi, S.; Garcia-Carbonero, R.; Elez Fernandez, M.E.; Yoshino, T.; Sobrero, A.F.; Yao, J.C.; García-Alfonso, P.; Kocsis, J.; Cubillo Gracian, A.; et al. FRESCO-2: A global phase III multiregional clinical trial (MRCT) evaluating the efficacy and safety of fruquintinib in patients with refractory metastatic colorectal cancer. Ann. Oncol. 2022, 33, S1392. [Google Scholar] [CrossRef]
- Tsukihara, H.; Nakagawa, F.; Sakamoto, K.; Ishida, K.; Tanaka, N.; Okabe, H.; Uchida, J.; Matsuo, K.; Takechi, T. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, together with bevacizumab, cetuximab, or panitumumab on human colorectal cancer xenografts. Oncol. Rep. 2015, 33, 2135–2142. [Google Scholar] [CrossRef]
- Nukatsuka, M.; Fujioka, A.; Nagase, H.; Tanaka, G.; Hayashi, H. Evaluation of a Novel Combination Therapy, based on Trifluridine/Tipiracil and Fruquintinib against Colorectal Cancer. Chemotherapy 2023, 68, 102–110. [Google Scholar] [CrossRef]
- National Cancer Institute Division of Cancer Treatment and Diagnosis. Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40 (accessed on 28 April 2022).
- Murakami, Y.; Kazuno, H.; Emura, T.; Tsujimoto, H.; Suzuki, N.; Fukushima, M. Different mechanisms of acquired resistance to fluorinated pyrimidines in human colorectal cancer cells. Int. J. Oncol. 2000, 17, 277–283. [Google Scholar] [CrossRef]
- Matsuoka, K.; Nakagawa, F.; Kobunai, T.; Takechi, T. Trifluridine/tipiracil overcomes the resistance of human gastric 5-fluorouracil-refractory cells with high thymidylate synthase expression. Oncotarget 2018, 9, 13438–13450. [Google Scholar] [CrossRef]
- Nukatsuka, M.; Nakagawa, F.; Takechi, T. Efficacy of combination chemotherapy using a novel Oral chemotherapeutic agent, TAS-102, with Oxaliplatin on human colorectal and gastric Cancer Xenografts. Anticancer Res. 2015, 35, 4605–4615. [Google Scholar] [PubMed]
- Nukatsuka, M.; Nakagawa, F.; Saito, H.; Sakata, M.; Uchida, J.; Takechi, T. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, with irinotecan hydrochloride on human colorectal and gastric cancer xenografts. Anticancer Res. 2015, 35, 1437–1445. [Google Scholar] [PubMed]
