Abstract
Background: Whole-body vibration exercises (WBVE), that are generated in systemic vibratory therapy (SVT), may benefit individuals with chronic obstructive pulmonary disease (COPD). This study evaluated acute effects of SVT on the flexibility, on the perception of exertion to perform the anterior trunk flexion (ATF), and on the handgrip strength (HG). Methods: Thirty-eight individuals, separated into two groups, performed a single session of SVT (five bouts, 25 Hz, 2.5 of amplitude) on a side-alternating vibrating platform (SAVP), in two postures: sitting (Sitting group-SitG, n = 21) or standing (Stand group-StandG, n = 17). In both positions, the feet were on the base of the SAVP. The HG and the AFT were performed before and after the session, and the perception of effort (RPE) was measured during the ATF. Results: The ATF in the SitG (p ≤ 0.05) and in the StandG (p ≤ 0.05) was significantly improved, but in the comparison between both groups, no significant reduction was found (p = 0.14). The RPE was not influenced by the session. A significant increase of the HG in StandG post session (33.49 ± 10.30 kgf) p = 0.03 was found, but not in the SitG (p = 0.12) or between the two groups (p = 0.55). Conclusions: SVT, in a single acute session, would be capable of promoting some functional benefits for the COPD individuals without altering the perception of exertion to perform the ATF. Trial Registration: 49219115.3.0000.5259, RBR-72dqtm.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a pulmonary disease characterized by persistent airflow limitation [1], and extrapulmonary symptoms (systemic effects) lead to comorbidities and may contribute to the severity of the COPD [2]. The comorbidities due to the systemic effects might be loss of muscle mass, which, in turn, leads to inactivity, physical deconditioning [3], and lower limb muscle function impairment [4].
It is suggested that a decrease in muscle mass leads to a reduction in quadriceps femoris muscle strength, which is related to disuse and consequently to muscle atrophy [5]. Muscle atrophy is one of the main causes of decreased skeletal muscle strength, and lower limb circumference can predict mortality from COPD [6,7]. Moreover, decreased skeletal muscle strength and endurance exist in patients with mild COPD, even before the presence of respiratory symptoms [8].
A loss of flexibility in the lower limbs is also associated with COPD and is reflected in very rigid or very irregular movement patterns [9]. It is described that there is a reduction of muscle strength and functionality in elderly with COPD, a consequent increased risk of fall, and an intolerance to exercise leading to the adoption of a sedentary lifestyle [10].
Considering the physical frailty observed by Abdulai et al. 2018 [11] in COPD individuals, rehabilitation is beneficial and serves as an essential component in the management of COPD, since it improves health-related quality of life and exercise capacity [5,12]. Pulmonary rehabilitation (PR) involves various kinds of interventions, such as exercise. PR brings clinical improvements, such as the exercise capacity and skeletal muscle function of COPD sufferers [13], relief from dyspnea and fatigue, and an increased sense of disease control. However, many COPD individuals have a fear of exacerbating dyspnea during the PR [14]. In PR, different stretching strategies and protocols have been used to enhance flexibility or maintain health, acting on the muscle tendon–unit to improve the range of motion of the joints [15].
One type of exercise on the PR is the whole-body vibration exercise (WBVE), which has been suggested to COPD individuals [16,17]. WBVE is generated in an individual in the systemic vibratory therapy (SVT) [18,19,20] when mechanical vibration produced in a vibrating platform (VP) is transmitted to an individual [21] who is in contact with the base of the PV, which is turned on [22]. Acute [23] and cumulative effects [24] of SVT have improved the flexibility and rating of perceived exertion [25], lower limb strength and power [26], muscle tone and hamstring flexibility [27], and metabolic parameters [28,29]. SVT also leads to sequential muscle contraction and relaxation [30] that can lead to a maximum voluntary muscle strength that can be measured by the handgrip strength (HG) [31].
Zhou J. et al. 2018 [32] in a systematic review and meta-analysis investigated if the WBVE may be used to treat COPD patients. It was verified that WBVE has beneficial effects for COPD patients when compared with the control group. An increased 6 min walking distance (6-MWD) (p < 0.001), the reduction of the time to finish the five repeated sit-to-stand tests (p = 0.04), and improvement of St George’s Respiratory Questionnaire score (p < 0.001) were found.
Cardiorespiratory responses were used to investigate effects of WBVE with different mechanical vibration frequencies and two types of squatting exercises (static and dynamic) in COPD individuals. A decrease in the ratio of minute ventilation to oxygen production (e/o2), and in the ratio of minute ventilation to carbon dioxide production were found during static and dynamic squats. There was a significant difference with a reduction in oxygen saturation (40 Hz when compared with 30 Hz). The WBVE represented in this study, a mild effort that promoted cardiorespiratory response in COPD, had no adverse effect [33].
Gloeckl, et al., 2021 [34] pointed out that the WBVE improved physical performance in COPD individuals, considering the static balance performance (p = 0.032), the muscular power (p = 0.001), the 6-MWD test (p < 0.001), and the 4 m gait speed test (p = 0.018), compared with a control group.
