Recent Advances in Understanding of the Etiopathogenesis, Diagnosis, and Management of Hair Loss Diseases
Abstract
1. Introduction
2. Diagnosis and Severity Evaluation
2.1. Diagnostic Criteria and Classification/Scoring Systems
| Hair Diseases | Diagnostic Criteria |
|---|---|
| Frontal fibrosing alopecia | (a) International FFA Cooperative Group Criteria for FFA (International guidelines for clinical trials of FFA [4]): Combination of clinical and pathological findings to diagnose classic FFA (Frontal hairline recession with loss of follicular ostia is necessary. Other items include positive biopsy result, eyebrow loss, perifollicular anterior scalp erythema, and perifollicular anterior scalp hyperkeratosis/scale) and probable FFA (frontal hairline recession is not necessary. Facial papules, preauricular hair loss, and absence of vellus hairs in affected hairline are added). Each item counts one or two points. Four or more points are needed to diagnose each condition. |
| Fibrosing alopecia in a pattern distribution | (b) “Proposed diagnostic criteria for FAPD” [5]: Criteria include seven major items (symmetric hair loss in androgen-dependent areas, sparing of androgen-independent areas, perifollicular casts/hyperkeratosis, loss of follicular ostia, hair density variability, lymphohistiocytic infiltrate around isthmus and infundibular region, and concentric perifollicular lamellar fibrosis) and six minor items (sparing of non-scalp hairs, perifollicular erythema, hair tufting, predominance of single hair follicles, interface dermatitis, and fibrosed follicular tracts) in three categories: clinical, trichoscopy, and histopathology. |
| Classification/scoring systems | |
| All hair loss diseases | (c) SALT (I, II, and III) [4,24]: Percentage of hair loss area (0–100) calculated by subdividing scalp surface area into four quadrants. |
| Alopecia areata | (d) Severity scale [23]: Severity subgrouping (S0–5) based on the SALT score: S0, no hair loss; S1, less than 25% hair loss; S2, 25–49% hair loss; S3, 50–74% hair loss; S4, 75–99% hair loss; and S5, 100%. (e) Severity scale for clinical use [25]: Mild AA, 20% or less scalp hair loss; moderate AA, 21–49% scalp hair loss; and severe AA, 50–100% scalp hair loss. If mild or moderate, increase AA severity rating by 1 level if 1 or more of the following is present: negative impact on psychosocial functioning resulting from AA, noticeable involvement of eyebrows or eyelashes, inadequate response after at least 6 months of treatment, and diffuse (multifocal) positive hair pull test consistent with rapidly progressive AA |
| Androgenetic alopecia | (f) Hamilton–Norwood classification [26]: The pattern and severity of hair loss is represented by seven categories (I to VII) and variants (e.g., III vertex) based on hair thinning of the vertex and recession of the frontal hair line. |
| (g) BASP classification [6]: The scale of pattern and severity of hair loss are composed of a basic type (L, M, C, and U) and specific type (V and F). | |
| Female pattern hair loss | |
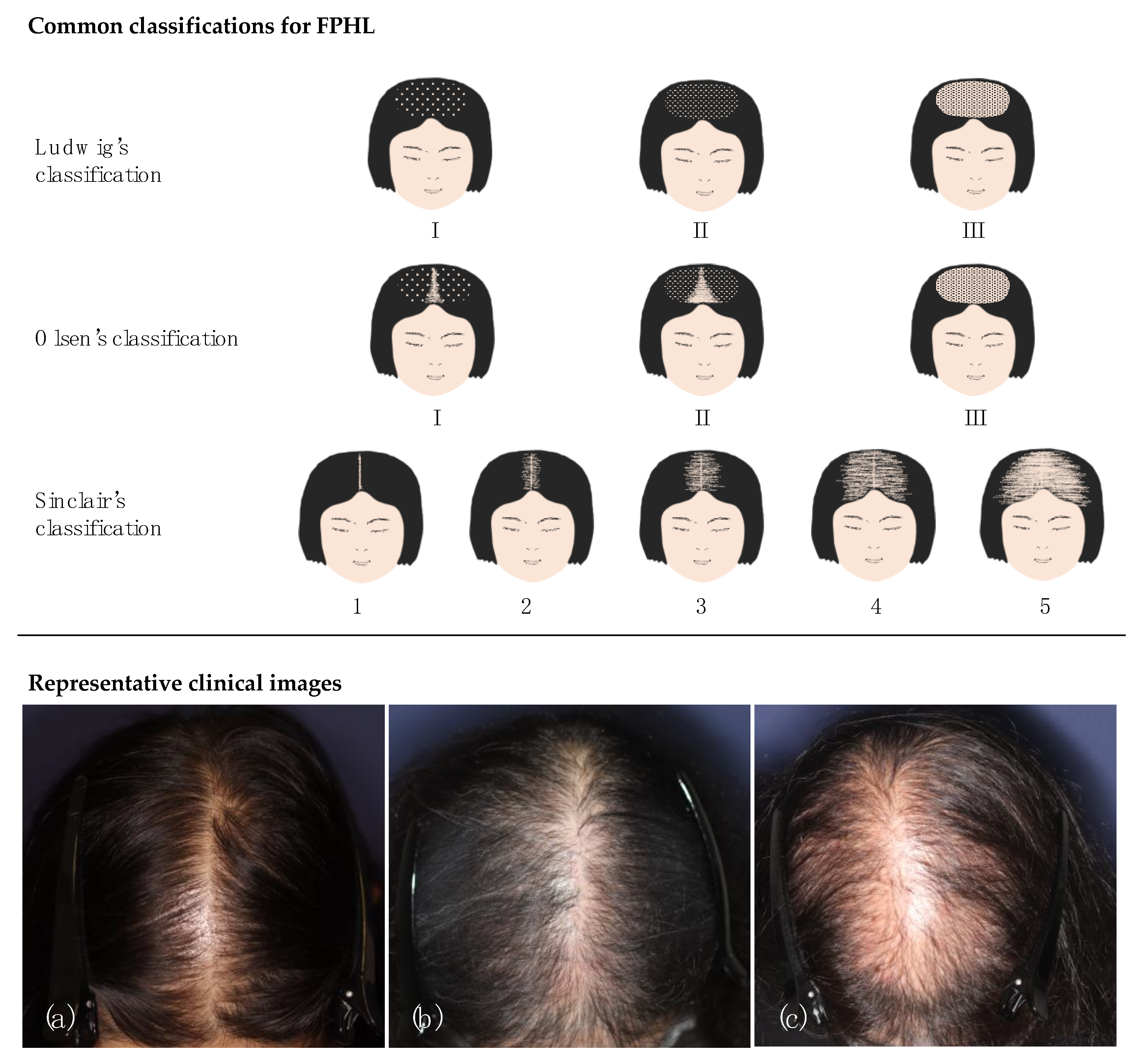
| (h) Ludwig’s classification [27]: severity scale based on degree of diffuse hair thinning on crown from I (mildest) to III (severest). (i) Olsen’s classification [28]: A severity scale of diffuse hair thinning on the crown (I to III), with the concept of frontal accentuation. (j) Sinclair’s classification (Woman’s Alopecia Severity Scale [WASS]) [29]: A severity scale from 1 (mildest) to 5 (severest) based on midline hair density. (k) Trichoscopy Derived Sinclair Scale [8]: A scale of hair density calculated by the formula of 3.9 × log (1/cumulative hair thickness density [total diameter of hair growing in scalp area unit]) as detected by trichoscopy) + 2.4. (l) Shiseido’s classification [31]: A grading system (Stages 1–6) based on the hair thinning on the crown. Stage 6 is comparable to Sinclair 2. (m) Kaneko’s classification [32]: A grading system (Grades 1–5) judged by the pattern of surface-reflected light, as detected by clinical picture recording. (n) FPHL-SI [7]: A severity scale for early FPHL (0–20) combining four measure items: the amount of hair shedding, midline hair density, and two trichoscopic findings. | |
| Lichen planopilaris | (o) LPPAI [34]: An activity index (0–10) calculated by the formula including six symptoms (pruritus, pain, burning, erythema, perifollicular erythema, and perifollicular scale), the result of pull test, and the tendency of spreading. |
| Frontal fibrosing alopecia | (p) FFASI [35]: A severity index (0–100) combining the extent of recession of hair line in five anatomical parts, existence of hair loss in five body parts, and six additional features. (q) FFASS [36]: A severity score (0–25) by summing the extent of alopecia score (hair line recession and loss of eyebrows, 0–21) and grade of inflammation score (pruritus and pain, 0–4) (r) Frontal fibrosing alopecia global staging score (international guidelines for clinical trials of FFA) [4]: A staging system comprising five common findings: scalp hair loss, eyebrow loss, facial papules, prominent forehead veins, and facial hyperpigmentation, each of which have a range of numbers (S0–4E0–2P0–1V0–1H0–1). |
2.2. Scalp and Hair Examination
2.3. Laboratory Examinations
2.4. Trichoscopy
2.5. Other Non-Invasive Imaging Technologies
2.6. Scalp Biopsy
3. Non-Cicatricial Alopecia
3.1. Androgenetic Alopecia and Female Pattern Hair Loss
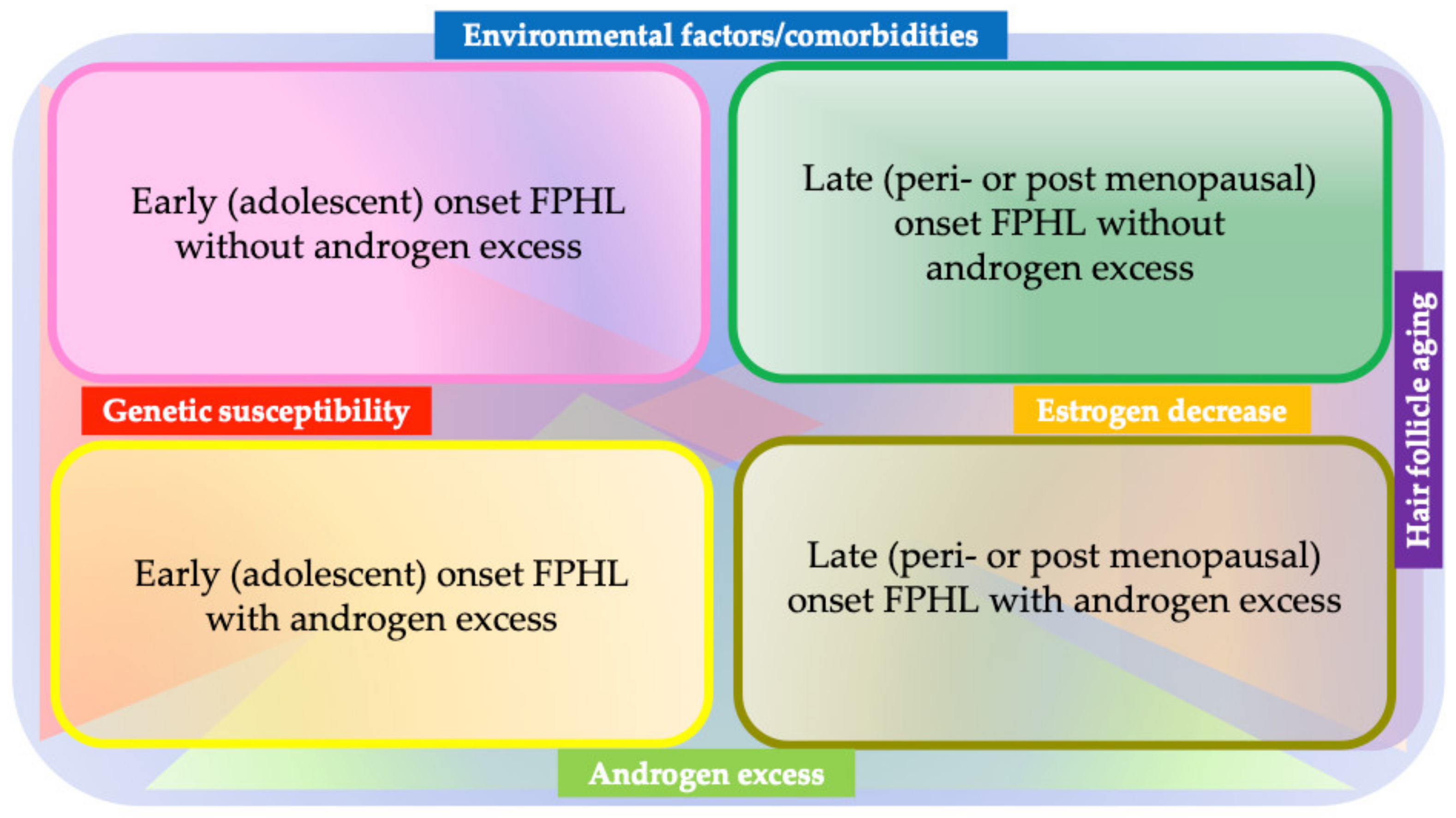
3.1.1. Etiopathogenesis
3.1.2. Treatment
5-α reductase Inhibitors
Minoxidil
Platelet-Rich Plasma
Spironolactone and Other Hormone-Modulating Therapies
Hair Transplantation
Other Modalities
3.2. Alopecia Areata
3.2.1. Etiopathogenesis
3.2.2. Treatment
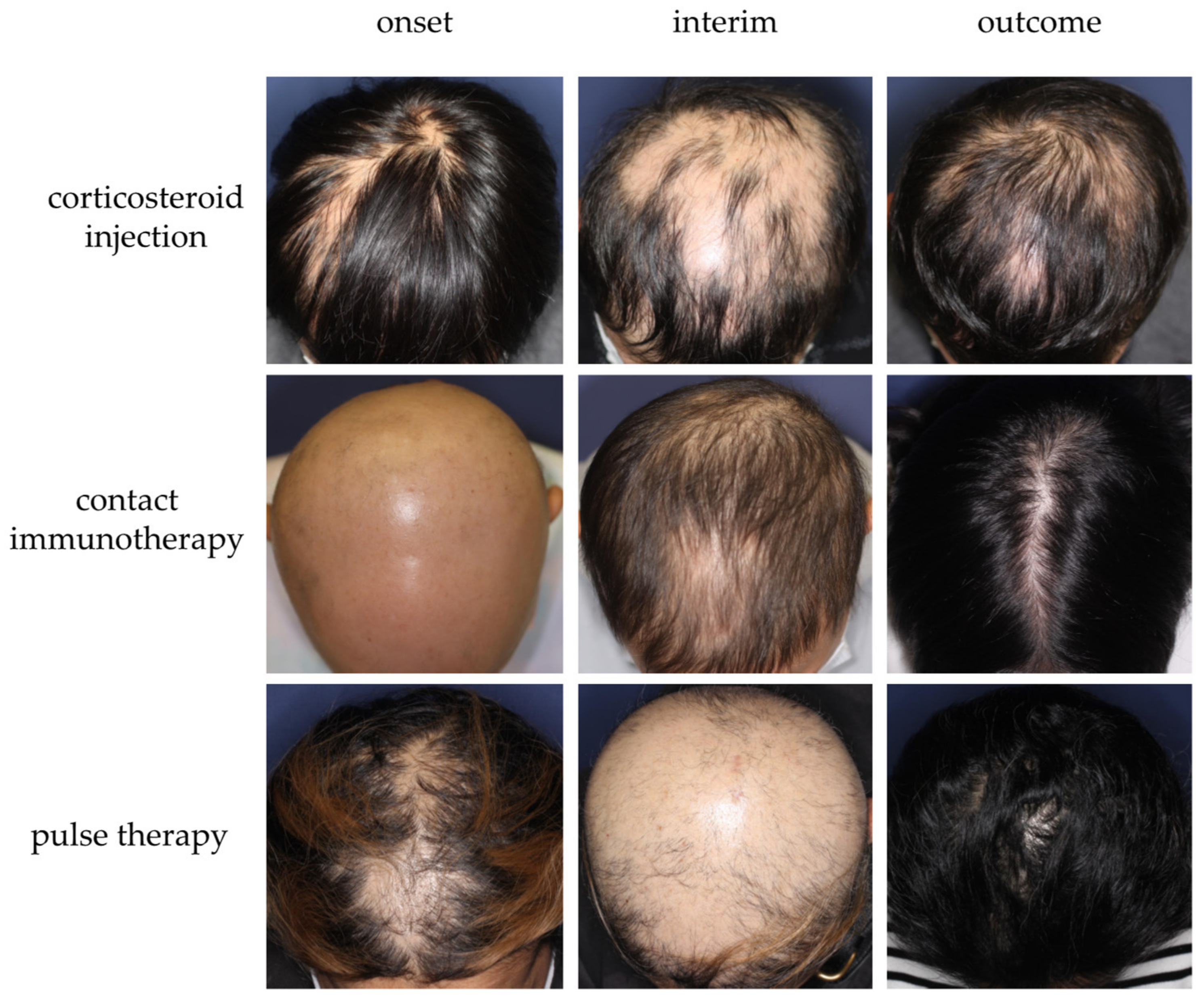
Optimization of Conventional Therapeutic Approaches
Janus Kinase (JAK) Inhibitor
Dupilumab
Platelet-Rich Plasma
Other Modalities
| Drug | Study | Patient | Dose | Outcome |
|---|---|---|---|---|
| Tofacitinib (pan-JAK) [148] | Phase 2, open label | 12 adult patients with moderate to severe AA | 5 mg to 10 mg twice daily for 6 months | 91% of the patients achieved SALT score improvement, and 67% patients reached SALT 50. |
| Ruxolitinib (JAK 1/2) [146] | Phase 2, open label | 12 adult patients with moderate to severe AA | 20 mg twice daily for 12–24 weeks | 75% of the patients were responsive with the average of 92% hair regrowth. |
| Delgocitinib ointment [149] | Phase 2, double blind | 31 adult patients with moderate to severe AA | 30 mg/g ointment applied twice daily or vehicle control for 12 weeks | The mean decrease in SALT was 3.8 in the delgocitinib group and 3.4 in the vehicle group. |
| Ritlecitinib (JAK 3) [144] | Phase 2B/3, double blind, placebo controlled | 718 patients (>12 years old) with moderate to severe AA | Range of daily oral dosing for a 4-week loading phase/20-week maintenance phase/24-week extension phase: 200 mg/50 mg/50 mg 200 mg/30 mg/30 mg 50 mg/50 mg/50 mg 30 mg/30 mg/30 mg 10 mg/10 mg/10 mg placebo | The estimated rate achieving a SALT score ≦20 was 31% with 200/50/50 mg, 22% with 200/30/30 mg, 24% with 50/50/50 mg, 14.5% with 30/30/30 mg, and 2% with placebo. |
| Brepocitinib (JAK1/TYK2) [147] | Phase 2a, double blind | 70 adult patients with moderate to severe AA | 60 mg daily for 4 weeks, subsequently 30 mg daily for 20 weeks or placebo | 64% of the patients receiving brepocitinib achieved SALT 30, and 34% achieved SALT 90 at 24 weeks. |
| Baricitinib (JAK1/2) [133] | Phase 3, double blind | 1200 adult patients with severe AA | 4 mg daily, 2 mg daily, or placebo | The estimated rate of the patients achieved SALT score of 20 or less at week 34 was 35.9–38.8% with 4 mg baricitinib, 19.4–22.8% with 2 mg baricitinib, and 3.3–6.2% with placebo. |
| CTP-543 (JAK1/2) [150] | Phase 2, randomized, dose-ranging trial | 149 adult patients with moderate to severe AA | 4 mg, 8 mg, or 12 mg twice daily or placebo for 24 weeks | The percentage of patients with ≥50% reduction of SALT score at week 24 was 58% with 12 mg CTP-543, 47% with 8 mg CTP-543, 21% with 4 mg CTP-543, and 9% with placebo. |
