The Follow-Up Necessity in Human Papilloma Virus-Positive vs. Human Papilloma Virus-Negative Oral Mucosal Lesions: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
- Traumatic fibroma;
- HPV-related lesions (squamous papilloma, condyloma acuminatum, and verruca vulgaris);
- Proliferative verrucous leukoplakia (PVL);
- Leukoplakia.
- Human papilloma virus generic-risk genotypes (HPV gr): 6, 11, 16, 18, 31, 33, 35, 39, 40, 42, 43, 45, 51, 52, 53, 54, 56, 58, 59, 66, 67, 68, 70, 71, 72, 73, 81, 82, 84, and 85;
- Human papilloma virus high-risk genotypes (HPV hr): 16, 18, 31, 33, 35, 52, and 58.
3. Results
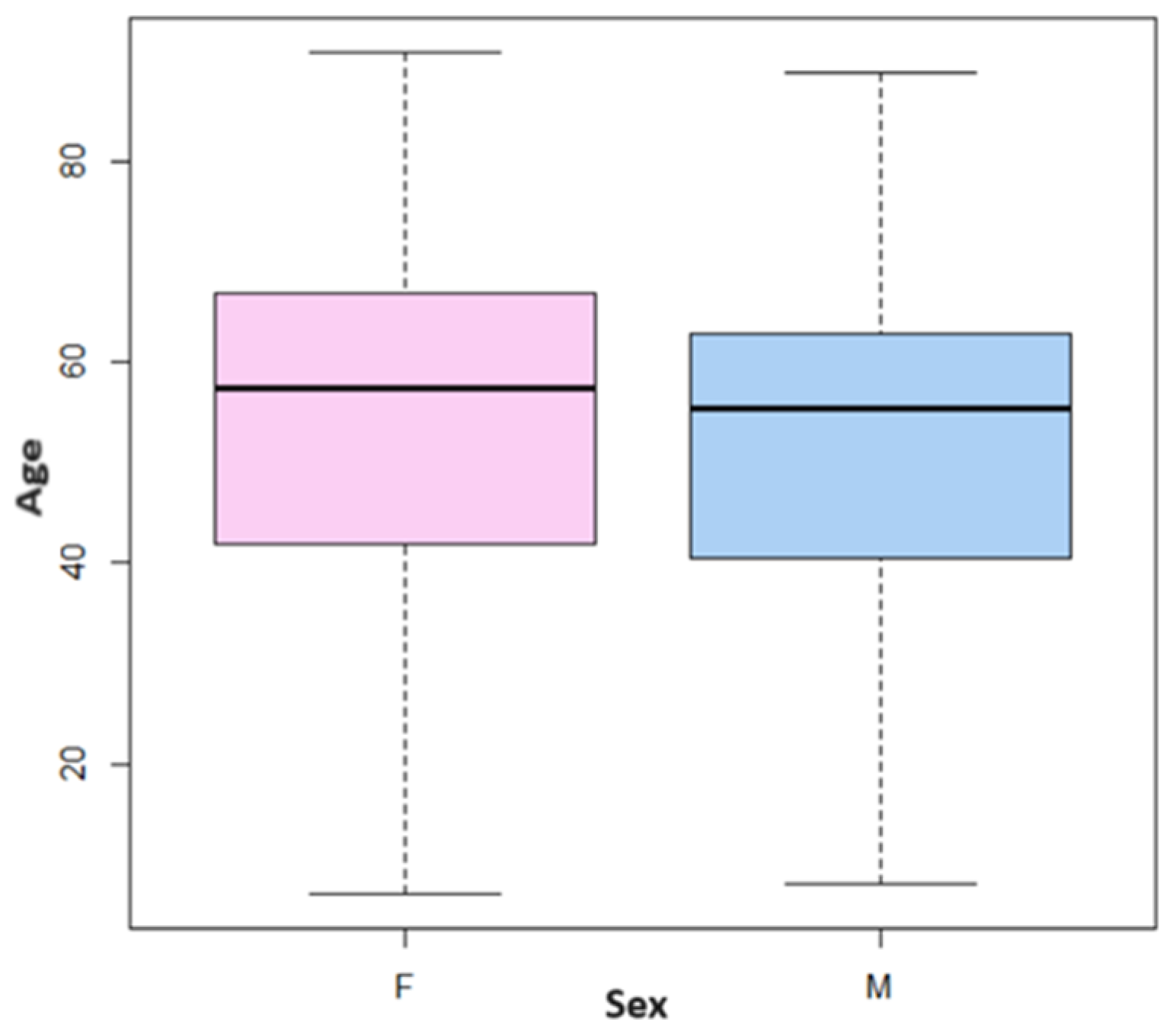
3.1. Sample Analysis
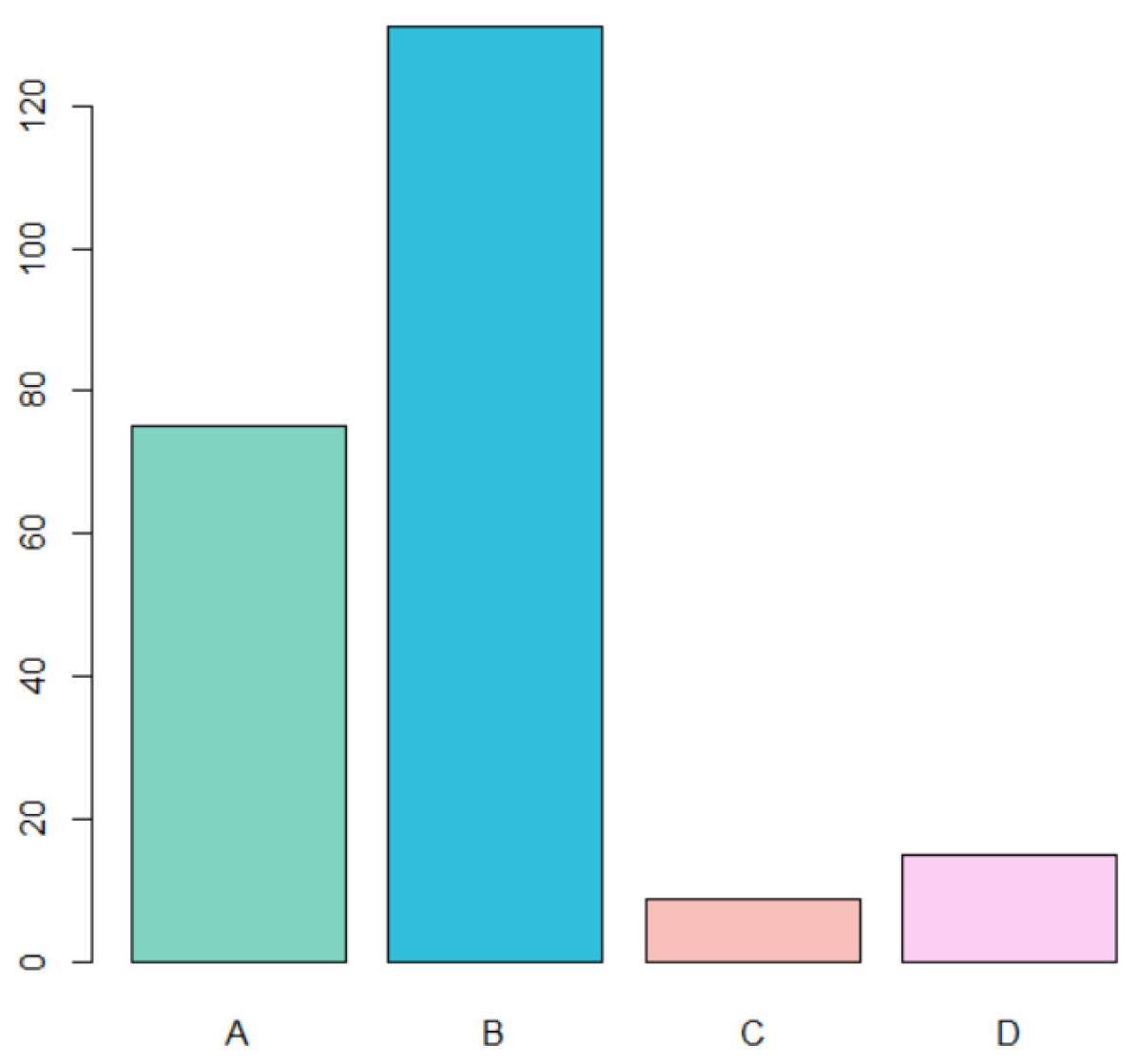
- A total of 75 cases of traumatic fibroma (A) (32.6% of total cases);
- A total of 131 cases of HPV-related lesions (B) (56.9% of total cases);
- A total of 9 cases of PVL (C) (3.9% of total cases);
- A total of 15 cases of leukoplakia (D) (6.5% of total cases).