The current study aimed to determine acute effects of SVT on the range of motion using the anterior trunk flexibility (ATF) test, on the perception of exertion across the Borg scale, and on the muscle strength across the handgrip strength in COPD elderly individuals. The hypothesis of this study was that the SVT could improve the functionality of COPD individuals.
2. Materials and Methods
2.1. Study Design
This is a quasi-experimental clinical trial. The recruitment of participants was carried out at Departamento de Pneumologia, Policlínica Universitária Piquet Carneiro (PPC), Universidade do Estado do Rio de Janeiro, Brazil and the clinical evaluation was performed by a medical staff. The subjects were referred to the Laboratório de Vibrações Mecânicas e Práticas Integrativas (LAVIMPI), also at PPC, to perform the protocol with SVT.
Inclusion and exclusion criteria were determined. The inclusion criteria were COPD individuals (i) of both gender, (ii) aged over 40 years old, (iii) being followed up at Departamento de Pneumologia of PPC with a diagnosis of COPD, (iv) stable (no exacerbation of the respiratory symptoms), (v) independent (able to stand on their own on the basis of the VP), and (vi) who signed the informed consent form (ICF).
The exclusion criteria were individuals with (a) exacerbation for less than three months, (b) presence of labyrinthitis, (c) reported osteoporosis, (d) other respiratory diseases, (e) use of a pacemaker, (f) previous history of fractures and/or other orthopedic diseases, (g) surgeries with implantation of metallic material, (h) peripheral vascular disease and/or thromboembolism, (i) severe tabagism and/or alcoholism, (j) decompensated cardiovascular disease, (k) aneurysm, (l) previous vitreous hemorrhage, (m) malnutrition, (n) recent postoperative period, (o) neurological disease that generates “fear” of movements in the VP, (p) severe or disabling clinical disease, and (q) at the discretion of the investigator.
2.2. Participants
Forty-eight COPD individuals were recruited. Thirty-eight concluded the acute SVT protocol and were allocated to two different postures on the SAVP: SitG (n = 21) e StandG (n = 17). Ten COPD individuals gave up for personal reasons (Figure 1).
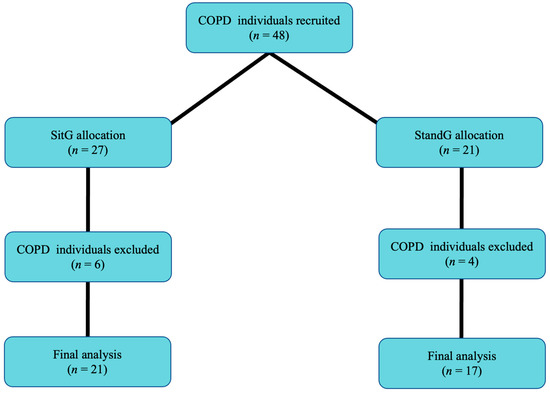
Figure 1.
Flow of participants through the study. Sitting group (SitG); Stand group (StandG).
2.3. Study Protocol (Procedures)
On the first day, the COPD individuals signed the ICF before any session. Personal data obtained in the anamnesis were collected: age, gender, tabagism, diabetes mellitus type 2 (DMT2), systemic arterial hypertension (SAH), time of illness diagnosis of COPD, other associated diseases, use of medications, physical inactivity, and family history. Functional tests and other clinical information were collated.
On the second day clinical and anthropometric data were collected before the session: height, body mass, body mass index (BMI), with bioimpedance device (InBody 370) partial oxygen saturation (pSO2), and respiratory rate (RR) with portable digital oximeter (G-tech). After this, the COPD individuals perfomed the acute session of the SVT protocol.
HG is a simple and commonly applied test to assess the person’s general strength level. HG can be used to predict the risk of cardiovascular disease and all-cause mortality in the general population [35], and also in COPD individuals [36]. HG strength was measured as proposed by American Society of Hand Therapists [37].
The individuals were in the seated position, arms in adduction, bent 90° forward at the elbow joint, forearm in neutral position, wrist with extension between 0° and 30°, and ulnar flexion between 0° and 15°. Subjects performed three maximum attempts with a manual dynamometer (EMG832WF, EMG System, São José dos Campos/SP) with the right hand, for 6 s each, verbal encouragement, and 30 s of rest. (Figure 2).

Figure 2.
COPD individual sitting in a chair with arm in adduction, with 90° forward bend at elbow joint, forearm in neutral position, wrist with extension between 0° and 30°, and ulnar flexion between 0° and 15°.
COPD individuals performed a single SVT acute session. The SVT protocol consisted of 5 bouts with a 1 min working time and resting time of 1 min after each working time, making a total of 25 min per session. Mechanical vibration with frequency of 25 Hz and peak-to-peak displacement of 2.5 mm on a side-alternating vibrating platform (SAVP) (Novaplate® Fitness Evolution, São Paulo, Brazil) was used.
The COPD individuals of the Sitting group (SitG) were sitting in an ancillary chair (Figure 3A) in front of the VP with the feet on the base of the VP and the hands maintained in contact with their knees. The individuals of the Stand group (StandG) were standing on the base of the SAVP (Figure 3B). The individuals in both groups had a squat position with the knees flexed at 120–130° (measured using a goniometer Carci®, São Paulo, Brazil).