| Apremilast [174] | A double-blind, placebo-controlled single-center pilot study | 30 adult patients with moderate to severe AA | 30 mg daily or placebo for 24 weeks | The percentage of patients with ≥50% reduction of SALT score at week 24 was 8.3% with apremilast and 12.5% with placebo. |
3.3. Telogen Effluvium
3.3.1. Etiopathogenesis
3.3.2. Treatment
3.4. COVID-19 Associated Hair Loss
3.4.1. Etiopathogenesis
3.4.2. Treatment
4. Cicatricial Alopecia
4.1. Etiopathogenesis
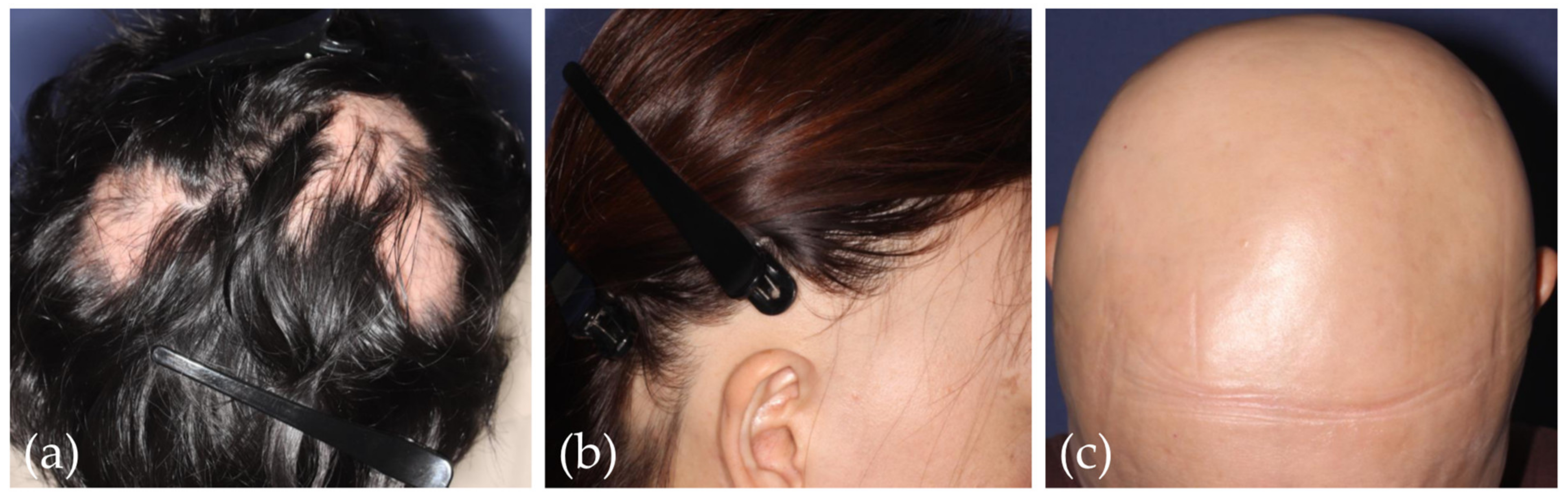
4.1.1. Lichen Planopilaris (LPP) and Frontal Fibrosing Alopecia (FFA)
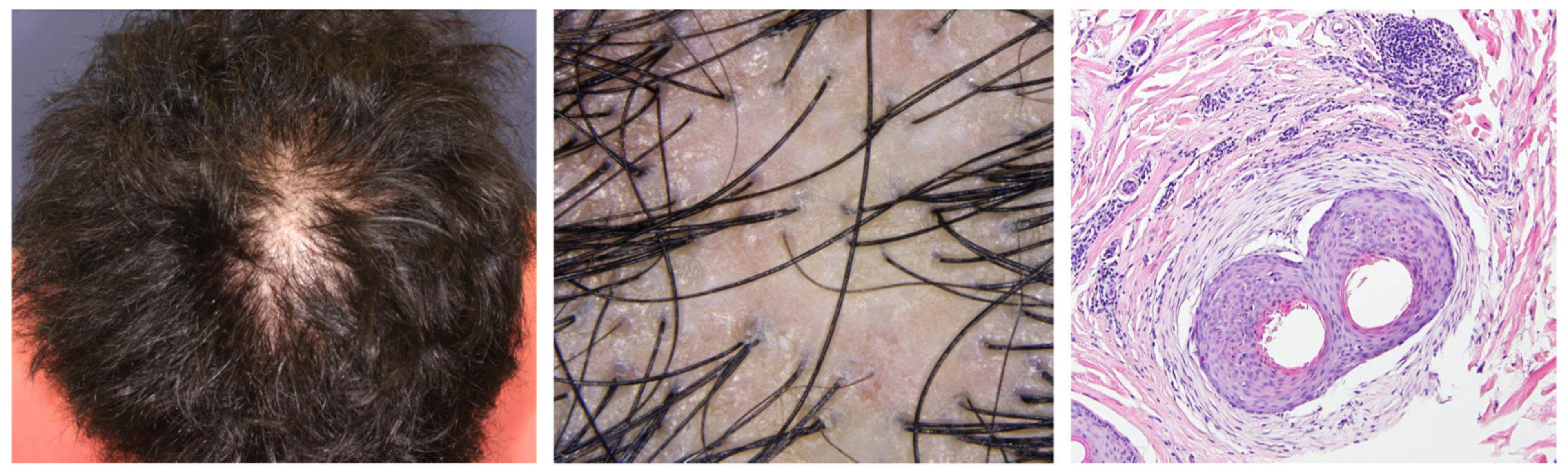
4.1.2. Fibrosing Alopecia in a Pattern Distribution (FAPD)
4.1.3. Central Centrifugal Cicatricial Alopecia (CCCA)
4.1.4. Folliculitis Decalvans (FD)
4.1.5. Other Types of Cicatricial Alopecia
4.2. Treatment
4.2.1. PPAR-γ Agonist
4.2.2. JAK Inhibitors
4.2.3. Biologics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fukuyama, M.; Ito, T.; Ohyama, M. Alopecia areata: Current understanding of the pathophysiology and update on therapeutic approaches, featuring the Japanese Dermatological Association guidelines. J. Dermatol. 2022, 49, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Chien Yin, G.O.; Siong-See, J.L.; Wang, E.C.E. Telogen Effluvium—A review of the science and current obstacles. J Dermatol Sci 2021, 101, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M. Primary cicatricial alopecia: Recent advances in evaluation and diagnosis based on trichoscopic and histopathological observation, including overlapping and specific features. J. Dermatol. 2022, 49, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Harries, M.; Tosti, A.; Bergfeld, W.; Blume-Peytavi, U.; Callender, V.; Chasapi, V.; Correia, O.; Cotsarelis, G.; Dhurat, R.; et al. Guidelines for clinical trials of frontal fibrosing alopecia: Consensus recommendations from the International FFA Cooperative Group (IFFACG). Br. J. Dermatol. 2021, 185, 1221–1231. [Google Scholar] [CrossRef]
- Griggs, J.; Trüeb, R.M.; Gavazzoni Dias, M.F.R.; Hordinsky, M.; Tosti, A. Fibrosing alopecia in a pattern distribution. J. Am. Acad. Dermatol. 2021, 85, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Ro, B.I.; Hong, S.P.; Bak, H.; Sim, W.Y.; Kim, D.W.; Park, J.K.; Ihm, C.W.; Eun, H.C.; Kwon, O.S.; et al. A new classification of pattern hair loss that is universal for men and women: Basic and specific (BASP) classification. J. Am. Acad. Dermatol. 2007, 57, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.; Tosti, A.; Bergfeld, W.; Blume-Peytavi, U.; Shapiro, J.; Lutz, G.; Messenger, A.; Sinclair, R.; Paus, R. Towards a consensus on how to diagnose and quantify female pattern hair loss—The ’Female Pattern Hair Loss Severity Index (FPHL-SI)’. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.; Sicińska, J.; Sinclair, R. The Trichoscopy Derived Sinclair Scale: Enhancing visual assessment through quantitative trichoscopy. Australas J. Dermatol. 2019, 60, 134–136. [Google Scholar] [CrossRef]
- Kinoshita-Ise, M.; Sachdeva, M. Update on trichoscopy: Integration of the terminology by systematic approach and a proposal of a diagnostic flowchart. J. Dermatol. 2022, 49, 4–18. [Google Scholar] [CrossRef]
- McDonald, K.A.; Shelley, A.J.; Maliyar, K.; Abdalla, T.; Beach, R.A.; Beecker, J. Hair pull test: A clinical update for patients with Asian- and Afro-textured hair. J. Am. Acad. Dermatol. 2021, 85, 1599–1601. [Google Scholar] [CrossRef]
- McDonald, K.A.; Shelley, A.J.; Colantonio, S.; Beecker, J. Hair pull test: Evidence-based update and revision of guidelines. J. Am. Acad. Dermatol. 2017, 76, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita-Ise, M.; Ohyama, M.; Ramjist, J.M.; Foster, F.S.; Yang, V.X.D.; Sachdeva, M.; Sade, S.; Shear, N.H. Ultra high-frequency ultrasound with seventy-MHz transducer in hair disorders: Development of a novel noninvasive diagnostic methodology. J. Dermatol. Sci. 2021, 102, 167–176. [Google Scholar] [CrossRef]
- Rajabi-Estarabadi, A.; Vasquez-Herrera, N.E.; Martinez-Velasco, M.A.; Tsatalis, J.; Verne, S.H.; Nouri, K.; Tosti, A. Optical coherence tomography in diagnosis of inflammatory scalp disorders. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2147–2151. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M. Hair follicle bulge: A fascinating reservoir of epithelial stem cells. J. Dermatol. Sci. 2007, 46, 81–89. [Google Scholar] [CrossRef]
- Lavker, R.M.; Sun, T.T.; Oshima, H.; Barrandon, Y.; Akiyama, M.; Ferraris, C.; Chevalier, G.; Favier, B.; Jahoda, C.A.; Dhouailly, D.; et al. Hair follicle stem cells. J. Investig. Derm. Symp. Proc. 2003, 8, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.J.; Jimenez, F.; Izeta, A.; Hardman, J.; Panicker, S.P.; Poblet, E.; Paus, R. Lichen Planopilaris and Frontal Fibrosing Alopecia as Model Epithelial Stem Cell Diseases. Trends Mol. Med. 2018, 24, 435–448. [Google Scholar] [CrossRef]
- Redler, S.; Brockschmidt, F.F.; Tazi-Ahnini, R.; Drichel, D.; Birch, M.P.; Dobson, K.; Giehl, K.A.; Herms, S.; Refke, M.; Kluck, N.; et al. Investigation of the male pattern baldness major genetic susceptibility loci AR/EDA2R and 20p11 in female pattern hair loss. Br. J. Dermatol. 2012, 166, 1314–1318. [Google Scholar] [CrossRef]
- Maghfour, J.; Ceresnie, M.; Olson, J.; Lim, H.W. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2022, 87, 395–396. [Google Scholar] [CrossRef]
- Betz, R.C.; Petukhova, L.; Ripke, S.; Huang, H.; Menelaou, A.; Redler, S.; Becker, T.; Heilmann, S.; Yamany, T.; Duvic, M.; et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat. Commun. 2015, 6, 5966. [Google Scholar] [CrossRef]
- Nguyen, B.; Tosti, A. Alopecia in patients with COVID-19: A systematic review and meta-analysis. JAAD Int. 2022, 7, 67–77. [Google Scholar] [CrossRef]
- Ladizinski, B.; Bazakas, A.; Selim, M.A.; Olsen, E.A. Frontal fibrosing alopecia: A retrospective review of 19 patients seen at Duke University. J. Am. Acad. Dermatol. 2013, 68, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Zinkernagel, M.S.; Trüeb, R.M. Fibrosing alopecia in a pattern distribution: Patterned lichen planopilaris or androgenetic alopecia with a lichenoid tissue reaction pattern? Arch. Dermatol. 2000, 136, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Hordinsky, M.K.; Price, V.H.; Roberts, J.L.; Shapiro, J.; Canfield, D.; Duvic, M.; King, L.E., Jr.; McMichael, A.J.; Randall, V.A.; et al. Alopecia areata investigational assessment guidelines--Part II. National Alopecia Areata Foundation. J. Am. Acad. Dermatol. 2004, 51, 440–447. [Google Scholar] [CrossRef]
- Olsen, E.A.; Canfield, D. SALT II: A new take on the Severity of Alopecia Tool (SALT) for determining percentage scalp hair loss. J. Am. Acad. Dermatol. 2016, 75, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- King, B.A.; Mesinkovska, N.A.; Craiglow, B.; Kindred, C.; Ko, J.; McMichael, A.; Shapiro, J.; Goh, C.; Mirmirani, P.; Tosti, A.; et al. Development of the alopecia areata scale for clinical use: Results of an academic-industry collaborative effort. J. Am. Acad. Dermatol. 2022, 86, 359–364. [Google Scholar] [CrossRef]
- Lesko, S.M.; Rosenberg, L.; Shapiro, S. A case-control study of baldness in relation to myocardial infarction in men. JAMA 1993, 269, 998–1003. [Google Scholar] [CrossRef]
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br. J. Dermatol. 1977, 97, 247–254. [Google Scholar] [CrossRef]
- Olsen, E.A. The midline part: An important physical clue to the clinical diagnosis of androgenetic alopecia in women. J. Am. Acad. Dermatol. 1999, 40, 106–109. [Google Scholar] [CrossRef]
- Biondo, S.; Goble, D.; Sinclair, R. Women who present with female pattern hair loss tend to underestimate the severity of their hair loss. Br. J. Dermatol. 2004, 150, 750–752. [Google Scholar] [CrossRef]
- Sinclair, R.; Wewerinke, M.; Jolley, D. Treatment of female pattern hair loss with oral antiandrogens. Br. J. Dermatol. 2005, 152, 466–473. [Google Scholar] [CrossRef]
- Tajima, M.; Hamada, C.; Arai, T.; Miyazawa, M.; Shibata, R.; Ishino, A. Characteristic features of Japanese women’s hair with aging and with progressing hair loss. J. Dermatol. Sci. 2007, 45, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, A.; Kaneko, T. A New Classification of Early Female Pattern Hair Loss. Int. J. Trichology 2018, 10, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Lee, H.J. Characteristics of androgenetic alopecia in asian. Ann. Dermatol. 2012, 24, 243–252. [Google Scholar] [CrossRef]