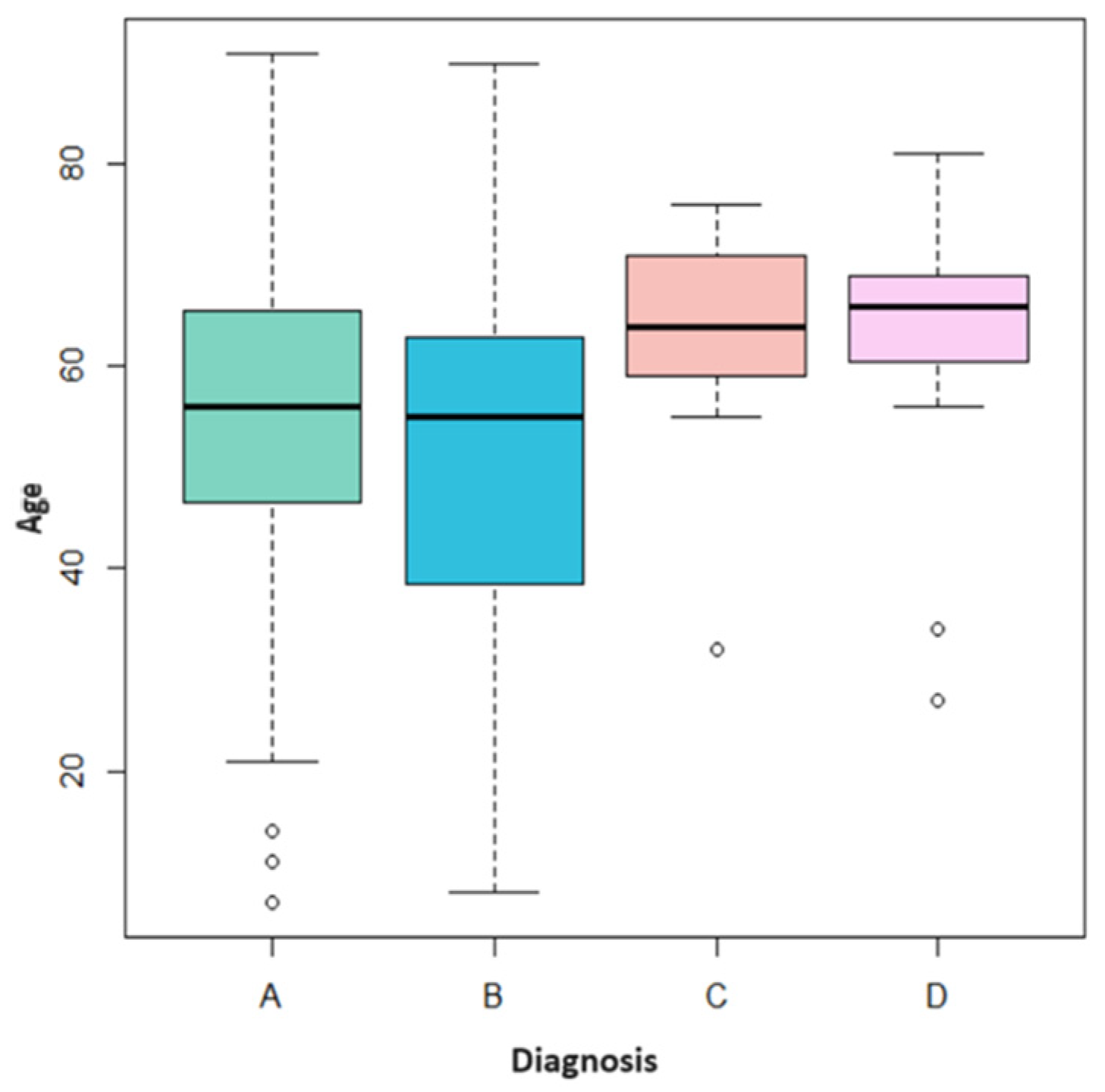
3.2. Follow-Up Comparison
- Patients diagnosed with traumatic fibroma (A) negative for both HPV hr and HPV gr were seen only after 1 month for a check-up visit. Patients with positive HPV hr received a further check-up after 6 months;
- Patients diagnosed with HPV-related (B) lesions negative for both HPV hr and HPV gr were seen on average only after 1 month; those who tested positive for HPV gr were seen after 1 and 6 months. The patients positive for HPV hr typing were generally seen at 1, 6, and 12 months for check-ups;
- Patients diagnosed with PVL (C) received, on average, periodic follow-ups every 4 months in case of HPV positivity and follow-up every 6 months in case of HPV negativity;
- Patients diagnosed with leukoplakia (D) received periodic follow-ups every 6 months regardless of the positivity or negativity of HPV.