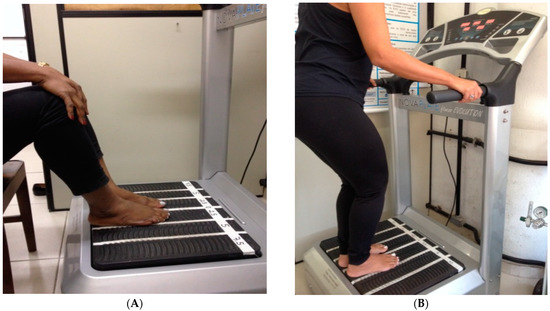
Figure 3.
(A) COPD individual sitting in a chair with the hands maintained in contact with the knees and the feet on the base of the platform. (B) COPD individual standing on the SAVP, with the feet on the base of the platform.
All procedures were supervised by a physiotherapist who followed the procedures and instructed the patient to report any discomfort.
The range of motion of the trunk in the anterior trunk flexibility evaluation was measured by the ATF test [30,38] that is also called the fingertip-to-floor distance (FTFD) test [39]. This test consisted in measuring the distance between of the tip of the third finger and the floor, expressed in centimeters, after an anterior trunk flexion, with feet together, without bending the knees [38]. The test was applied before and after the SVT session (Figure 4).

Figure 4.
COPD individual perfoming the anterior trunk flexion to measure the range of motion of the trunk (flexibiity).
The Rating of Perception of Effort (RPE) scale is a numerical scale from 6 to 20, ranging from “mild” (6 points) to “maximum effort” (20 points) [40] and aims to assess the individual’s perception of exertion, before and after the session of SVT [25].
The strength of muscles were analyzed across the HG strength of the right hand, using a dynamometer (EMG832WF, EMG System, São José dos Campos, Brazil), with standardized positioning and instruction [41,42]. The COPD individuals were in an upright position with the shoulder adducted and neutrally rotated, forearm in neutral position, and wrist between 0° and 30° dorsiflexion and between 0° and 15° of ulnar deviation. Three trials were performed with a 60-s rest in between and the average of the values (kgf) was used for the analysis [43].
2.4. Statistical Analysis
The sample size was calculated considering a type I error (α) of 5% and a type II error (β) of 20%. A standard deviation of the observed difference before and after the session of 2.5 was also considered, and the minimum difference to be detected was 2 (hypothetically defined according to preliminary results). Sixteen individuals were calculated for each group [44]. Data were entered and stored using Microsoft® Excel 365. The statistical analysis was performed using GraphPad Prism 6.0. The Shapiro–Wilk test was used to evaluate the normality of the data. Confirming the normality of data, a paired Student t test was performed (intragroup). A Mann–Whitney U test was used for intergroup analysis. Pearson correlation analysis was performed. The characteristics of the participants were summarized using means and standard deviations (SD) or frequencies and percentages, as appropriate. For categorical variables, the Chi-square test was used. The significance level of p ≤ 0.05 was considered.
3. Results
Individual Characteristics
The anthropometric characteristics and clinical data of the COPD individuals of both groups in the baseline are presented in Table 1. No differences were found between the groups.

Table 1.
Anthropometric characteristics and clinical data of the COPD individuals.
About the range of motion of the trunk (ATF), a significant reduction of FTFD was found for the SitG, pre-session (19.62 ± 4.88 cm) and post session (17.29 ± 5.38 cm), p < 0.05. It was also found in the StandG, pre-session (16.55 ± 10.50 cm) and post session (13.41 ± 10.16 cm), p < 0.05. However, comparing both groups, no significant reduction was found (p = 0.14).
The RPE was measured during the ATF. No significant difference was found in both the paired groups (SitG—9.38 ± 3.26 × 8.61 ± 2.95, p = 0.13 and StandG—8.29 ± 3.07 × 8.05 ± 2.90, p = 0.62), or between the groups, (p = 0.48).
Analyzing the HG in the SitG pre-session (30.24 ± 8.44 kgf) and post session (31.61 ± 8.91 kgf), p = 0.12, no significant increase was observed. Comparing the StandG pre-session (28.64 ± 8.46 kgf) and post session (33.49 ± 10.30 kgf), p = 0.03, a significant increase of the HG was found (Figure 5).
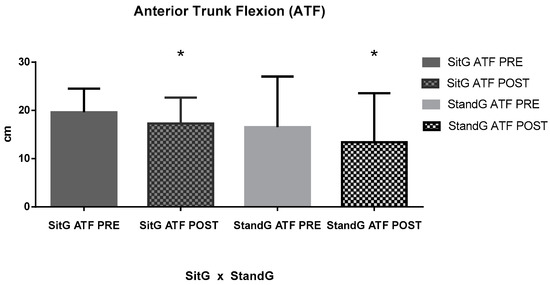
Figure 5.
Anterior trunk flexion (ATF); Sitting group (SitG); Stand group (StandG). ATF measure of the Sitting group in pre-session (SiTG ATF PRE); ATF measure of the Sitting group in post-session (SiTG ATF POST); ATF measure of the Standing group in pre-session (StandG ATF PRE); ATF measure of the Standing group in post-session (StandG ATF POST). * p ≤ 0.05 pre × post session.