- Chiang, C.; Sah, D.; Cho, B.K.; Ochoa, B.E.; Price, V.H. Hydroxychloroquine and lichen planopilaris: Efficacy and introduction of Lichen Planopilaris Activity Index scoring system. J. Am. Acad. Dermatol. 2010, 62, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.; Ryan, T.; Young, D.; Harries, M. Frontal Fibrosing Alopecia Severity Index (FFASI): A validated scoring system for assessing frontal fibrosing alopecia. Br. J. Dermatol. 2016, 175, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Saceda-Corralo, D.; Moreno-Arrones, Ó.M.; Fonda-Pascual, P.; Pindado-Ortega, C.; Buendía-Castaño, D.; Alegre-Sánchez, A.; Segurado-Miravalles, G.; Rodrigues-Barata, A.R.; Jaén-Olasolo, P.; Vaño-Galván, S. Development and validation of the Frontal Fibrosing Alopecia Severity Score. J. Am. Acad. Dermatol. 2018, 78, 522–529. [Google Scholar] [CrossRef]
- Dhurat, R.; Saraogi, P. Hair evaluation methods: Merits and demerits. Int. J. Trichology 2009, 1, 108–119. [Google Scholar] [CrossRef]
- Mubki, T.; Rudnicka, L.; Olszewska, M.; Shapiro, J. Evaluation and diagnosis of the hair loss patient: Part I. History and clinical examination. J. Am. Acad. Dermatol. 2014, 71, e411–e415. [Google Scholar] [CrossRef]
- Daly, T.; Daly, K. Telogen Effluvium With Dysesthesia (TED) Has Lower B12 Levels and May Respond to B12 Supplementation. J. Drugs Dermatol. 2018, 17, 1236–1240. [Google Scholar]
- Blume-Peytavi, U.; Blumeyer, A.; Tosti, A.; Finner, A.; Marmol, V.; Trakatelli, M.; Reygagne, P.; Messenger, A. S1 guideline for diagnostic evaluation in androgenetic alopecia in men, women and adolescents. Br. J. Dermatol. 2011, 164, 5–15. [Google Scholar] [CrossRef]
- Conic, R.R.Z.; Piliang, M.; Bergfeld, W.; Atanaskova-Mesinkovska, N. Vitamin D status in scarring and nonscarring alopecia. J. Am. Acad. Dermatol. 2021, 85, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Nosewicz, J.; Spaccarelli, N.; Roberts, K.M.; Hart, P.A.; Kaffenberger, J.A.; Trinidad, J.C.; Kaffenberger, B.H. The epidemiology, impact, and diagnosis of micronutrient nutritional dermatoses part 1: Zinc, selenium, copper, vitamin A, and vitamin C. J. Am. Acad. Dermatol. 2022, 86, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Nosewicz, J.; Spaccarelli, N.; Roberts, K.M.; Hart, P.A.; Kaffenberger, J.A.; Trinidad, J.C.; Kaffenberger, B.H. The epidemiology, impact, and diagnosis of micronutrient nutritional dermatoses. Part 2: B-complex vitamins. J. Am. Acad. Dermatol. 2022, 86, 281–292. [Google Scholar] [CrossRef]
- Shim, W.H.; Jwa, S.W.; Song, M.; Kim, H.S.; Ko, H.C.; Kim, B.S.; Kim, M.B. Dermoscopic approach to a small round to oval hairless patch on the scalp. Ann. Dermatol. 2014, 26, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Inui, S. Trichoscopy for common hair loss diseases: Algorithmic method for diagnosis. J. Dermatol. 2011, 38, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Doshi, B.; Khopkar, U. Trichoscopy in alopecias: Diagnosis simplified. Int. J. Trichology 2013, 5, 170–178. [Google Scholar] [CrossRef]
- Waśkiel, A.; Rakowska, A.; Sikora, M.; Olszewska, M.; Rudnicka, L. Trichoscopy of alopecia areata: An update. J. Dermatol. 2018, 45, 692–700. [Google Scholar] [CrossRef]
- Waśkiel-Burnat, A.; Rakowska, A.; Sikora, M.; Ciechanowicz, P.; Olszewska, M.; Rudnicka, L. Trichoscopy of Tinea Capitis: A Systematic Review. Dermatol. Ther. 2020, 10, 43–52. [Google Scholar] [CrossRef]
- Mathur, M.; Acharya, P. Trichoscopy of primary cicatricial alopecias: An updated review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 473–484. [Google Scholar] [CrossRef]
- Żychowska, M.; Żychowska, M. Dermoscopy of discoid lupus erythematosus—A systematic review of the literature. Int. J. Dermatol. 2021, 60, 818–828. [Google Scholar] [CrossRef]
- Kaczorowska, A.; Rudnicka, L.; Stefanato, C.M.; Waskiel-Burnat, A.; Warszawik-Hendzel, O.; Olszewska, M.; Rakowska, A. Diagnostic Accuracy of Trichoscopy in Trichotillomania: A Systematic Review. Acta Derm. Venereol. 2021, 101, adv00565. [Google Scholar] [CrossRef] [PubMed]
- Almuhanna, N.; Wortsman, X.; Wohlmuth-Wieser, I.; Kinoshita-Ise, M.; Alhusayen, R. Overview of Ultrasound Imaging Applications in Dermatology [Formula: See text]. J. Cutan. Med. Surg. 2021, 25, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Herrera, N.E.; Eber, A.E.; Martinez-Velasco, M.A.; Perper, M.; Cervantes, J.; Verne, S.H.; Magno, R.J.; Nouri, K.; Tosti, A. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Ekelem, C.; Feil, N.; Csuka, E.; Juhasz, M.; Lin, J.; Choi, F.; Asghari, A.; Heydarlou, D.; Mesinkovska, N.A. Optical Coherence Tomography in the Evaluation of the Scalp and Hair: Common Features and Clinical Utility. Lasers Surg. Med. 2021, 53, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Sun, Y.; Liu, X.; Lin, D.; Lu, D. Characteristic findings by phototrichogram in southern Chinese women with Female pattern hair loss. Skin Res. Technol. 2019, 25, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Magri, F.; Caro, G.; Michelini, S.; Di Fraia, M.; Fortuna, M.C.; Pellacani, G.; Carlesimo, M. Fluorescence advanced videodermoscopy: A new method of hairs and scalp evaluation. Comparison with trichoscopy. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2317–2323. [Google Scholar] [CrossRef]
- Napolitano, M.; Cantelli, M.; Potestio, L.; Ocampo-Garza, S.S.; Vastarella, M.; Nappa, P.; Scalvenzi, M.; Fabbrocini, G.; Patruno, C. Clinical, trichoscopic and in vivo reflectance confocal microscopy evaluation of alopecia areata in atopic dermatitis patients treated with dupilumab. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e561–e563. [Google Scholar] [CrossRef]
- Kurzeja, M.; Czuwara, J.; Walecka, I.; Olszewska, M.; Rudnicka, L. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: A preliminary study. Skin Res. Technol. 2021, 27, 266–271. [Google Scholar] [CrossRef]
- Frishberg, D.P.; Sperling, L.C.; Guthrie, V.M. Transverse scalp sections: A proposed method for laboratory processing. J. Am. Acad. Dermatol. 1996, 35, 220–222. [Google Scholar] [CrossRef]
- Garcia, C.; Poletti, E. Scalp biopsy specimens: Transverse vs. vertical sections. Arch. Dermatol. 2007, 143, 268. [Google Scholar] [CrossRef]
- Ko, J.H.; Huang, Y.H.; Kuo, T.T. Hair counts from normal scalp biopsy in Taiwan. Dermatol. Surg. 2012, 38, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ha, S.J.; Lee, J.H.; Kim, J.W.; Kim, H.O.; Whiting, D.A. Hair counts from scalp biopsy specimens in Asians. J. Am. Acad. Dermatol. 2002, 46, 218–221. [Google Scholar] [CrossRef]
- Aslani, F.S.; Dastgheib, L.; Banihashemi, B.M. Hair counts in scalp biopsy of males and females with androgenetic alopecia compared with normal subjects. J. Cutan. Pathol. 2009, 36, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Sperling, L.C. Hair density in African Americans. Arch. Dermatol. 1999, 135, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.A. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J. Am. Acad. Dermatol. 1993, 28, 755–763. [Google Scholar] [CrossRef]
- Manabe, M.; Tsuboi, R.; Itami, S.; Osada, S.I.; Amoh, Y.; Ito, T.; Inui, S.; Ueki, R.; Ohyama, M.; Kurata, S.; et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version. J. Dermatol. 2018, 45, 1031–1043. [Google Scholar] [CrossRef]
- Rebora, A. Pathogenesis of androgenetic alopecia. J. Am. Acad. Dermatol. 2004, 50, 777–779. [Google Scholar] [CrossRef]
- Garza, L.A.; Liu, Y.; Yang, Z.; Alagesan, B.; Lawson, J.A.; Norberg, S.M.; Loy, D.E.; Zhao, T.; Blatt, H.B.; Stanton, D.C.; et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci. Transl. Med. 2012, 4, 126ra134. [Google Scholar] [CrossRef]
- Leirós, G.J.; Attorresi, A.I.; Balañá, M.E. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br. J. Dermatol. 2012, 166, 1035–1042. [Google Scholar] [CrossRef]
- Chew, E.G.Y.; Lim, T.C.; Leong, M.F.; Liu, X.; Sia, Y.Y.; Leong, S.T.; Yan-Jiang, B.C.; Stoecklin, C.; Borhan, R.; Heilmann-Heimbach, S.; et al. Observations that suggest a contribution of altered dermal papilla mitochondrial function to androgenetic alopecia. Exp. Dermatol. 2022, 31, 906–917. [Google Scholar] [CrossRef]