3.3. Recurrence Rate of HPV-Related Lesions
4. Discussion
4.1. Oral Lesions Follow-Up
4.2. Recurrence Rate of HPV-Related Oral Lesions
4.3. Limits of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, S.; Kumar, P.; Das, B.C. HPV: Molecular pathways and targets. Curr. Probl. Cancer 2018, 42, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Berman, T.A.; Schiller, J.T. Human papillomavirus in cervical cancer and oropharyngeal cancer: One cause, two diseases. Cancer 2017, 123, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.-U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; zur Hausen, H.; de Villiers, E.-M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, A.; Serpico, R.; Guida, A.; Crincoli, V.; Scully, C.; Kanduc, D. Interkeratin Peptide-Protein Interactions That Promote HPV16 E7 Gene Expression. Int. J. Immunopathol. Pharmacol. 2010, 23, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Pyeon, D.; Pearce, S.M.; Lank, S.M.; Ahlquist, P.; Lambert, P.F. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog. 2009, 5, e1000318. [Google Scholar] [CrossRef]
- Stacey, S.N.; Jordan, D.; Williamson, A.J.K.; Brown, M.; Coote, J.H.; Arrand, J.R. Leaky Scanning Is the Predominant Mechanism for Translation of Human Papillomavirus Type 16 E7 Oncoprotein from E6/E7 Bicistronic mRNA. J. Virol. 2000, 74, 7284–7297. [Google Scholar] [CrossRef]
- Graham, S.V. The human papillomavirus replication cycle, and its links to cancer progression: A comprehensive review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar] [CrossRef]
- Giacinti, C.; Giordano, A. RB and cell cycle progression. Oncogene 2006, 25, 5220–5227. [Google Scholar] [CrossRef]
- Sritippho, T.; Chotjumlong, P.; Iamaroon, A. Roles of human papillomaviruses and p16 in oral cancer. Asian Pac. J. Cancer Prev. 2015, 16, 6193–6200. [Google Scholar] [CrossRef] [PubMed]
- Skelin, J.; Sabol, I.; Tomaić, V. Do or Die: HPV E5, E6 and E7 in Cell Death Evasion. Pathogens 2022, 11, 1027. [Google Scholar] [CrossRef] [PubMed]
- Basukala, O.; Banks, L. The not-so-good, the bad and the ugly: HPV E5, E6 and E7 oncoproteins in the orchestration of carcinogenesis. Viruses 2021, 13, 1892. [Google Scholar] [CrossRef] [PubMed]
- Estêvão, D.; Costa, N.R.; Gil da Costa, R.M.; Medeiros, R. Hallmarks of HPV carcinogenesis: The role of E6, E7 and E5 oncoproteins in cellular malignancy. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2019, 1862, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Vats, A.; Trejo-Cerro, O.; Thomas, M.; Banks, L. Human papillomavirus E6 and E7: What remains? Tumour Virus Res. 2021, 11, 200213. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Campbell, C.M.P.; Lin, H.-Y.; Fulp, W.; Papenfuss, M.R.; Abrahamsen, M.; Hildesheim, A.; Villa, L.L.; Salmerón, J.J.; Lazcano-Ponce, E.; et al. Incidence and clearance of oral human papillomavirus infection in men: The HIM cohort study. Lancet 2013, 382, 877–887. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Bhatia, R.K.; Messeguer, A.L.; González, P.; Herrero, R.; Giuliano, A.R. Oral Human Papillomavirus in Healthy Individuals: A Systematic Review of the Literature. Sex. Transm. Dis. 2010, 37, 386–391. Available online: https://journals.lww.com/stdjournal/Fulltext/2010/06000/Oral_Human_Papillomavirus_in_Healthy_Individuals_.10.aspx (accessed on 6 July 2023). [CrossRef]
- Esquenazi, D.; Filho, I.B.; Carvalho, M.d.G.d.C.; de Barros, F.S. The frequency of human papillomavirus findings in normal oral mucosa of healthy people by PCR. Braz. J. Otorhinolaryngol. 2010, 76, 78–84. [Google Scholar] [CrossRef]
- Tam, S.; Fu, S.; Xu, L.; Krause, K.J.; Lairson, D.R.; Miao, H.; Sturgis, E.M.; Dahlstrom, K.R. The epidemiology of oral human papillomavirus infection in healthy populations: A systematic review and meta-analysis. Oral Oncol. 2018, 82, 91–99. [Google Scholar] [CrossRef]