Pearson correlation analysis (r) between HG parameters and the ATF and the RPE of the ATF was performed. Considering the correlation between ATF and HG, SitG presented a negative correlation (r = −0.17, p = 0.46) while the StandG presented a positive correlation between these variables (r = 0.34, p = 0.17). The correlation analysis between RPE and ATF showed a positive correlation for SitG (r = 0.20, p = 0.37) and negative for StandG (r = −0.26, p = 0.30). A negative correlation was observed between the RPE and HG in SitG (r = −0.002, p = 0.99), while a positive correlation was found in StandG (r = 0.13, p = 0.61) (Figure 6).
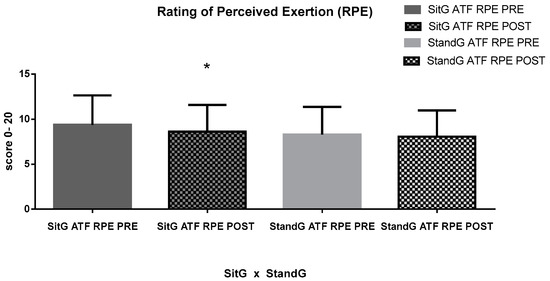
Figure 6.
RPE of the ATF. Rating of Perceived Exertion (RPE); ATF—anterior trunk flexion (ATF); Sitting group (SitG); Stand group (StandG). Rating of Perceived Exertion of the anterior trunk flexion by the Sitting group during the pre-session (SitG ATF RPE PRE); Rating of Perceived Exertion of the anterior trunk flexion by the Sitting group during the post-session (SitG ATF RPE POST); Rating of Perceived Exertion of the anterior trunk flexion by the Standing group during the pre-session (StandG ATF RPE PRE); Rating of Perceived Exertion of the anterior trunk flexion by the Sitting group during the post-session (StandG ATF RPE POST). * p ≤ 0.05 pre vs. post session.
Figure 7 shows the muscle strength of the COPD individuals in both groups, across the HG measures. Analyzing the SitG pre session (30.24 ± 8.44 kgf) vs. the SitG post session (31.61 ± 8.91 kgf), p = 0.12, no significant increase of the HG is observed. However, comparing the StandG pre session (28.64 ± 8.46 kgf) vs. StandG post session (33.49 ± 10.30 kgf), p = 0.03, a significant increase of the HG was found. The unpaired t-test showed that no significant difference was observed between the two groups (p = 0.55).
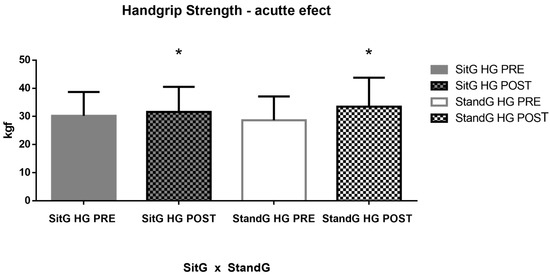
Figure 7.
Acute effects of the SVT on the muscle strength by the HG. Handgrip (HG); Sitting group (SitG); Stand group (StandG). Handgrip measure of the Sitting group at the pre-session (SitG HG PRE); Handgrip measure of the Sitting group at the post-session (SitG HG POST); Handgrip measure of the Standing group at the pre-session (StandG HG PRE); Handgrip measure of the Standing group at the post-session (StandG HG POST). * p ≤ 0.05 pre vs. post session.
Pearson correlation analysis (r) between HG parameters and the ATF and the RPE of the ATF was performed and is represented in Figure 8A–F. Considering the correlation between ATF and HG (Figure 8A,B), SitG presented a negative correlation (r = − 0.17, p = 0.46) while the StandG presented a correlation between these variables (r = 0.34, p = 0.17). The correlation analysis between RPE and ATF (Figure 8C,D) showed a positive correlation for SitG (r = 0.20, p = 0.37) and negative for StandG (r = − 0.26, p = 0.30). A negative correlation was observed between the RPE and HG in SitG (Figure 8E, r = − 0.002, p = 0.99), while a positive correlation was found between the RPE and HG in StandG (Figure 8F, r = 0.13, p = 0.61).
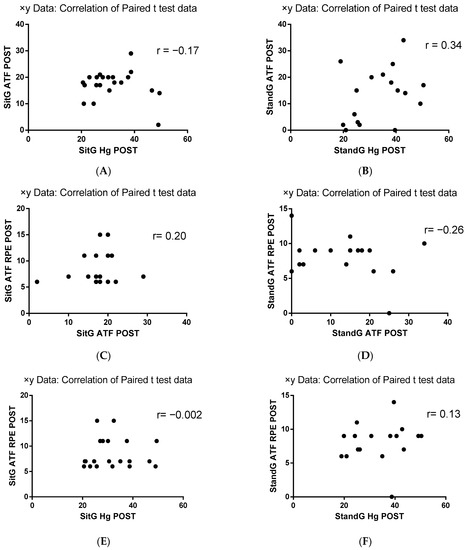
Figure 8.