- Trüeb, R.M. The impact of oxidative stress on hair. Int. J. Cosmet. Sci. 2015, 37 (Suppl. S2), 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.S.; Vaz, C.; Ramasamy, S.; Chew, E.G.Y.; Mohamed, J.S.; Jaffar, H.; Hillmer, A.; Tanavde, V.; Bigliardi-Qi, M.; Bigliardi, P.L. Progressive expression of PPARGC1α is associated with hair miniaturization in androgenetic alopecia. Sci. Rep. 2019, 9, 8771. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, K.; Wang, G.; Yang, L.; Qu, Q.; Fan, Z.; Sun, Y.; Huang, J.; Miao, Y.; Hu, Z. Impairment of autophagy may be associated with follicular miniaturization in androgenetic alopecia by inducing premature catagen. J. Dermatol. 2021, 48, 289–300. [Google Scholar] [CrossRef]
- Su, L.H.; Chen, T.H. Association of androgenetic alopecia with metabolic syndrome in men: A community-based survey. Br. J. Dermatol. 2010, 163, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Y.J.; Saqib, N.U.; Latif, I.; Hassan, I. Female Pattern Hair Loss-An Update. Indian Derm. Online J. 2020, 11, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; Cantelli, M.; Masarà, A.; Annunziata, M.C.; Marasca, C.; Cacciapuoti, S. Female pattern hair loss: A clinical, pathophysiologic, and therapeutic review. Int. J. Womens Dermatol. 2018, 4, 203–211. [Google Scholar] [CrossRef]
- Paik, J.H.; Yoon, J.B.; Sim, W.Y.; Kim, B.S.; Kim, N.I. The prevalence and types of androgenetic alopecia in Korean men and women. Br. J. Dermatol. 2001, 145, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Ohn, J.; Son, H.Y.; Yu, D.A.; Kim, M.S.; Kwon, S.; Park, W.S.; Kim, J.I.; Kwon, O. Early onset female pattern hair loss: A case-control study for analyzing clinical features and genetic variants. J. Dermatol. Sci. 2022, 106, 21–28. [Google Scholar] [CrossRef]
- Olsen, E.A.; Messenger, A.G.; Shapiro, J.; Bergfeld, W.F.; Hordinsky, M.K.; Roberts, J.L.; Stough, D.; Washenik, K.; Whiting, D.A. Evaluation and treatment of male and female pattern hair loss. J. Am. Acad. Dermatol. 2005, 52, 301–311. [Google Scholar] [CrossRef]
- Endo, Y.; Takahashi, M.; Obayashi, Y.; Serizawa, T.; Murakoshi, M.; Ohyama, M. The ovariectomized mouse simulates the pathophysiology of postmenopausal female pattern hair loss. J. Dermatol. Sci. 2017, 87, 79–82. [Google Scholar] [CrossRef]
- Endo, Y.; Obayashi, Y.; Ono, T.; Serizawa, T.; Murakoshi, M.; Ohyama, M. Reversal of the hair loss phenotype by modulating the estradiol-ANGPT2 axis in the mouse model of female pattern hair loss. J. Dermatol. Sci. 2018, 91, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mahé, Y.F.; Michelet, J.F.; Billoni, N.; Jarrousse, F.; Buan, B.; Commo, S.; Saint-Léger, D.; Bernard, B.A. Androgenetic alopecia and microinflammation. Int. J. Dermatol. 2000, 39, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.S.; Ho, E.X.P.; Chu, C.W.; Ramasamy, S.; Bigliardi-Qi, M.; de Sessions, P.F.; Bigliardi, P.L. Microbiome in the hair follicle of androgenetic alopecia patients. PLoS ONE 2019, 14, e0216330. [Google Scholar] [CrossRef] [PubMed]
- Özkoca, D.; Aşkın, Ö.; Engin, B. The comparison of demographics and comorbidities of female pattern hair loss according to the clinical subtype and stage. J. Am. Acad. Dermatol. 2022, 87, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Santas, M.; Diaz-Guimaraens, B.; Saceda-Corralo, D.; Hermosa-Gelbard, A.; Muñoz-Moreno Arrones, O.; Pindado-Ortega, C.; Fernandez-Nieto, D.; Jimenez-Cauhe, J.; Ortega-Quijano, D.; Suarez-Valle, A.; et al. The state-of-the-art in the management of androgenetic alopecia: A review of new therapies and treatment algorithms. JEADV Clin. Pract. 2022, 1, 176–185. [Google Scholar] [CrossRef]
- Gupta, A.K.; Venkataraman, M.; Talukder, M.; Bamimore, M.A. Relative Efficacy of Minoxidil and the 5-α Reductase Inhibitors in Androgenetic Alopecia Treatment of Male Patients: A Network Meta-analysis. JAMA Dermatol. 2022, 158, 266–274. [Google Scholar] [CrossRef]
- Choi, G.S.; Sim, W.Y.; Kang, H.; Huh, C.H.; Lee, Y.W.; Shantakumar, S.; Ho, Y.F.; Oh, E.J.; Duh, M.S.; Cheng, W.Y.; et al. Long-Term Effectiveness and Safety of Dutasteride versus Finasteride in Patients with Male Androgenic Alopecia in South Korea: A Multicentre Chart Review Study. Ann. Dermatol. 2022, 34, 349–359. [Google Scholar] [CrossRef]
- Shum, K.W.; Cullen, D.R.; Messenger, A.G. Hair loss in women with hyperandrogenism: Four cases responding to finasteride. J. Am. Acad. Dermatol. 2002, 47, 733–739. [Google Scholar] [CrossRef]
- Won, Y.Y.; Lew, B.L.; Sim, W.Y. Clinical efficacy of oral administration of finasteride at a dose of 2.5 mg/day in women with female pattern hair loss. Dermatol. Ther. 2018, 31, e12588. [Google Scholar] [CrossRef]
- Olsen, E.A.; Hordinsky, M.; Whiting, D.; Stough, D.; Hobbs, S.; Ellis, M.L.; Wilson, T.; Rittmaster, R.S. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride. J. Am. Acad. Dermatol. 2006, 55, 1014–1023. [Google Scholar] [CrossRef]
- Sung, C.T.; Juhasz, M.L.; Choi, F.D.; Mesinkovska, N.A. The Efficacy of Topical Minoxidil for Non-Scarring Alopecia: A Systematic Review. J. Drugs Dermatol. 2019, 18, 155–160. [Google Scholar] [PubMed]
- Caserini, M.; Radicioni, M.; Leuratti, C.; Terragni, E.; Iorizzo, M.; Palmieri, R. Effects of a novel finasteride 0.25% topical solution on scalp and serum dihydrotestosterone in healthy men with androgenetic alopecia. Int. J. Clin. Pharmacol. Ther. 2016, 54, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Hillmann, K.; Dietz, E.; Canfield, D.; Garcia Bartels, N. A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J. Am. Acad. Dermatol. 2011, 65, 1126–1134.e2. [Google Scholar] [CrossRef]
- Lachgar, S.; Charveron, M.; Gall, Y.; Bonafe, J.L. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br. J. Dermatol. 1998, 138, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Pietrauszka, K.; Bergler-Czop, B. Sulfotransferase SULT1A1 activity in hair follicle, a prognostic marker of response to the minoxidil treatment in patients with androgenetic alopecia: A review. Postępy Dermatol. Alergol. 2022, 39, 472–478. [Google Scholar] [CrossRef]
- Chitalia, J.; Dhurat, R.; Goren, A.; McCoy, J.; Kovacevic, M.; Situm, M.; Naccarato, T.; Lotti, T. Characterization of follicular minoxidil sulfotransferase activity in a cohort of pattern hair loss patients from the Indian Subcontinent. Dermatol. Ther. 2018, 31, e12688. [Google Scholar] [CrossRef]
- Randolph, M.; Tosti, A. Oral minoxidil treatment for hair loss: A review of efficacy and safety. J. Am. Acad. Dermatol. 2021, 84, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, R.D. Female pattern hair loss: A pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int. J. Dermatol. 2018, 57, 104–109. [Google Scholar] [CrossRef]
- Shapiro, J.; Ho, A.; Sukhdeo, K.; Yin, L.; Lo Sicco, K. Evaluation of platelet-rich plasma as a treatment for androgenetic alopecia: A randomized controlled trial. J. Am. Acad. Dermatol. 2020, 83, 1298–1303. [Google Scholar] [CrossRef]
- Evans, A.G.; Mwangi, J.M.; Pope, R.W.; Ivanic, M.G.; Botros, M.A.; Glassman, G.E.; Pearce, F.B., Jr.; Kassis, S. Platelet-rich plasma as a therapy for androgenic alopecia: A systematic review and meta-analysis. J. Dermatol. Treat. 2022, 33, 498–511. [Google Scholar] [CrossRef]
- Elena, E.P.; Irina, O.S. Combination therapy with platelet-rich plasma and minoxidil leads to better clinical results than monotherapy with these methods in men with androgenetic alopecia. Int. J. Trichology 2022, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Grimalt, R. Platelet-Rich Plasma in Combination With 5% Minoxidil Topical Solution and 1 mg Oral Finasteride for the Treatment of Androgenetic Alopecia: A Randomized Placebo-Controlled, Double-Blind, Half-Head Study. Dermatol. Surg. 2018, 44, 126–130. [Google Scholar] [CrossRef]
- Gabbard, R.D.; Hoopes, R.R.; Kemp, M.G. Spironolactone and XPB: An Old Drug with a New Molecular Target. Biomolecules 2020, 10, 756. [Google Scholar] [CrossRef]
- Aguilar Medina, D.A.; Cazarin, J.; Magana, M. Spironolactone in dermatology. Dermatol. Ther. 2022, 35, e15321. [Google Scholar] [CrossRef] [PubMed]