- Taberna, M.; Mena, M.; Pavón, M.A.; Alemany, L.; Gillison, M.L.; Mesía, R. Human papillomavirus-related oropharyngeal cancer. Ann. Oncol. 2017, 28, 2386–2398. [Google Scholar] [CrossRef]
- de la Cour, C.D.; Sperling, C.D.; Belmonte, F.; Syrjänen, S.; Kjaer, S.K. Human papillomavirus prevalence in oral potentially malignant disorders: Systematic review and meta-analysis. Oral Dis. 2021, 27, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, S.; Lodi, G.; von Bültzingslöwen, I.; Aliko, A.; Arduino, P.; Campisi, G.; Challacombe, S.; Ficarra, G.; Flaitz, C.; Zhou, H.; et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: A systematic review. Oral Dis. 2011, 17, 58–72. [Google Scholar] [CrossRef]
- Feller, L.; Lemmer, J. Oral Leukoplakia as It Relates to HPV Infection: A Review. Int. J. Dent. 2012, 2012, 540561. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol. Biomark. Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Giraldi, L.; Collatuzzo, G.; Hashim, D.; Franceschi, S.; Herrero, R.; Chen, C.; Schwartz, S.M.; Smith, E.; Kelsey, K.; McClean, M.; et al. Infection with Human Papilloma Virus (HPV) and risk of subsites within the oral cancer. Cancer Epidemiol. 2021, 75, 102020. [Google Scholar] [CrossRef]
- Guida, A.; Maglione, M.; Crispo, A.; Perri, F.; Villano, S.; Pavone, E.; Aversa, C.; Longo, F.; Feroce, F.; Botti, G.; et al. Oral lichen planus and other confounding factors in narrow band imaging (NBI) during routine inspection of oral cavity for early detection of oral squamous cell carcinoma: A retrospective pilot study. BMC Oral Health 2019, 19, 70. [Google Scholar] [CrossRef]
- Ndiaye, C.; Mena, M.; Alemany, L.; Arbyn, M.; Castellsagué, X.; Laporte, L.; Bosch, F.X.; de Sanjosé, S.; Trottier, H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014, 15, 1319–1331. [Google Scholar] [CrossRef]
- Wierzbicka, M.; San Giorgi, M.R.M.; Dikkers, F.G. Transmission and clearance of human papillomavirus infection in the oral cavity and its role in oropharyngeal carcinoma—A review. Rev. Med. Virol. 2023, 33, e2337. [Google Scholar] [CrossRef]
- Villa, A.; Bin Woo, S. Leukoplakia—A Diagnostic and Management Algorithm. J. Oral Maxillofac. Surg. 2017, 75, 723–734. [Google Scholar] [CrossRef]
- Saldivia-Siracusa, C.; González-Arriagada, W.A. Difficulties in the Prognostic Study of Oral Leukoplakia: Standardisation Proposal of Follow-Up Parameters. Front. Oral Health 2021, 2, 614045. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Ariyawardana, A. Malignant transformation of oral leukoplakia: A systematic review of observational studies. J. Oral Pathol. Med. 2016, 45, 155–166. [Google Scholar] [CrossRef]
- Proaño-Haro, A.; Bagan, L.; Bagan, J.V. Recurrences following treatment of proliferative verrucous leukoplakia: A systematic review and meta-analysis. J. Oral Pathol. Med. 2021, 50, 820–828. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Surace, G.; De Stefano, R.; Laino, L.; D’amico, C.; Fiorillo, M.T.; Meto, A.; Herford, A.S.; Arzukanyan, A.V.; et al. Human Papilloma Virus: Current Knowledge and Focus on Oral Health. BioMed Res. Int. 2021, 2021, 6631757. [Google Scholar] [CrossRef] [PubMed]
- Van Der Waal, I.; Schepman, K.P.; Van Der Meij, E.H.; Smeele, L.E. Oral Leukoplakia: A Clinicopathological Review. Oral Oncol. 1997, 33, 291–301. [Google Scholar] [CrossRef]
- Bacci, C.; Donolato, L.; Stellini, E.; Berengo, M.; Valente, M. A comparison between histologic and clinical diagnoses of oral lesions. Quintessence Int. 2014, 45, 789–794. [Google Scholar] [CrossRef]
- Suradi, D.; Abdullah, H.; Pang, P.; Yi, E.E. The Prevalence of Fibroma in Oral Mucosa Among Patient Attending USM Dental Clinic Year 2006–2010. Indones. J. Dent. Res. 2010, 1, 61–66. Available online: http://the-indonesian-jdr.fkg.ugm.ac.id (accessed on 6 July 2023).