- Elamin, Y.Y.; Rafee, S.; Osman, N.; Byrne, K.J.O.; Gately, K. Thymidine Phosphorylase in Cancer; Enemy or Friend? Cancer Microenviron. 2016, 9, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Arao, T.; Matsumoto, K.; Kimura, H.; Togashi, Y.; Hirashima, Y.; Horita, Y.; Iwasa, S.; Okita, T.N.; Honma, Y.; et al. Biomarkers of reactive resistance and early disease progression during chemotherapy plus bevacizumab treatment for colorectal carcinoma. Oncotarget 2014, 5, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Loupakis, F.; Antonuzzo, L.; Bachet, J.B.; Kuan, F.C.; Macarulla, T.; Pietrantonio, F.; Xu, R.H.; Taniguchi, H.; Winder, T.; Yuki, S.; et al. Practical considerations in the use of regorafenib in metastatic colorectal cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920956862. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, J.; Li, K.; Zhang, L.; Ren, H.; Guo, L.; Sai, Y.; Zhang, W.; Su, W. Preclinical pharmacokinetics and disposition of a novel selective VEGFR inhibitor fruquintinib (HMPL-013) and the prediction of its human pharmacokinetics. Cancer Chemother. Pharmacol. 2014, 74, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Li, H.; Xiong, X.; Peng, C.; Wang, N.; Ma, X.; Ma, A. Cost-effectiveness analysis of fruquintinib versus regorafenib as the third-line therapy for metastatic colorectal cancer in China. J. Med. Econ. 2021, 24, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.L.; Warner, S.; Ito, K.; Raoof, M.; Wu, G.X.; Kessler, J.; Kim, J.Y.; Fong, Y. Cytoreduction for Colorectal Metastases: Liver, Lung, Peritoneum, Lymph Nodes, Bone, Brain. When Does it Palliate, Prolong Survival, and Potentially Cure? Curr. Probl. Surg. 2018, 55, 330–379. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.A.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D.J. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: A pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J. Clin. Oncol. 2012, 30, 263–267. [Google Scholar] [CrossRef]