- van Zuuren, E.J.; Fedorowicz, Z.; Schoones, J. Interventions for female pattern hair loss. Cochrane Database Syst. Rev. 2016, 2016, CD007628. [Google Scholar] [CrossRef]
- Burns, L.J.; De Souza, B.; Flynn, E.; Hagigeorges, D.; Senna, M.M. Spironolactone for treatment of female pattern hair loss. J. Am. Acad. Dermatol. 2020, 83, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, H.; Aly, U.F.; Medhat, W.; Ahmed, S.S.; Abdel-Aziz, R.T.A. A novel topical combination of minoxidil and spironolactone for androgenetic alopecia: Clinical, histopathological, and physicochemical study. Dermatol. Ther. 2021, 34, e14678. [Google Scholar] [CrossRef]
- Ammar, A.M.; Elshahid, A.R.; Abdel-Dayem, H.A.; Mohamed, A.A.; Elsaie, M.L. Dermoscopic evaluation of the efficacy of combination of topical spironolactone 5% and minoxidil 5% solutions in the treatment of androgenetic alopecia: A cross sectional-comparative study. J. Cosmet. Dermatol. 2022, 21, 5790–5799. [Google Scholar] [CrossRef]
- Wei, C.; Bovonratwet, P.; Gu, A.; Moawad, G.; Silverberg, J.I.; Friedman, A.J. Spironolactone use does not increase the risk of female breast cancer recurrence: A retrospective analysis. J. Am. Acad. Dermatol. 2020, 83, 1021–1027. [Google Scholar] [CrossRef]
- Vexiau, P.; Chaspoux, C.; Boudou, P.; Fiet, J.; Jouanique, C.; Hardy, N.; Reygagne, P. Effects of minoxidil 2% vs. cyproterone acetate treatment on female androgenetic alopecia: A controlled, 12-month randomized trial. Br. J. Dermatol. 2002, 146, 992–999. [Google Scholar] [CrossRef]
- Azarchi, S.; Bienenfeld, A.; Lo Sicco, K.; Marchbein, S.; Shapiro, J.; Nagler, A.R. Androgens in women: Hormone-modulating therapies for skin disease. J. Am. Acad. Dermatol. 2019, 80, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Magri, F.; D’Arino, A.; Pigliacelli, F.; Muscianese, M.; Leoncini, P.; Caro, G.; Federico, A.; Fortuna, M.C.; Carlesimo, M. Efficacy of Topical Finasteride 0.5% vs 17alpha-Estradiol 0.05% in the Treatment of Postmenopausal Female Pattern Hair Loss: A Retrospective, Single-Blind Study of 119 Patients. Dermatol. Pract. Concept. 2020, 10, e2020039. [Google Scholar] [CrossRef]
- Egger, A.; Resnik, S.R.; Aickara, D.; Maranda, E.; Kaiser, M.; Wikramanayake, T.C.; Jimenez, J.J. Examining the Safety and Efficacy of Low-Level Laser Therapy for Male and Female Pattern Hair Loss: A Review of the Literature. Ski. Appendage Disord. 2020, 6, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.K.; Mysore, V. Role of Low-Level Light Therapy (LLLT) in Androgenetic Alopecia. J. Cutan. Aesthetic Surg. 2021, 14, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Lee, S.J.; Kim, W.S. The effect of a 1550 nm fractional erbium-glass laser in female pattern hair loss. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1450–1454. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Chalermroj, N.; Khunkhet, S. Low-level laser therapy for the treatment of androgenetic alopecia in Thai men and women: A 24-week, randomized, double-blind, sham device-controlled trial. Lasers Med. Sci. 2019, 34, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Campo, D.; Fortuna, M.C.; Garelli, V.; Pranteda, G.; De Vita, G.; Sorriso-Valvo, L.; Di Nunno, D.; Carlesimo, M. A preliminary study on topical cetirizine in the therapeutic management of androgenetic alopecia. J. Dermatolog. Treat. 2018, 29, 149–151. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, H.; Yang, M. Topical cetirizine for treating androgenetic alopecia: A systematic review. J. Cosmet Dermatol. 2022, 21, 5519–5526. [Google Scholar] [CrossRef]
- Tsuboi, R.; Niiyama, S.; Irisawa, R.; Harada, K.; Nakazawa, Y.; Kishimoto, J. Autologous cell-based therapy for male and female pattern hair loss using dermal sheath cup cells: A randomized placebo-controlled double-blinded dose-finding clinical study. J. Am. Acad. Dermatol. 2020, 83, 109–116. [Google Scholar] [CrossRef]
- Piérard-Franchimont, C.; De Doncker, P.; Cauwenbergh, G.; Piérard, G.E. Ketoconazole shampoo: Effect of long-term use in androgenic alopecia. Dermatology 1998, 196, 474–477. [Google Scholar] [CrossRef]
- Fischer, T.W.; Trüeb, R.M.; Hänggi, G.; Innocenti, M.; Elsner, P. Topical melatonin for treatment of androgenetic alopecia. Int. J. Trichology 2012, 4, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.J.; Hughes, O.B.; Canazza, A.; Zaiac, M.N. An Open-Label Evaluator Blinded Study of the Efficacy and Safety of a New Nutritional Supplement in Androgenetic Alopecia: A Pilot Study. J. Clin. Aesthet. Dermatol. 2017, 10, 52–56. [Google Scholar] [PubMed]
- Ocampo-Garza, S.S.; Fabbrocini, G.; Ocampo-Candiani, J.; Cinelli, E.; Villani, A. Micro needling: A novel therapeutic approach for androgenetic alopecia, A Review of Literature. Dermatol. Ther. 2020, 33, e14267. [Google Scholar] [CrossRef] [PubMed]
- McElwee, K.J.; Shapiro, J.S. Promising therapies for treating and/or preventing androgenic alopecia. Ski. Ther. Lett. 2012, 17, 1–4. [Google Scholar]
- Gilhar, A.; Etzioni, A.; Paus, R. Alopecia areata. N. Engl. J. Med. 2012, 366, 1515–1525. [Google Scholar] [CrossRef]
- Rossi, A.; Muscianese, M.; Piraccini, B.M.; Starace, M.; Carlesimo, M.; Mandel, V.D.; Alessandrini, A.; Calvieri, S.; Caro, G.; D’Arino, A.; et al. Italian Guidelines in diagnosis and treatment of alopecia areata. G. Ital. Di Dermatol. E Venereol. 2019, 154, 609–623. [Google Scholar] [CrossRef]
- Mirzoyev, S.A.; Schrum, A.G.; Davis, M.D.P.; Torgerson, R.R. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990–2009. J. Investig. Dermatol. 2014, 134, 1141–1142. [Google Scholar] [CrossRef]
- Safavi, K.H.; Muller, S.A.; Suman, V.J.; Moshell, A.N.; Melton, L.J., III. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin. Proc. 1995, 70, 628–633. [Google Scholar] [CrossRef]
- Aranishi, T.; Ito, T.; Fukuyama, M.; Isaka, Y.; Mackie, D.S.; King-Concialdi, K.; Senglaub, S.S.; Jaffe, D.H.; Shimomura, Y.; Ohyama, M. Prevalence of alopecia areata in Japan: Estimates from a nationally representative sample. J. Dermatol. 2022, 50, 26–36. [Google Scholar] [CrossRef]
- Ito, T.; Ito, N.; Bettermann, A.; Tokura, Y.; Takigawa, M.; Paus, R. Collapse and restoration of MHC class-I-dependent immune privilege: Exploiting the human hair follicle as a model. Am. J. Pathol. 2004, 164, 623–634. [Google Scholar] [CrossRef]
- Ito, T.; Ito, N.; Saatoff, M.; Hashizume, H.; Fukamizu, H.; Nickoloff, B.J.; Takigawa, M.; Paus, R. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J. Investig. Dermatol. 2008, 128, 1196–1206. [Google Scholar] [CrossRef]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- King, B.; Ohyama, M.; Kwon, O.; Zlotogorski, A.; Ko, J.; Mesinkovska, N.A.; Hordinsky, M.; Dutronc, Y.; Wu, W.S.; McCollam, J.; et al. Two Phase 3 Trials of Baricitinib for Alopecia Areata. N. Engl. J. Med. 2022, 386, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, F.; Drake, L.A.; Senna, M.M.; Rezaei, N. Alopecia areata: A review of disease pathogenesis. Br. J. Dermatol. 2018, 179, 1033–1048. [Google Scholar] [CrossRef]
- Loh, S.H.; Moon, H.N.; Lew, B.L.; Sim, W.Y. Role of T helper 17 cells and T regulatory cells in alopecia areata: Comparison of lesion and serum cytokine between controls and patients. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1028–1033. [Google Scholar] [CrossRef]
- Fuentes-Duculan, J.; Gulati, N.; Bonifacio, K.M.; Kunjravia, N.; Zheng, X.; Suárez-Fariñas, M.; Shemer, A.; Guttman-Yassky, E.; Krueger, J.G. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp. Dermatol. 2016, 25, 282–286. [Google Scholar] [CrossRef]
- Suárez-Fariñas, M.; Ungar, B.; Noda, S.; Shroff, A.; Mansouri, Y.; Fuentes-Duculan, J.; Czernik, A.; Zheng, X.; Estrada, Y.D.; Xu, H.; et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J. Allergy Clin. Immunol. 2015, 136, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Cai, Y.; Zhao, Y.; Yang, Y. The gut microbiome and Alopecia areata: Implications for early diagnostic biomarkers and novel therapies. Front. Nutr. 2022, 9, 979876. [Google Scholar] [CrossRef]
- Ramos, P.M.; Anzai, A.; Duque-Estrada, B.; Melo, D.F.; Sternberg, F.; Santos, L.D.N.; Alves, L.D.; Mulinari-Brenner, F. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An. Bras. De Dermatol. 2020, 95 (Suppl. S1), 39–52. [Google Scholar] [CrossRef]