- Valério, R.A.; de Queiroz, A.M.; Romualdo, P.C.; Brentegani, L.G.; de Paula-Silva, F.W.G. Mucocele and Fibroma: Treatment and Clinical Features for Differential Diagnosis. Braz. Dent. J. 2013, 24, 537–541. [Google Scholar] [CrossRef]
- Orrù, G.; Mameli, A.; Demontis, C.; Rossi, P.; Ratto, D.; Occhinegro, A.; Piras, V.; Kuqi, L.; Berretta, M.; Taibi, R.; et al. Oral human papilloma virus infection: An overview of clinical-laboratory diagnosis and treatment. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8148–8157. [Google Scholar]
- Guida, A.; Ionna, F.; Farah, C.S. Narrow-band imaging features of oral lichenoid conditions: A multicentre retrospective study. Oral Dis. 2023, 29, 764–771. [Google Scholar] [CrossRef]
- Ries, J.; Agaimy, A.; Vairaktaris, E.; Kwon, Y.; Neukam, F.W.; Strassburg, L.H.; Nkenke, E. Evaluation of MAGE-A expression and grade of dysplasia for predicting malignant progression of oral leukoplakia. Int. J. Oncol. 2012, 41, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Gandara-Vila, P.; Sayáns, M.P.; Suarez-Penaranda, J.; Gallas-Torreira, M.; Martín, J.M.S.; Lopez, R.; Blanco-Carrion, A.; Garcia-Garcia, A. Survival study of leukoplakia malignant transformation in a region of northern Spain. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e413–e420. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Urizar, J.M.; Lafuente-Ibáñez de Mendoza, I.; Warnakulasuriya, S. Malignant transformation of oral leukoplakia: Systematic review and meta-analysis of the last 5 years. Oral Dis. 2021, 27, 1881–1895. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, T.E.; Marinho, S.A.; Verli, F.D.; Mesquita, A.T.M.; Lima, N.L.; Miranda, J.L. Oral squamous papilloma: Clinical, histologic and immunohistochemical analyses. J. Oral Sci. 2009, 51, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Abbey, L.M.; Page, D.G.; Sawyer, D.R. The clinical and histopathologic features of a series of 464 oral squamous cell papillomas. Oral Surg. Oral Med. Oral Pathol. 1980, 49, 419–428. [Google Scholar] [CrossRef]
- Pringle, G.A. The Role of Human Papillomavirus in Oral Disease. Dent. Clin. N. Am. 2014, 58, 385–399. [Google Scholar] [CrossRef]
- Kumar, P.; Gupta, S.; Das, B.C. Saliva as a potential non-invasive liquid biopsy for early and easy diagnosis/prognosis of head and neck cancer. Transl. Oncol. 2023, 40, 101827. [Google Scholar] [CrossRef]