- Qin, S.; Li, J.; Bai, Y.; Deng, Y.; Yang, L.; Xu, R.H.; Zhong, H.; Chen, Z.; Pan, H.; Guo, W.; et al. Quality-adjusted survival in patients with metastatic colorectal cancer treated with fruquintinib in the FRESCO trial. Future Oncol. 2021, 17, 1923–1931. [Google Scholar] [CrossRef]
- Liu, G.; Franssen, E.; Fitch, M.I.; Warner, E. Patient preferences for oral versus intravenous palliative chemotherapy. J. Clin. Oncol. 1997, 15, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Nakagawa, F.; Tanaka, N.; Okabe, H.; Matsuo, K.; Takechi, T. Effective Sequential Combined Chemotherapy with Trifluridine/Tipiracil and Regorafenib in Human Colorectal Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2915. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Yokogawa, T.; Ueno, H.; Oguchi, K.; Kazuno, H.; Ishida, K.; Tanaka, N.; Osada, A.; Yamada, Y.; Okabe, H.; et al. Crucial roles of thymidine kinase 1 and deoxyUTPase in incorporating the antineoplastic nucleosides trifluridine and 2’-deoxy-5-fluorouridine into DNA. Int. J. Oncol. 2015, 46, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Marmorino, F.; Salvatore, L.; Barbara, C.; Allegrini, G.; Antonuzzo, L.; Masi, G.; Loupakis, F.; Borelli, B.; Chiara, S.; Banzi, M.C.; et al. Serum LDH predicts benefit from bevacizumab beyond progression in metastatic colorectal cancer. Br. J. Cancer 2017, 116, 318–323. [Google Scholar] [CrossRef]
- Iorio, J.; Lastraioli, E.; Tofani, L.; Petroni, G.; Antonuzzo, L.; Messerini, L.; Perrone, G.; Caputo, D.; Francesconi, M.; Amato, M.M.; et al. hERG1 and HIF-2 Behave as Biomarkers of Positive Response to Bevacizumab in Metastatic Colorectal Cancer Patients. Transl. Oncol. 2020, 13, 100740. [Google Scholar] [CrossRef] [PubMed]
- Antoniotti, C.; Marmorino, F.; Boccaccino, A.; Martini, S.; Antista, M.; Rossini, D.; Zuco, V.; Prisciandaro, M.; Conca, V.; Zucchelli, G.; et al. Early modulation of Angiopoietin-2 plasma levels predicts benefit from regorafenib in patients with metastatic colorectal cancer. Eur. J. Cancer 2022, 165, 116–124. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Bai, Y.; Deng, Y.; Yang, L.; Xu, R.; Chen, Z.; Zhong, H.; Pan, H.; Guo, W.; et al. Association between hand-foot skin reaction (HFSR) and survival benefit of fruquintinib in FRESCO trial. J. Clin. Oncol. 2019, 37, e15012. [Google Scholar] [CrossRef]