- Cranwell, W.C.; Lai, V.W.; Photiou, L.; Meah, N.; Wall, D.; Rathnayake, D.; Joseph, S.; Chitreddy, V.; Gunatheesan, S.; Sindhu, K.; et al. Treatment of alopecia areata: An Australian expert consensus statement. Australas. J. Dermatol. 2019, 60, 163–170. [Google Scholar] [CrossRef]
- Messenger, A.G.; McKillop, J.; Farrant, P.; McDonagh, A.J.; Sladden, M. British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br. J. Dermatol. 2012, 166, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Meah, N.; Wall, D.; York, K.; Bhoyrul, B.; Bokhari, L.; Asz-Sigall, D.; Bergfeld, W.F.; Betz, R.C.; Blume-Peytavi, U.; Callender, V.; et al. The Alopecia Areata Consensus of Experts (ACE) study part II: Results of an international expert opinion on diagnosis and laboratory evaluation for alopecia areata. J. Am. Acad. Dermatol. 2021, 84, 1594–1601. [Google Scholar] [CrossRef]
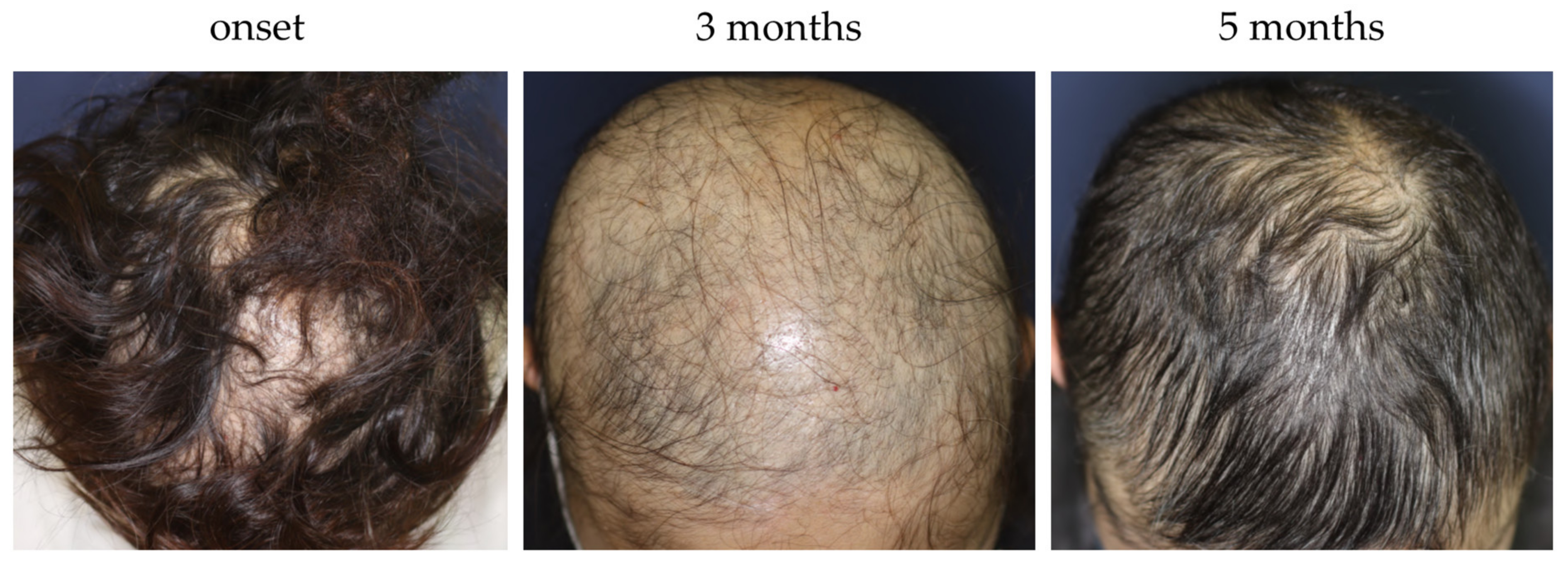
- Fukuyama, M.; Kinoshita-Ise, M.; Sato, Y.; Ohyama, M. Elucidation of demographic, clinical and trichoscopic features for early diagnosis of self-healing acute diffuse and total alopecia. J. Dermatol. 2020, 47, 583–591. [Google Scholar] [CrossRef]
- Lensing, M.; Jabbari, A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front. Immunol. 2022, 13, 955035. [Google Scholar] [CrossRef] [PubMed]
- Kennedy Crispin, M.; Ko, J.M.; Craiglow, B.G.; Li, S.; Shankar, G.; Urban, J.R.; Chen, J.C.; Cerise, J.E.; Jabbari, A.; Winge, M.C.; et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight 2016, 1, e89776. [Google Scholar] [CrossRef] [PubMed]
- Mackay-Wiggan, J.; Jabbari, A.; Nguyen, N.; Cerise, J.E.; Clark, C.; Ulerio, G.; Furniss, M.; Vaughan, R.; Christiano, A.M.; Clynes, R. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight 2016, 1, e89790. [Google Scholar] [CrossRef]
- King, B.; Guttman-Yassky, E.; Peeva, E.; Banerjee, A.; Sinclair, R.; Pavel, A.B.; Zhu, L.; Cox, L.A.; Craiglow, B.; Chen, L.; et al. A phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral Janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24-week results. J. Am. Acad. Dermatol. 2021, 85, 379–387. [Google Scholar] [CrossRef]
- Jabbari, A.; Sansaricq, F.; Cerise, J.; Chen, J.C.; Bitterman, A.; Ulerio, G.; Borbon, J.; Clynes, R.; Christiano, A.M.; Mackay-Wiggan, J. An Open-Label Pilot Study to Evaluate the Efficacy of Tofacitinib in Moderate to Severe Patch-Type Alopecia Areata, Totalis, and Universalis. J. Investig. Dermatol. 2018, 138, 1539–1545. [Google Scholar] [CrossRef]
- Mikhaylov, D.; Glickman, J.W.; Del Duca, E.; Nia, J.; Hashim, P.; Singer, G.K.; Posligua, A.L.; Florek, A.G.; Ibler, E.; Hagstrom, E.L.; et al. A phase 2a randomized vehicle-controlled multi-center study of the safety and efficacy of delgocitinib in subjects with moderate-to-severe alopecia areata. Arch. Dermatol. Res. 2023, 315, 181–189. [Google Scholar] [CrossRef]
- King, B.; Mesinkovska, N.; Mirmirani, P.; Bruce, S.; Kempers, S.; Guttman-Yassky, E.; Roberts, J.L.; McMichael, A.; Colavincenzo, M.; Hamilton, C.; et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus Kinase inhibitor, in moderate-to-severe alopecia areata. J. Am. Acad. Dermatol. 2022, 87, 306–313. [Google Scholar] [CrossRef]
- Guo, L.; Feng, S.; Sun, B.; Jiang, X.; Liu, Y. Benefit and risk profile of tofacitinib for the treatment of alopecia areata: A systemic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 192–201. [Google Scholar] [CrossRef]
- McKenzie, P.L.; Castelo-Soccio, L. Alopecia areata flare patterns in children and young adults while on systemic tofacitinib. J. Am. Acad. Dermatol. 2021, 86, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Irisawa, R.; Ito, T.; Uchiyama, M.; Tsuboi, R. The effectiveness of dupilumab in patients with alopecia areata who have atopic dermatitis: A case series of seven patients. Br. J. Dermatol. 2020, 183, 396–397. [Google Scholar] [CrossRef]
- Uchida, H.; Kamata, M.; Watanabe, A.; Agematsu, A.; Nagata, M.; Fukaya, S.; Hayashi, K.; Fukuyasu, A.; Tanaka, T.; Ishikawa, T.; et al. Dupilumab Improved Alopecia Areata in a Patient with Atopic Dermatitis: A Case Report. Acta Derm. Venereol. 2019, 99, 675–676. [Google Scholar] [CrossRef] [PubMed]
- Ushida, M.; Ohshita, A.; Arakawa, Y.; Kanehisa, F.; Katoh, N.; Asai, J. Dupilumab therapy rapidly improved alopecia areata associated with trichotillomania in an atopic dermatitis patient. Allergol. Int. 2020, 69, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Renert-Yuval, Y.; Bares, J.; Chima, M.; Hawkes, J.E.; Gilleaudeau, P.; Sullivan-Whalen, M.; Singer, G.K.; Garcet, S.; Pavel, A.B.; et al. Phase 2a randomized clinical trial of dupilumab (anti-IL-4Rα) for alopecia areata patients. Allergy 2022, 77, 897–906. [Google Scholar] [CrossRef]
- Yoshimasu, T.; Uede, M.; Kanazawa, N.; Mikita, N.; Yamamoto, Y.; Ito, T.; Furukawa, F. Involvement of FcɛR1α immunopositive cells in alopecia areata with atopic dermatitis and a high titer of serum immunoglobulin E. Eur. J. Dermatol. 2014, 24, 500–503. [Google Scholar] [CrossRef]
- Renert-Yuval, Y.; Pavel, A.B.; Del Duca, E.; Facheris, P.; Pagan, A.D.; Bose, S.; Gómez-Arias, P.J.; Angelov, M.; Bares, J.; Chima, M.; et al. Scalp Biomarkers During Dupilumab Treatment Support Th2 Pathway Pathogenicity in Alopecia Areata. Allergy 2022, 78, 1047–1059. [Google Scholar] [CrossRef]
- Chung, J.; Slaught, C.L.; Simpson, E.L. Alopecia areata in 2 patients treated with dupilumab: New onset and worsening. JAAD Case Rep. 2019, 5, 643–645. [Google Scholar] [CrossRef]
- Kageyama, R.; Ito, T.; Hanai, S.; Morishita, N.; Nakazawa, S.; Fujiyama, T.; Honda, T.; Tokura, Y. Immunological Properties of Atopic Dermatitis-Associated Alopecia Areata. Int. J. Mol. Sci. 2021, 22, 2618. [Google Scholar] [CrossRef]
- Almohanna, H.M.; Ahmed, A.A.; Griggs, J.W.; Tosti, A. Platelet-Rich Plasma in the Treatment of Alopecia Areata: A Review. J. Investig. Dermatol. Symp. Proc. 2020, 20, S45–S49. [Google Scholar] [CrossRef]
- Meznerics, F.A.; Illés, K.; Dembrovszky, F.; Fehérvári, P.; Kemény, L.V.; Kovács, K.D.; Wikonkál, N.M.; Csupor, D.; Hegyi, P.; Bánvölgyi, A. Platelet-Rich Plasma in Alopecia Areata-A Steroid-Free Treatment Modality: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Biomedicines 2022, 10, 1829. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Joy, B.; Thyvalappil, A.; Mathew, P.; Sreenivasan, A.; Sridharan, R. A Comparative Study of Therapeutic Response to Intralesional Injections of Platelet-Rich Plasma Versus Triamcinolone Acetonide in Alopecia Areata. Indian Derm. Online J. 2020, 11, 920–924. [Google Scholar] [CrossRef]
- Hegde, P.; Relhan, V.; Sahoo, B.; Garg, V.K. A randomized, placebo and active controlled, split scalp study to evaluate the efficacy of platelet-rich plasma in patchy alopecia areata of the scalp. Dermatol. Ther. 2020, 33, e14388. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Kumar, S.; Brar, B.K.; Kukar, N.; Arora, H.; Brar, S.K. Comparative Evaluation of Therapeutic Efficacy of Intralesional Injection of Triamcinolone Acetonide versus Intralesional Autologous Platelet-rich Plasma Injection in Alopecia Areata. J. Cutan. Aesthetic Surg. 2020, 13, 103–111. [Google Scholar] [CrossRef]
- Cervantes, J.; Jimenez, J.J.; DelCanto, G.M.; Tosti, A. Treatment of Alopecia Areata with Simvastatin/Ezetimibe. J. Investig. Dermatol. Symp. Proc. 2018, 19, S25–S31. [Google Scholar] [CrossRef]
- Shin, J.M.; Jung, K.E.; Yim, S.H.; Rao, B.; Hong, D.; Seo, Y.J.; Kim, C.D.; Lee, Y. Putative therapeutic mechanisms of simvastatin in the treatment of alopecia areata. J. Am. Acad. Dermatol. 2021, 84, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Gherardini, J.; Rivas, K.E.; Chéret, J.; Strbo, N.; Paus, R. Downregulation of pathogenic MICA-NKG2D interactions as a novel strategy in alopecia areata management: A new rationale for adjunct statin therapy? J. Eur. Acad. Dermatol. Venereol. 2022, 36, e1013–e1015. [Google Scholar] [CrossRef]
- Lattouf, C.; Jimenez, J.J.; Tosti, A.; Miteva, M.; Wikramanayake, T.C.; Kittles, C.; Herskovitz, I.; Handler, M.Z.; Fabbrocini, G.; Schachner, L.A. Treatment of alopecia areata with simvastatin/ezetimibe. J. Am. Acad. Dermatol. 2015, 72, 359–361. [Google Scholar] [CrossRef]