| Patient | Age | Sex | Biopsy Date | Histological Exam | Anatomical Site | HPV hr | HPV gr | Follow Up 4 w | 2 Months | 3 Months | 4 Months |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 122 | 61 | M | 11/01/21 | D | b | 0 | 0 | 1 | 0 | 0 | 0 |
| Patient 123 | 56 | M | 12/01/21 | B | b | 0 | 0 | 1 | 0 | 0 | 0 |
| Patient 124 | 47 | F | 15/01/21 | A | a | 0 | 0 | 1 | 0 | 0 | 0 |
| Patient 125 | 62 | M | 19/01/21 | B | d | 0 | 1 | 1 | 0 | 0 | 0 |
| Patient 126 | 23 | F | 04/02/21 | B | a | 1 | 1 | 1 | 0 | 0 | 0 |
| Patient 127 | 70 | M | 09/02/21 | A | k | 0 | 0 | 1 | 0 | 0 | 0 |
| Patient 128 | 64 | F | 09/02/21 | B | e | 0 | 0 | 1 | 0 | 0 | 0 |
| Patient 129 | 49 | M | 09/02/21 | B | h | 0 | 1 | 1 | 0 | 0 | 0 |
| Patient 130 | 65 | F | 09/02/21 | A | e | 0 | 0 | 1 | 0 | 0 | 0 |
| Patient 131 | 30 | F | 10/02/21 | B | g | 1 | 1 | 1 | 0 | 0 | 0 |
| Patient 132 | 18 | M | 23/02/21 | B | b | 0 | 1 | 1 | 0 | 0 | 0 |
| Patient 133 | 47 | F | 23/02/21 | B | f | 1 | 1 | 1 | 0 | 0 | 0 |
| Patient 134 | 48 | M | 02/03/21 | B | i | 1 | 1 | 1 | 0 | 0 | 0 |
| Patient 135 | 32 | M | 09/03/21 | C | a | 1 | 0 | 1 | 1 | 0 | 0 |
| Patient 136 | 59 | F | 15/03/21 | B | a | 0 | 0 | 1 | 0 | 0 | 0 |
| Patient 137 | 39 | M | 15/03/21 | B | c | 0 | 0 | 1 | 0 | 0 | 0 |
| Patient 138 | 16 | M | 16/03/21 | B | d | 0 | 1 | 1 | 0 | 0 | 0 |
| Code | Diagnosis | |
|---|---|---|
| 1 | A | Clinical–histological picture compatible with traumatic fibroma of the oral mucosa |
| 2 | B | Clinical–histological picture compatible with HPV-related lesions 1 [Morphological findings compatible with viral cytopathic alterations (HPV) of the oral mucosa: fragments of oral mucosa with epithelial hyperplasia and focal hyperkeratosis and koilocytosis] |
| 3 | C | Clinical–histological picture compatible with proliferative verrucous leukoplakia of the oral mucosa (PVL, proliferative verrucous leukoplakia) |
| 4 | D | Clinical–histological picture compatible with leukoplakia |
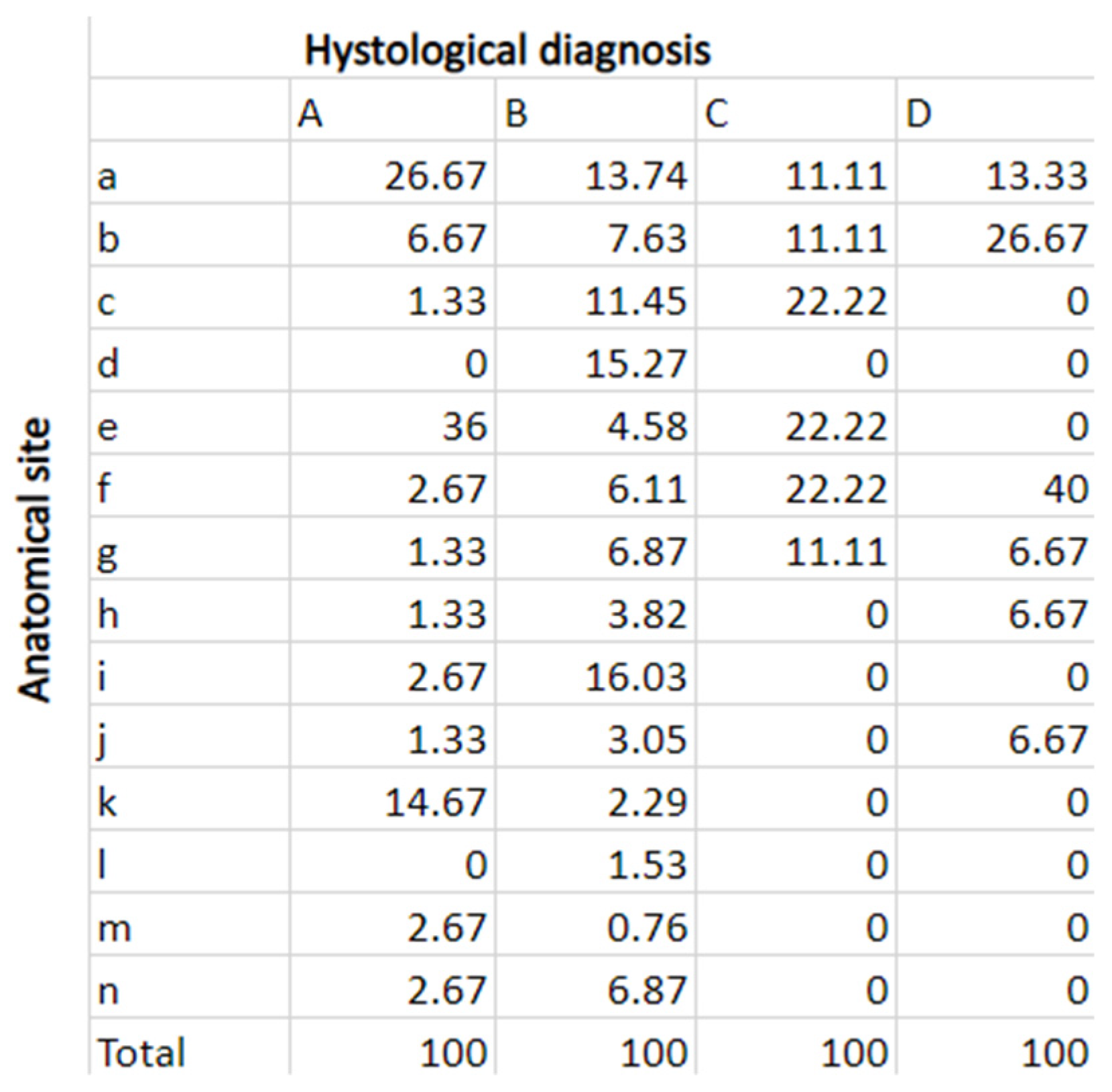
| Code | Anatomical Site | |
|---|---|---|
| 1 | a | Lateral margin of the tongue |
| 2 | b | Retromolar trigone |
| 3 | c | Hard palate mucosa |
| 4 | d | Soft palate mucosa |
| 5 | e | Buccal mucosa |
| 6 | f | Vestibular gingiva of the upper maxilla |