Pearson correlation (r) analysis. RPE—Rating of Perceived Exertion; ATF—anterior trunk flexion (ATF); Handgrip strength (HG); Sitting group (SitG); Stand group (StandG). (A) Pearson correlation of ATF × HG of the SitG. (B) Pearson correlation of ATF x HG of the StandG. (C) Pearson correlation of ATF × ATF RPE of the SitG. (D) Pearson correlation of ATF × ATF RPE of the StandG (StandG ATF POST). (E) Pearson correlation of ATF x HG of the SitG. (F) Pearson correlation of ATF × HG of the StandG.
4. Discussion
As was hypothesized, the current study demonstrates the possible benefits of SVT to increase the functionality of COPD individuals.
In the analysis of the acute effects of the SVT on the muscle strength of the COPD individuals, across the HG measures, StandG presented a significant increase (pre session versus post session). However, no significant difference was observed between the two groups.
According to Coelho-Oliveira et al., 2021 [42], SVT probably reduces the muscle activation levels after the SVT session, suggesting that fewer motor units were required to perform the same handgrip activity. However, a study carried out by Strandkvist et al., 2020 [45] evaluated 389 individuals with COPD in a sitting position and concluded that there was no clear association between HG strength and the level of physical activity. Cheung et al., 2013 [46] investigated the relationship of handgrip strength and chronic diseases and multimorbidity in an aged population analyzing data from 748 men and 397 women. The study concluded handgrip strength is associated with multiple chronic diseases in men and women. HG would be a biomarker of multiple physiological systems. Its augmentation may be a feasible strategy to improve general health and decrease likelihood of having multiple chronic diseases and, hence, premature mortality. In addition, handgrip strength appeared to be a more useful marker of multimorbidity than chronological age in men.
Sá-Caputo et al., 2019 [29] concluded an exploratory study with the aim to assess effects of SVT on functional parameters with chronic disease individuals. The hypothesis was that the SVT could improve functionality in these individuals. Corroborating our study, Sá-Caputo et al., 2019 [29] showed a significant difference (p = 0.04), with an increase in the HS, found after the SVT protocol.
The relevance of the increase of the HG strength in this current study can be attributed to some physiological results pointed out in some studies involving this same population. Strandkvist et al., 2020 [45] reported that, among men with COPD, HG strength was associated with fatigue independent of physical activity level. Tsuburai et al., 2022 [47] pointed out that HG strength might be more useful for predicting peak inspiratory flow (PIF) than other parameters. Turan et al., 2019 [48] reinforced the idea that HG strength may be used as a measure of muscle performance in COPD exacerbation, especially when the 6-MWD test cannot be performed.
The range of motion of the trunk (trunk flexibility) was also assessed in this current study. Across the ATF, a significant reduction of this distance between the finger to the ground was found in both groups separately, considering the pre session and the post session. Pfeifer et al., 2022 [49] pointed out that flexibility is an important factor for individuals to successfully perform activities of daily living, and decreased flexibility is not only associated with a greater risk of falling, but also difficulty performing body movements.
Cardinale and Bosco, 2003 [50] verified that the muscle activation due to SVT can induce improvements in muscle performance and an increase in flexibility due to exercise that is generated through the vibration produced in VP. In addition, there is strong evidence of the effects of SVT on fitness, including improvements in the flexibility of the lower limbs [38,51,52].
For the population investigated in this current study, an increase in the range of the motion of the trunk (flexibility) assessed by the FTFD [39] can be identified as an acute effect of SVT, helping to improve the functional capacity of COPD individuals, considering the biomechanical limitations in the thoracic region and spine due to pathological pulmonary mechanics of the COPD individuals [53].
The RPE during the ATF presented no significant difference, considering the pre session and the post session. It was not influenced by the session and the SVT can be considered a safe and feasible type of exercise. Furness et al., 2014 [16] also investigated COPD individuals and concluded that SVT was an efficacious mode of exercise for this population. SVT does not negatively affect the exercise tolerance or exacerbate the disease, while concurrently improving the functional performance of the lower limbs. SVT would increase neuromuscular spindle activity, triggering a reflex–stretch response [54] and consequently create a small and rapid change in muscle length [55]. Thus, SVT seems to benefit COPD individuals by improving the functional exercise capacity, without producing adverse effects [56].
Although it is understood that sarcopenia may be present in most of the individuals analyzed, there were no reports of falls or unpleasant symptoms that may have arisen during SVT. The sitting posture proposed in this study and evaluated in other studies involving populations with chronic disorders [42,57] reinforce the relevance of the SVT and it would be recommended that an exercise modality be included in PR.
The reduced functional exercise capacity and musculoskeletal function have been investigated as important extrapulmonary consequences of the COPD, and SVT has been suggested as an intervention with positive impacts in the COPD individuals. Gloeckl et al., 2021 [34] pointed out that the SVT can significantly improve the physical performance in COPD patients. Furness et al., 2014 [16] found positive effects of SVT on muscle strength, physical performance, and quality of life for COPD individuals. It is also reinforced that the SVT did not exacerbate symptoms of COPD that can be associated with physical inactivity. Lage et al., 2018 [58] reported that SVT has gained prominence in the rehabilitation of COPD individuals because it is a safe low-intensity exercise that leads to beneficial effects on physical performance and quality of life.