- Gorcey, L.; Gordon Spratt, E.A.; Leger, M.C. Alopecia universalis successfully treated with adalimumab. JAMA Dermatol. 2014, 150, 1341–1344. [Google Scholar] [CrossRef]
- Skurkovich, S.; Korotky, N.G.; Sharova, N.M.; Skurkovich, B. Treatment of alopecia areata with anti-interferon-gamma antibodies. J. Investig. Dermatol. Symp. Proc. 2005, 10, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Price, V.H.; Hordinsky, M.K.; Olsen, E.A.; Roberts, J.L.; Siegfried, E.C.; Rafal, E.S.; Korman, N.J.; Altrabulsi, B.; Leung, H.M.; Garovoy, M.R.; et al. Subcutaneous efalizumab is not effective in the treatment of alopecia areata. J. Am. Acad. Dermatol. 2008, 58, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Strober, B.E.; Siu, K.; Alexis, A.F.; Kim, G.; Washenik, K.; Sinha, A.; Shupack, J.L. Etanercept does not effectively treat moderate to severe alopecia areata: An open-label study. J. Am. Acad. Dermatol. 2005, 52, 1082–1084. [Google Scholar] [CrossRef]
- Mikhaylov, D.; Pavel, A.; Yao, C.; Kimmel, G.; Nia, J.; Hashim, P.; Vekaria, A.S.; Taliercio, M.; Singer, G.; Karalekas, R.; et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch. Dermatol. Res. 2019, 311, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Telogen Effluvium: Is There a Need for a New Classification? Ski. Appendage Disord. 2016, 2, 39–44. [Google Scholar] [CrossRef]
- Kligman, A.M. Pathologic dynamics of human hair loss. I. Telogen effuvium. Arch. Dermatol. 1961, 83, 175–198. [Google Scholar] [CrossRef]
- Grover, C.; Khurana, A. Telogen effluvium. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 591–603. [Google Scholar] [CrossRef]
- Malkud, S. Telogen Effluvium: A Review. J. Clin. Diagn Res. 2015, 9, WE01–WE03. [Google Scholar] [CrossRef]
- Headington, J.T. Telogen effluvium. New concepts and review. Arch. Dermatol. 1993, 129, 356–363. [Google Scholar] [CrossRef]
- Rebora, A. Proposing a Simpler Classification of Telogen Effluvium. Ski. Appendage Disord. 2016, 2, 35–38. [Google Scholar] [CrossRef]
- Whiting, D.A. Chronic telogen effluvium. Dermatol. Clin. 1996, 14, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Seleit, I.; Bakry, O.A.; Badr, E.; Hassan, E.H. Vitamin D Receptor Gene Polymorphism In Chronic Telogen Effluvium; A Case-Control Study. Clin. Cosmet. Investig. Dermatol. 2019, 12, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, G.M.; Crollick, J.S.; Hassett, J.M., Jr. Postfebrile telogen effluvium in critically ill patients. Crit. Care Med. 1988, 16, 98–99. [Google Scholar] [CrossRef]
- Ohyama, M.; Matsudo, K.; Fujita, T. Management of hair loss after severe acute respiratory syndrome coronavirus 2 infection: Insight into the pathophysiology with implication for better management. J. Dermatol. 2022, 49, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Starace, M.; Piraccini, B.M.; Evangelista, V.; Bruni, F.; Alessandrini, A. Acute telogen effluvium due to Dengue fever mimicking androgenetic alopecia. Ital. J. Dermatol. Venerol. 2023, 158, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Systematic approach to hair loss in women. J. Der Dtsch. Dermatol. Ges. 2010, 8, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Pujol, J.C.; Spector, T.D.; Ourselin, S.; Steves, C.J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022, 399, 2263–2264. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Goren, A.; Wambier, C.G.; Herrera, S.; McCoy, J.; Vaño-Galván, S.; Gioia, F.; Comeche, B.; Ron, R.; Serrano-Villar, S.; Ramos, P.M.; et al. Anti-androgens may protect against severe COVID-19 outcomes: Results from a prospective cohort study of 77 hospitalized men. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e13–e15. [Google Scholar] [CrossRef]
- Montopoli, M.; Zumerle, S.; Vettor, R.; Rugge, M.; Zorzi, M.; Catapano, C.V.; Carbone, G.M.; Cavalli, A.; Pagano, F.; Ragazzi, E.; et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: A population-based study (N = 4532). Ann. Oncol. 2020, 31, 1040–1045. [Google Scholar] [CrossRef]
- Kim, J.; Hong, K.; Gómez Gómez, R.E.; Kim, S.; Chun, B.C. Lack of Evidence of COVID-19 Being a Risk Factor of Alopecia Areata: Results of a National Cohort Study in South Korea. Front. Med. 2021, 8, 758069. [Google Scholar] [CrossRef] [PubMed]
- Ganjei, Z.; Yazdan Panah, M.; Rahmati, R.; Zari Meidani, F.; Mosavi, A. COVID-19 vaccination and alopecia areata: A case report and literature review. Clin. Case Rep. 2022, 10, e6039. [Google Scholar] [CrossRef] [PubMed]
- Michelini, S.; Caro, G.; Di Fraia, M.; Fortuna, M.; Magri, F.; Gomes, V.V.; Grieco, T.; Carlesimo, M.; Rossi, A.; Pellacani, G. Telogen effluvium in SARS-CoV-2 infection: Histological aspects. J. Eur. Acad. Dermatol. Venereol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Senna, M.M.; Peterson, E.; Jozic, I.; Chéret, J.; Paus, R. Frontiers in Lichen Planopilaris and Frontal Fibrosing Alopecia Research: Pathobiology Progress and Translational Horizons. JID Innov. 2022, 2, 100113. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.; Hardman, J.; Chaudhry, I.; Poblet, E.; Paus, R. Profiling the human hair follicle immune system in lichen planopilaris and frontal fibrosing alopecia: Can macrophage polarization differentiate these two conditions microscopically? Br. J. Dermatol. 2020, 183, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.H.C.; Monga, I.; Sallee, B.N.; Chen, J.C.; Abdelaziz, A.R.; Perez-Lorenzo, R.; Bordone, L.A.; Christiano, A.M. Primary cicatricial alopecias are characterized by dysregulation of shared gene expression pathways. PNAS Nexus 2022, 1, pgac111. [Google Scholar] [CrossRef]
- Pavlovsky, L.; Israeli, M.; Sagy, E.; Berg, A.L.; David, M.; Shemer, A.; Klein, T.; Hodak, E. Lichen planopilaris is associated with HLA DRB1*11 and DQB1*03 alleles. Acta Derm. Venereol. 2015, 95, 177–180. [Google Scholar] [CrossRef]
- Tziotzios, C.; Petridis, C.; Dand, N.; Ainali, C.; Saklatvala, J.R.; Pullabhatla, V.; Onoufriadis, A.; Pramanik, R.; Baudry, D.; Lee, S.H.; et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun 2019, 10, 1150. [Google Scholar] [CrossRef]
- Cuenca-Barrales, C.; Ruiz-Villaverde, R.; Molina-Leyva, A. Familial Frontal Fibrosing Alopecia: Report of a case and systematic review of the literature. Sultan Qaboos Univ. Med. J. 2021, 21, e320–e323. [Google Scholar] [CrossRef]
- Dlova, N.; Goh, C.L.; Tosti, A. Familial frontal fibrosing alopecia. Br. J. Dermatol. 2013, 168, 220–222. [Google Scholar] [CrossRef]
- Ranasinghe, G.C.; Piliang, M.P.; Bergfeld, W.F. Prevalence of hormonal and endocrine dysfunction in patients with lichen planopilaris (LPP): A retrospective data analysis of 168 patients. J. Am. Acad. Dermatol. 2017, 76, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita-Ise, M.; Fukuyama, M.; Ohyama, M. Distinctive age distribution and hair loss pattern putatively highlighting uniqueness of Japanese cases of fibrosing alopecia in a pattern distribution. J. Dermatol. 2022, 49, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Malki, L.; Sarig, O.; Romano, M.T.; Méchin, M.C.; Peled, A.; Pavlovsky, M.; Warshauer, E.; Samuelov, L.; Uwakwe, L.; Briskin, V.; et al. Variant PADI3 in Central Centrifugal Cicatricial Alopecia. N. Engl. J. Med. 2019, 380, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Strowd, L.C.; Subash, J.; McGregor, S.; McMichael, A. Cicatricial Alopecia in Identical Twin Lumbee Native American Women. Ski. Appendage Disord. 2018, 4, 108–111. [Google Scholar] [CrossRef]
- Kyei, A.; Bergfeld, W.F.; Piliang, M.; Summers, P. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: A population study. Arch. Dermatol. 2011, 147, 909–914. [Google Scholar] [CrossRef]
- Dina, Y.; Okoye, G.A.; Aguh, C. Association of Uterine Leiomyomas With Central Centrifugal Cicatricial Alopecia. JAMA Dermatol. 2018, 154, 213–214. [Google Scholar] [CrossRef]
- Jamerson, T.A.; Talbot, C.C., Jr.; Dina, Y.; Aguh, C. Presence of Uterine Leiomyomas Has No Significant Impact on Gene Expression Profile in the Scalp of Patients with Central Centrifugal Cicatricial Alopecia. JID Innov. 2022, 2, 100060. [Google Scholar] [CrossRef]