- Ciardiello, F.; Ciardiello, D.; Martini, G.; Napolitano, S.; Tabernero, J.; Cervantes, A. Clinial management of metastatic colorectal cancer in the era of precision medicine. CA Cancer. J. Clin. 2022, 72, 372–401. [Google Scholar] [CrossRef]

| Characteristic | Total (n = 32) n (%) | TAS-102 Plus Fruquintinib 4 mg (n = 21) n (%) | TAS-102 Plus Fruquintinib3 mg (n = 11) n (%) | p |
|---|---|---|---|---|
| Age (median, range) | 64 (39–78) | 58 (39–74) | 67 (46–78) | |
| Sex | 1 | |||
| Male | 19 (59.4) | 12 (57.1) | 7 (63.6) | |
| Female | 13 (40.6) | 9 (42.9) | 4 (36.4) | |
| ECOG | 0.02 | |||
| 0 | 7 (21.9) | 7 (33.3) | 0 (0) | |
| 1 | 20 (62.5) | 13 (61.9) | 7 (63.6) | |
| 2 | 5 (15.6) | 1 (4.8) | 4 (36.4) | |
| Primary location | 1 | |||
| Right colon | 11 (34.4) | 7 (33.3) | 4 (36.4) | |
| Left colon | 21 (65.6) | 14 (66.7) | 7 (63.6) | |
| Number of metastatic sites | 1 | |||
| 1–2 | 19 (59.4) | 12 (57.1) | 7 (63.6) | |
| ≥3 | 13 (40.6) | 9 (42.9) | 4 (36.4) | |
| Metastatic sites | 0.45 | |||
| Liver | 22 (68.8) | 16 (76.2) | 6 (54.5) | |
| Lung | 20 (62.5) | 10 (47.6) | 10 (90.9) | |
| Peritoneum | 8 (25) | 5 (23.8) | 3 (27.3) | |
| Lymph node | 19 (59.4) | 11 (52.4) | 8 (72.7) | |
| Others | 2 (6.25) | 2 (9.5) | 0 (0) | |
| RAS status | 0.47 | |||
| Wild-type | 14 (44) | 8 (38.1) | 6 (54.5) | |
| Mutant | 18 (56) | 13 (61.9) | 5 (45.5) | |
| BRAF status | 1 | |||
| Wild-type | 30 (93.8) | 20 (95.2) | 10 (90.9) | |
| Mutant | 2 (6.2) | 1 (4.8) | 1 (9.1) | |
| MMR status | ||||
| MSS | 32 (100) | 21 (100) | 11 (100) | |
| Unknown | 0 (0) | 0 (0) | 0 (0) | |
| Treatment line | 0.06 | |||
| 3 | 22 (68.8) | 17 (81) | 5 (45.5) | |
| ≥4 | 10 (31.2) | 4 (19) | 6 (54.5) | |
| Previous treatment agents | 0.99 | |||
| Fluorouracil | 32 (100) | 21 (100) | 11 (100) | |