Exercise training has great potential benefits that can improve functional status [59] of COPD individuals. However, starting a physical program is challenging for patients after an exacerbation, and more time may be needed to find the appropriate exercise protocol for an individual patient [60]. Then, the SVT has been gaining prominence as an exercise modality option in PR [16,17,56].
Considering the correlation analysis between ATF and HG, a positive correlation was found for StandG (both p ≤ 0.05), indicating that in this group, the improvement of the range of motion can be associated with better HG parameters; between RPE and ATF (Figure 8C,D) (r = 0.20, p = 0.37), a positive correlation was found for SitG, confirming that the perceived effort during the trunk range of motion test did not increase among SitG participants. It is relevant to highlight that all correlations were low or very low.
Still, the performance of the SVT in the sitting posture (SitG) has seemed to be more adequate regarding the RPE, while performing the SVT in the standing posture (StandG) seems to favor the gain of HG strength in the COPD individuals.
Limitations
The current study has some limitations such as the study design being without a control group. Moreover, the fingertip-to-floor distance was used. However, the sit-and-reach or straight-leg-rise test would be recommended due to their validity and reliability.
5. Conclusions
The COPD individuals may benefit from the SVT promoting physiological responses that contribute to improved functional parameters, such as muscle strength and flexibility, in a single session of SVT. It is suggested that WBV exercise would be suitable for COPD individuals and justify the inclusion of this type of exercise in PR protocols.
Author Contributions
Conceptualization, E.d.O.G.-A.; methodology, B.B.M.-O.; software, L.L.P.-D.; validation, E.d.O.G.-A., B.B.M.-O. and L.L.P.-D.; formal analysis, E.d.O.G.-A.; investigation, E.d.O.G.-A.; resources, D.d.C.d.S.-C. and M.B.-F.; data curation, E.d.O.G.-A.; writing—original draft preparation, E.d.O.G.-A.; writing—review and editing, R.T.; visualization, R.T.; supervision, R.T.; project administration, D.d.C.d.S.-C. and M.B.-F.; funding acquisition, D.d.C.d.S.-C. and M.B.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The current study was approved by the Ethics Committee on Human Research of the Hospital Universitário Pedro Ernesto (HUPE) of Universidade do Estado do Rio de Janeiro (UERJ) and registered in Plataforma Brasil under number (CAAE: 49219115.3.0000.5259) and in the Registro de Brasileiro de Ensaios Clínicos (ReBEC) under number RBR-72dqtm. The principles from the Declaration of Helsinki were followed.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Universidade do Estado do Rio de Janeiro (UERJ) and the Fundação de Amparo à pesquisa do Estado do Rio de Janeiro (FAPERJ) for the academic support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Global Initiative for Chronic Obstructive Lung Disease—Report. Global Initiative for Chronic Obstructive Lung Disease. 2023. Available online: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf (accessed on 6 July 2022).
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 15, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Pascual, S.; Casadevall, C.; Orozco-Levi, M.; Barreiro, E. Muscle dysfunction in chronic obstructive pulmonary disease: Update on causes and biological findings. J. Thorac. Dis. 2015, 7, E418–E438. [Google Scholar] [CrossRef] [PubMed]
- Rassler, B. Impaired function of upper limb muscles in patients with chronic obstructive pulmonary disease. Indian J. Med. Res. 2013, 138, 443–445. [Google Scholar] [PubMed]
- Barreiro, E.; Jaitovich, A. Muscle atrophy in chronic obstructive pulmonary disease: Molecular basis and potential therapeutic targets. J. Thorac. Dis. 2018, 10 (Suppl. S12), S1415–S1424. [Google Scholar] [CrossRef]
- Ho, G.J.; Liew, S.M.; Ng, C.J.; Hisham Shunmugam, R.; Glasziou, P. Development of a search strategy for an evidence based retrieval service. PLoS ONE 2016, 11, e0167170. [Google Scholar] [CrossRef]
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigaré, R.; Dekhuijzen, P.N.R.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. An official american thoracic society/european respiratory society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189, 15–62. [Google Scholar] [CrossRef]