- Olsen, E.A.; Bergfeld, W.F.; Cotsarelis, G.; Price, V.H.; Shapiro, J.; Sinclair, R.; Solomon, A.; Sperling, L.; Stenn, K.; Whiting, D.A.; et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J. Am. Acad. Dermatol. 2003, 48, 103–110. [Google Scholar] [CrossRef]
- Yip, L.; Barrett, T.H.; Harries, M.J. Folliculitis decalvans and lichen planopilaris phenotypic spectrum: A case series of biphasic clinical presentation and theories on pathogenesis. Clin. Exp. Dermatol. 2020, 45, 63–72. [Google Scholar] [CrossRef]
- Egger, A.; Stojadinovic, O.; Miteva, M. Folliculitis Decalvans and Lichen Planopilaris Phenotypic Spectrum-A Series of 7 New Cases With Focus on Histopathology. Am. J. Dermatopathol. 2020, 42, 173–177. [Google Scholar] [CrossRef]
- Matard, B.; Cavelier Balloy, B.; Assouly, P.; Reygagne, P. It has the Erythema of a Lichen Planopilaris, it has the Hyperkeratosis of a Lichen Planopilaris, but it is Not a Lichen Planopilaris: About the "Lichen Planopilaris-Like" Form of Folliculitis Decalvans. Am. J. Dermatopathol. 2021, 43, 235–236. [Google Scholar] [CrossRef] [PubMed]
- Shavit, E.; Cohen, A.; Zoller, L.; Onn, E.; Kridin, K. The burden of gout in acne keloidalis nuchae-Insights from a population-based study. J. Cosmet. Dermatol. 2023, 22, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Lullo, J.J.; Carter, M.J.; Shitabata, P.K.; Lee, D.J. Acne Keloidalis Nuchae is Associated with Cutis Verticis Gyrata. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1421–1427. [Google Scholar] [CrossRef]
- Zagelbaum Ward, N.K.; Jun, J.A.; Vecerek, N.; Donaldson, M.; Quismorio, F.P., Jr. Dissecting cellulitis of the scalp associated with peripheral and axial spondyloarthritis: Report of a case and review of the literature. Clin. Rheumatol. 2022, 41, 2553–2560. [Google Scholar] [CrossRef]
- Thomas, J.; Aguh, C. Approach to treatment of refractory dissecting cellulitis of the scalp: A systematic review. J. Dermatolog. Treat. 2021, 32, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Starace, M.; Vano-Galvan, S.; Piraccini, B.M.; Reygagne, P.; Rudnicka, L.; Silyuk, T.; Sinclair, R.; Tosti, A. Refractory folliculitis decalvans treated with adalimumab: A case series of 23 patients. J. Am. Acad. Dermatol. 2022, 87, 666–669. [Google Scholar] [CrossRef]
- Rambhia, P.H.; Conic, R.R.Z.; Murad, A.; Atanaskova-Mesinkovska, N.; Piliang, M.; Bergfeld, W. Updates in therapeutics for folliculitis decalvans: A systematic review with evidence-based analysis. J. Am. Acad. Dermatol. 2019, 80, 794–801.e1. [Google Scholar] [CrossRef]
- Mesinkovska, N.A.; Tellez, A.; Dawes, D.; Piliang, M.; Bergfeld, W. The use of oral pioglitazone in the treatment of lichen planopilaris. J. Am. Acad. Dermatol. 2015, 72, 355–356. [Google Scholar] [CrossRef]
- Mirmirani, P.; Karnik, P. Lichen planopilaris treated with a peroxisome proliferator-activated receptor gamma agonist. Arch. Dermatol. 2009, 145, 1363–1366. [Google Scholar] [CrossRef]
- Peterson, E.L.; Gutierrez, D.; Brinster, N.K.; Lo Sicco, K.I.; Shapiro, J. Response of Lichen Planopilaris to Pioglitazone Hydrochloride. J. Drugs Dermatol. 2019, 18, 1276–1279. [Google Scholar]
- Spring, P.; Spanou, Z.; de Viragh, P.A. Lichen planopilaris treated by the peroxisome proliferator activated receptor-γ agonist pioglitazone: Lack of lasting improvement or cure in the majority of patients. J. Am. Acad. Dermatol. 2013, 69, 830–832. [Google Scholar] [CrossRef]
- Lajevardi, V.; Ghiasi, M.; Balighi, K.; Daneshpazhooh, M.; Azar, P.M.; Kianfar, N.; Dasdar, S.; Peymanfar, A.A. Efficacy and safety of oral pioglitazone in the management of lichen planopilaris in comparison with clobetasol: A randomized clinical trial. Dermatol. Ther. 2022, 35, e15868. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Khanna, T.; Sallee, B.; Christiano, A.M.; Bordone, L.A. Tofacitinib for the treatment of lichen planopilaris: A case series. Dermatol. Ther. 2018, 31, e12656. [Google Scholar] [CrossRef] [PubMed]
- Plante, J.; Eason, C.; Snyder, A.; Elston, D. Tofacitinib in the treatment of lichen planopilaris: A retrospective review. J. Am. Acad. Dermatol. 2020, 83, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Moussa, A.; Bhoyrul, B.; Asfour, L.; Kazmi, A.; Eisman, S.; Sinclair, R.D. Treatment of lichen planopilaris with baricitinib: A retrospective study. J. Am. Acad. Dermatol. 2022, 87, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Tsoi, L.C.; Sarkar, M.K.; Xing, X.; Xue, K.; Uppala, R.; Berthier, C.C.; Zeng, C.; Patrick, M.; Billi, A.C.; et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci. Transl. Med. 2019, 11, eaav7561. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, M.; Coste, V.; Konstantinou, M.P.; Reguiai, Z.; Villani, A.; Hotz, C.; Viguier, M.; Pruvost-Balland, C.; Dupuy, A.; Wolkenstein, P.; et al. Treatment of dissecting cellulitis of the scalp with tumor necrosis factor inhibitors: A retrospective multicenter study. Clin. Exp. Dermatol. 2023, 48, 528–530. [Google Scholar] [CrossRef]
- Sobell, J.M. Update on TNF Inhibitors in Dermatology. Semin. Cutan. Med. Surg. 2016, 35, S104–S106. [Google Scholar] [CrossRef]



| Findings | Definitions | Suggestive Diseases * |
|---|---|---|
| Changes in hair shafts | ||
| Black dot | Fractured hair with dotted shape seen in hair ostia | AA |
| Broken hair | Short hair with dystrophic (or broken) peripheral ending | AA |
| Coudability hair (tapered hair) | Terminal hair with tapered proximal shaft | AA |
| Exclamation-mark hair (tapering hair) | Short and fractured hair with gradual thinning of proximal shaft | AA |
| Pohl-Pinkus constriction | Irregular narrowing of hair shaft | AA |
| Short vellus hair | Thin and hypopigmented short hair | AA (recovering phase) |
| Upright regrowing hair | Short regrowing hair with straight-up position and tapered distal ending | AA (recovering phase) |
| Changes in hair follicles | ||
| Absence of follicular openings | Disappearance of follicular openings | CA |
| Follicular keratotic plugging | Thick keratotic material filling follicular ostia | CA (DLE) |
| Follicular red dot | Erythematous polycyclic structure observed in and around follicular openings | CA (DLE) |
| White dots | Whitish fibrotic dotted area corresponding to disappeared follicular openings | CA (LPP) |
| Yellow dots | Yellowish keratotic material and/or sebum filling follicular ostia | AA, AGA/FPHL |
| Changes in perifollicular area | ||
| Blue-gray dot | Blue-to-grey-pigmented dot annularly distributed or randomly scattered around hair shaft | CA (LPP) |
| Follicular pustule | Pustule corresponding to hair follicle | CA (FD and DC) |
| Perifollicular erythema | Erythema observed around hair shafts | CA (LPP) |
| Perifollicular scale | Scale observed around hair shafts | CA (LPP) |
| Perifollicular whitish halo | Fibrotic whitish halo around hair shaft | CA |
| Changes on scalp | ||
| Pinpoint white dot | Small (<0.3 mm) white dot seen in interfollicular space | CCCA ** |
| White patch | Non-structured and hypopigmented fibrotic area | CA |
| Changes in hair distribution | ||
| Hair diameter diversity | Distribution of randomly thinned hairs within the same field | AGA/FPHL |
| Focal atrichia | Small area lacking hairs | AGA/FPHL |
| Tufted hairs | Multiple terminal hairs from one hair ostia | CA (FD) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinoshita-Ise, M.; Fukuyama, M.; Ohyama, M. Recent Advances in Understanding of the Etiopathogenesis, Diagnosis, and Management of Hair Loss Diseases. J. Clin. Med. 2023, 12, 3259. https://doi.org/10.3390/jcm12093259
Kinoshita-Ise M, Fukuyama M, Ohyama M. Recent Advances in Understanding of the Etiopathogenesis, Diagnosis, and Management of Hair Loss Diseases. Journal of Clinical Medicine. 2023; 12(9):3259. https://doi.org/10.3390/jcm12093259
Chicago/Turabian StyleKinoshita-Ise, Misaki, Masahiro Fukuyama, and Manabu Ohyama. 2023. "Recent Advances in Understanding of the Etiopathogenesis, Diagnosis, and Management of Hair Loss Diseases" Journal of Clinical Medicine 12, no. 9: 3259. https://doi.org/10.3390/jcm12093259
APA StyleKinoshita-Ise, M., Fukuyama, M., & Ohyama, M. (2023). Recent Advances in Understanding of the Etiopathogenesis, Diagnosis, and Management of Hair Loss Diseases. Journal of Clinical Medicine, 12(9), 3259. https://doi.org/10.3390/jcm12093259