| Oxaliplatin | 32 (100) | 21 (100) | 11 (100) | |
| Irinotecan | 30 (93.8) | 20 (95.2) | 10 (90.9) | |
| Raltitrexed | 3 (9.4) | 2 (9.5) | 1 (9.1) | |
| Bevacizumab | 29 (90.6) | 20 (95.2) | 9 (81.8) | |
| Cetuximab | 8 (25) | 5 (23.8) | 3 (27.3) | |
| Regorafenib | 4 (12.5) | 2 (9.5) | 2 (18.2) |
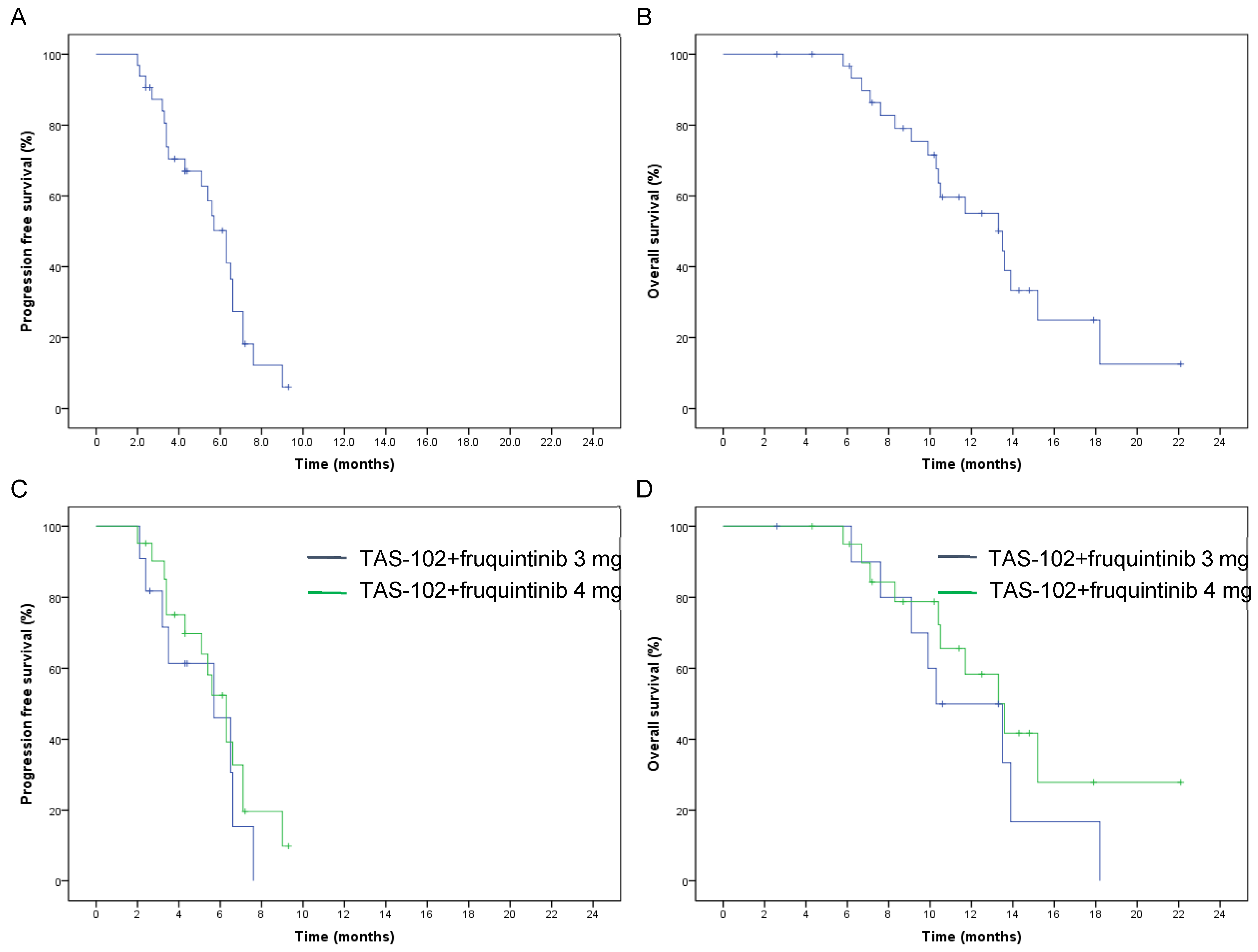
| ORR | p | DCR | p | Median PFS (95% CI) | p | Median OS (95% CI) | p | |
|---|---|---|---|---|---|---|---|---|
| Total | 9.4% | 75% | 6.3 (5.3–7.3) | 13.5 (9.5–17.5) | ||||
| Treatment program | 0.53 | 0.40 | 0.40 | 0.12 | ||||
| TAS-102 + fruquintinib 3 mg | 0 | 63.6% | 5.7 (2.3–9.1) | 10.3 (9.2–11.4) | ||||
| TAS-102 + fruquintinib 4 mg | 14.3% | 81% | 6.3 (5.2–7.4) | 13.6 (11.8–15.4) | ||||
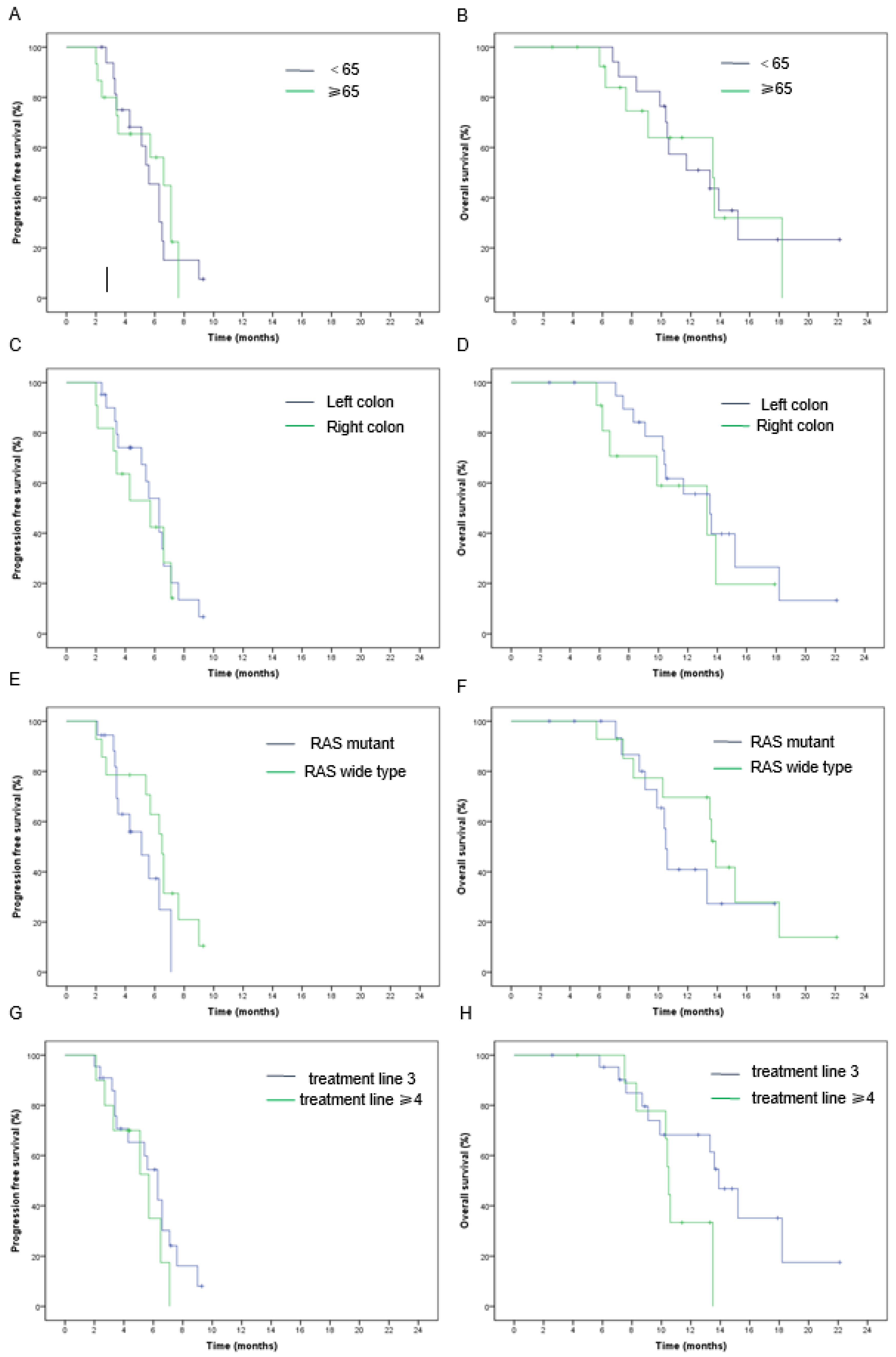
| Age | 0.59 | 0.69 | 0.85 | 0.87 | ||||
| <65 | 5.9% | 70.6% | 5.4 (4.9–5.9) | 13.3 (8.2–13.4) | ||||
| ≥65 | 13.3% | 80% | 6.5 (5.0–8.0) | 13.5 (10.2–16.8) | ||||
| Tumor site | 1 | 0.40 | 0.61 | 0.46 | ||||
| Left | 9.5% | 81% | 6.3 (5.2–7.4) | 13.5 (8.4–18.6) | ||||
| Right | 9.1% | 63.6% | 5.7 (2.4–9.0) | 13.3 (6.6–20) | ||||
| RAS status | 0.57 | 0.70 | 0.17 | 0.86 | ||||
| Wild | 14.3% | 71.4% | 6.5 (5.5–7.5) | 13.6 (8.5–18.7) | ||||
| Mutant | 5.6% | 77.8% | 5.4 (3.7–7.1) | 10.6 (6.9–14.3) | ||||
| Metastatic site | 0.22 | 1 | 0.03 | 0.01 | ||||
| Liver | 4.5% | 72.7% | 5.6 (3.2–8.0) | 10.4 (9.5–11.3) | ||||
| Without liver | 20% | 80% | 7.1 (6.3–7.9) | 15.2 (10.9–19.5) | ||||
| Metastatic site | 0.56 | 0.38 | 0.04 | 0.03 | ||||
| Peritoneum | 0 | 62.5% | 3.4 (3.0–3.8) | 7.1 (4.1–10.1) | ||||
| Without peritoneum | 12% | 79.2% | 6.3 (4.7–7.9) | 13.6 (12.9–14.3) | ||||
| Number of metastatic sites | 1 | 0.22 | 0.000 | 0.000 | ||||
| 1–2 | 10.5% | 84.2% | 6.6 (6.4–6.8) | 15.2 (13–17.4) | ||||
| ≥3 | 7.7% | 61.5% | 3.4 (3.2–3.6) | 9.1 (6.6–11.6) | ||||
| Treatment line | 0.53 | 0.68 | 0.34 | 0.22 | ||||
| 3 | 13.6% | 77.3% | 6.3 (5.1–7.5) | 13.6 (11–16.2) | ||||
| ≥4 | 0 | 70% | 5.7 (3.0–8.4) | 10.5 (10.0–11.1) |
| Adverse Event | All Grades | ≥Grade 3 |
|---|---|---|