- Rejc, E.; Floreani, M.; Taboga, P.; Botter, A.; Toniolo, L.; Cancellara, L.; Narici, M.; Šimunič, B.; Pišot, R.; Biolo, G.; et al. Loss of maximal explosive power of lower limbs after 2 weeks of disuse and incomplete recovery after retraining in older adults. J. Physiol. 2018, 15, 647–665. [Google Scholar] [CrossRef]
- Janssens, T.; Van de Moortel, Z.; Geidl, W.; Carl, J.; Pfeifer, K.; Lehbert, N.; Wittmann, M.; Schultz, K.; von Leupoldt, A. Impact of disease-specific fears on pulmonary rehabilitation trajectories in patients with COPD. J. Clin. Med. 2019, 8, 1460. [Google Scholar] [CrossRef]
- Adolfo, J.R.; Dhein, W.; Sbruzzi, G. Intensity of physical exercise and its effect on functional capacity in COPD: Systematic review and meta-analysis. J. Bras. Pneumol. 2019, 45, 20180011. [Google Scholar] [CrossRef]
- Abdulai, R.M.; Jensen, T.J.; Patel, N.R.; Polkey, M.I.; Jansson, P.; Celli, B.R.; Rennard, S.I. Deterioration of limb muscle function during acute exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 15, 433–449. [Google Scholar] [CrossRef]
- Mccarthy, B.; Casey, D.; Devane, D.; Murphy, K.; Murphy, E.; Lacasse, Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2020, 16, CD012626. [Google Scholar] [CrossRef]
- Wouters, E.F.; Posthuma, R.; Koopman, M.; Liu, W.-Y.; Sillen, M.J.; Hajian, B.; Sastry, M.; Spruit, M.A.; Franssen, F.M. An update on pulmonary rehabilitation techniques for patients with chronic obstructive pulmonary disease. Expert Rev. Respir. Med. 2020, 14, 149–161. [Google Scholar] [CrossRef]
- Corhay, J.L.; Nguyen, D.; Van Cauwenberge, H.; Louis, R. Pulmonary rehabilitation and COPD: Providing patients a good environment for optimizing therapy. Int. J. Chron Obstruct. Pulm. Dis. 2014, 9, 27–39. [Google Scholar] [CrossRef]
- Opplert, J.; Babault, N. Acute effects of dynamic stretching on muscle flexibility and performance: An analysis of the current literature. Sports Med. 2018, 48, 299–325. [Google Scholar] [CrossRef]
- Furness, T.; Joseph, C.; Naughton, G.; Welsh, L.; Lorenzen, C. Benefits of whole-body vibration to people with COPD: A community-based efficacy trial. BMC Pulm. Med. 2014, 8, 14–38. [Google Scholar] [CrossRef]
- Gloeckl, R.; Heinzelmann, I.; Baeuerle, S.; Damm, E.; Schwedhelm, A.-L.L.; Diril, M.; Buhrow, D.; Jerrentrup, A.; Kenn, K. Effects of whole body vibration in patients with chronic obstructive pulmonary disease—A randomized controlled trial. Respir. Med. 2012, 1, 75–83. [Google Scholar] [CrossRef]
- Bernardo-Filho, M.; Taiar, R.; Sañudo, B.; Furness, T. Clinical Approaches of Whole Body Vibration Exercises. Rehabil. Res. Pract. 2018, 21, 9123625. [Google Scholar] [CrossRef]
- Sañudo, B.; Seixas, A.; Gloeckl, R.; Rittweger, J.; Rawer, R.; Taiar, R.; van der Zee, E.A.; van Heuvelen, M.J.; Lacerda, A.C.; Sartorio, A.; et al. Potential application of whole body vibration exercise for improving the clinical conditions of COVID-19 infected individuals: A narrative review from the world association of vibration exercise experts (wavex) panel. Int. J. Environ. Res. Public Health 2020, 17, 3650. [Google Scholar] [CrossRef]
- Sá-Caputo, D.C.; Seixas, A.; Taiar, R.; Bernardo-Filho, M. Vibration therapy for health promotion. In Complementary Therapies; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- van Heuvelen, M.J.G.; Rittweger, J.; Judex, S.; Sañudo, B.; Seixas, A.; Fuermaier, A.B.M.; Tucha, O.; Nyakas, C.; Marín, P.J.; Taiar, R.; et al. Reporting guidelines for whole-body vibration studies in humans, animals and cell cultures: A consensus statement from an international group of experts. Biology 2021, 10, 965. [Google Scholar] [CrossRef]
- Rittweger, J. Vibration as an exercise modality: How it may work, and what its potential might be. Eur. J. Appl. Physiol. 2010, 108, 877–904. [Google Scholar] [CrossRef]
- Sá-Caputo, D.C.; Paineiras-Domingos, L.L.; Oliveira, R.; Neves, M.F.T.; Brandão, A.; Marin, P.J.; Sañudo, B.; Furness, T.; Taiar, R.; Bernardo-Filho, M. Acute effects of whole-body vibration on the pain level, flexibility, and cardiovascular responses in individuals with metabolic syndrome. Dose-Response 2018, 16, 1559325818802139. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Marconi, E.; Dionello, C.F.; Morel, D.S.; Sá-Caputo, D.C.; Sousa-Gonçalves, C.R.; Paineiras-Domingos, L.L.; Teixeira-Silva, Y.; Pereira, M.J.S.; Bernardo-Filho, M. Whole body vibration and auriculotherapy improve handgrip strength in individuals with knee osteoarthritis. J. Tradit. Chin. Med. 2019, 39, 707–715. [Google Scholar]