| 7 | g | Vestibular gingiva of the inferior jaw |
| 8 | h | Tongue tip |
| 9 | i | Dorsal surface of the tongue |
| 10 | j | Ventral surface of the tongue |
| 11 | k | Retrocommissure of lower lip |
| 12 | l | Lingual gingiva of the upper maxilla |
| 13 | m | Upper lip mucosa |
| 14 | n | Lower lip mucosa |
| Cases | Age | Sex | Histological Diagnosis | Anatomical Site | HPV hr | HPV gr | Recurrence | |
|---|---|---|---|---|---|---|---|---|
| 1 | Patient 22 | 58 | F | B | a | + | + | 12 months |
| 2 | Patient 23 | 84 | M | B | c | + | + | 16 months |
| 3 | Patient 168 | 81 | F | B | b | + | + | 12 months |
| 4 | Patient 176 | 41 | M | B | c | − | + | 12 months |
| 5 | Patient 179 | 63 | F | B | g | + | + | 6 months |
| 6 | Patient 194 | 20 | M | B | c | + | + | 6 months |
| Year | Maximum Follow-Up Period (Starting from the Date of Diagnosis until 1 June 2023) |
|---|---|
| 2018 | 66 |
| 2019 | 54 |
| 2020 | 42 |
| 2021 | 30 |
| 2022 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rushiti, A.; Castellani, C.; Cerrato, A.; Fedrigo, M.; Sbricoli, L.; Bressan, E.; Angelini, A.; Bacci, C. The Follow-Up Necessity in Human Papilloma Virus-Positive vs. Human Papilloma Virus-Negative Oral Mucosal Lesions: A Retrospective Study. J. Clin. Med. 2024, 13, 58. https://doi.org/10.3390/jcm13010058
Rushiti A, Castellani C, Cerrato A, Fedrigo M, Sbricoli L, Bressan E, Angelini A, Bacci C. The Follow-Up Necessity in Human Papilloma Virus-Positive vs. Human Papilloma Virus-Negative Oral Mucosal Lesions: A Retrospective Study. Journal of Clinical Medicine. 2024; 13(1):58. https://doi.org/10.3390/jcm13010058
Chicago/Turabian StyleRushiti, Armina, Chiara Castellani, Alessia Cerrato, Marny Fedrigo, Luca Sbricoli, Eriberto Bressan, Annalisa Angelini, and Christian Bacci. 2024. "The Follow-Up Necessity in Human Papilloma Virus-Positive vs. Human Papilloma Virus-Negative Oral Mucosal Lesions: A Retrospective Study" Journal of Clinical Medicine 13, no. 1: 58. https://doi.org/10.3390/jcm13010058
APA StyleRushiti, A., Castellani, C., Cerrato, A., Fedrigo, M., Sbricoli, L., Bressan, E., Angelini, A., & Bacci, C. (2024). The Follow-Up Necessity in Human Papilloma Virus-Positive vs. Human Papilloma Virus-Negative Oral Mucosal Lesions: A Retrospective Study. Journal of Clinical Medicine, 13(1), 58. https://doi.org/10.3390/jcm13010058