| Neutropenia | 26 (81.3) | 15 (46.9) |
| Anemia | 17 (53.1) | 7 (21.9) |
| Decreased platelet | 13 (40.6) | 2 (6.3) |
| Elevated ALT/AST | 7 (21.9) | 0 |
| Elevated bilirubin | 4 (12.5) | 1 (3.1) |
| Fatigue | 16 (50) | 2 (6.3) |
| Nausea | 17 (53.1) | 4 (12.5) |
| Diarrhea | 14 (43.8) | 5 (15.6) |
| Oral mucositis | 10 (31.3) | 2 (6.3) |
| Hypertension | 15 (46.9) | 3 (9.4) |
| Proteinuria | 10 (31.3) | 2 (6.3) |
| HFS | 9 (28.1) | 4 (12.5) |
| dysphonia | 6 (18.8) | 3 (9.4) |
| Abdominal pain | 3 (9.4) | 0 |
| Constipation | 4 (12.5) | 0 |
| Hemorrhage | 5 (15.6) | 0 |
| Number of the Patients with Dose Reduction | TAS-102 | Fruquintinib |
|---|---|---|
| One-level dose reduction | 8 (25%) | 6 (28.6%) |
| Two-level dose reduction | 5 (15.6%) | 0 (0%) |
| Number of patients with treatment interruption | 9 (28.1%) | 9 (28.1%) |
| Number of patients with treatment discontinuation | 5 (15.6%) | 2 (6.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Wang, Y.; Xu, J.; Li, J.; Wang, T.; Zhang, Y.; Bai, Y. A Retrospective Study of Trifluridine/Tipiracil with Fruquintinib in Patients with Chemorefractory Metastatic Colorectal Cancer. J. Clin. Med. 2024, 13, 57. https://doi.org/10.3390/jcm13010057
Zou J, Wang Y, Xu J, Li J, Wang T, Zhang Y, Bai Y. A Retrospective Study of Trifluridine/Tipiracil with Fruquintinib in Patients with Chemorefractory Metastatic Colorectal Cancer. Journal of Clinical Medicine. 2024; 13(1):57. https://doi.org/10.3390/jcm13010057
Chicago/Turabian StyleZou, Jiayun, Yuanyuan Wang, Jiayu Xu, Jinna Li, Tianzhuo Wang, Ying Zhang, and Yibo Bai. 2024. "A Retrospective Study of Trifluridine/Tipiracil with Fruquintinib in Patients with Chemorefractory Metastatic Colorectal Cancer" Journal of Clinical Medicine 13, no. 1: 57. https://doi.org/10.3390/jcm13010057
APA StyleZou, J., Wang, Y., Xu, J., Li, J., Wang, T., Zhang, Y., & Bai, Y. (2024). A Retrospective Study of Trifluridine/Tipiracil with Fruquintinib in Patients with Chemorefractory Metastatic Colorectal Cancer. Journal of Clinical Medicine, 13(1), 57. https://doi.org/10.3390/jcm13010057