- Paiva, P.C.; Figueiredo, C.A.; Reis-Silva, A.; Francisca-Santos, A.; Paineiras-Domingos, L.L.; Martins-Anjos, E.; Melo-Oliveira, M.E.S.; Moreira-Marconi, E.; Guedes-Aguiar, E.O.; Xavier, V.L.; et al. Acute and cumulative effects with whole-body vibration exercises using 2 biomechanical conditions on the flexibility and rating of perceived exertion in individuals with metabolic syndrome: A randomized clinical trial pilot study. Dose-Response 2019, 17, 1559325819886495. [Google Scholar] [CrossRef] [PubMed]
- Karatrantou, K.; Bilios, P.; Bogdanis, G.C.; Ioakimidis, P.; Soulas, E.; Gerodimos, V. Effects of whole-body vibration training frequency on neuromuscular performance: A randomized controlled study. Biol. Sport 2019, 36, 273–282. [Google Scholar] [CrossRef]
- Saldıran, T.Ç.; Atıcı, E.; Rezaei, D.A.; Öztürk, Ö.; Uslu, B.; Özcan, B.A.; Melo-Oliveira, M.E.S.; Lourenço-Revelles, G.M.G.; Moreira-Marconi, E.; Guedes-Aguiar, E.O.; et al. The acute effects of different intensity whole-body vibration exposure on muscle tone and strength of the lower legs, and hamstring flexibility: A pilot study. J. Sport Rehabil. 2021, 30, 235–241. [Google Scholar] [CrossRef]
- Paineiras-Domingos, L.L.; da Cunha Sá-Caputo, D.; Reis, A.S.; Francisca Santos, A.; Sousa-Gonçalves, C.R.; dos Anjos, E.M.; Pereira, M.J.d.S.; Sartorio, A.; Bernardo-Filho, M. Assessment through the short physical performance battery of the functionality in individuals with metabolic syndrome exposed to whole-body vibration exercises. Dose-Response 2018, 1, 16. [Google Scholar] [CrossRef]
- Sá-Caputo, D.C.; Paineiras-Domingos, L.L.; Francisca-Santos, A.; dos Anjos, E.M.; Reis, A.S.; Neves, M.F.T.; Oigman, W.; Oliveira, R.; Brandão, A.; Machado, C.B.; et al. Whole-body vibration improves the functional parameters of individuals with metabolic syndrome: An exploratory study. BMC Endocr. Disord. 2019, 19, 6. [Google Scholar] [CrossRef]
- Lamari, N.; Marino, L.C.; Cordeiro, J.A.; Pellegrini, A.M. Flexibilidade anterior do tronco no adolecente após o pico da velocidade de crescimento em estatura. Acta Ortop. Bras. 2007, 15, 25–29. [Google Scholar] [CrossRef]
- Lee, S.Y. Handgrip strength: An irreplaceable indicator of muscle function. Ann. Rehabil. Med. 2021, 45, 167–169. [Google Scholar] [CrossRef]
- Zhou, J.; Pang, L.; Chen, N.; Wang, Z.; Wang, C.; Hai, Y.; Lyu, M.; Lai, H.; Lin, F. Whole-body vibration training—Better care for COPD patients: A systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulm. Dis. 2018, 10, 3243–3254. [Google Scholar] [CrossRef]
- Lage, V.K.S.; Lacerda, A.C.R.; Neves, C.D.C.; Chaves, M.G.A.; Soares, A.A.; Lima, L.P.; Matos, M.A.; Leite, H.R.; Fernandes, J.S.C.; Oliveira, V.C.; et al. Cardiorespiratory responses in different types of squats and frequencies of whole body vibration in patients with chronic obstructive pulmonary disease. J. Appl. Physiol. 2019, 126, 23–29. [Google Scholar] [CrossRef]
- Gloeckl, R.; Schneeberger, T.; Leitl, D.; Reinold, T.; Nell, C.; Jarosch, I.; Kenn, K.; Koczulla, A.R. Whole-body vibration training versus conventional balance training in patients with severe COPD—A randomized, controlled trial. Resp. Res. 2021, 4, 138. [Google Scholar] [CrossRef]
- Jeong, M.; Kang, H.K.; Song, P.; Park, H.K.; Jung, H.; Lee, S.-S.; Koo, H.-K. Hand grip strength in patients with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulm. Dis. 2017, 12, 2385–2390. [Google Scholar] [CrossRef]
- Fonseca, J.; Machado, F.V.C.; Santin, L.C.; Medeiros, L.; Andrello, A.C.; Hernandes, N.A.; Pitta, F. Use of different reference values for handgrip strength in individuals with COPD: Analysis of agreement, discriminative capacity, and main clinical implications. J. Bras. Pneumol. 2022, 48, e20210510. [Google Scholar] [CrossRef]
- Severijns, D.; Lamers, I.; Kerkhofs, L.; Feys, P. Hand grip fatigability in persons with multiple sclerosis according to hand dominance and disease progression. J. Rehabil. Med. 2015, 47, 154–160. [Google Scholar] [CrossRef]
- Sá-Caputo, D.C.; Ronikeili-Costa, P.; Carvalho-Lima, R.P.P.; Bernardo, L.C.C.; Bravo-Monteiro, M.O.O.; Costa, R.; De Moraes-Silva, J.; Paiva, D.N.; Machado, C.B.; Mantilla-Giehl, P.; et al. Whole-body vibration exercises and the improvement of the flexibility in patient with metabolic syndrome. Rehabil. Res. Pract. 2014, 2014, 628518. [Google Scholar] [CrossRef]
- Perret, C.; Poiraudeau, S.; Fermanian, J.; Colau, M.M.; Benhamou, M.A.; Revel, M. Validity, reliability, and responsiveness of the fingertip-to-floor test. Arch. Phys. Med. Rehabil. 2001, 82, 566–570. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Fess, E.E. A method for checking Jamar dynamometer calibration. J. Hand Ther. 1987, 1, 28–32. [Google Scholar] [CrossRef]
- Coelho-Oliveira, A.C.; Lacerda, A.C.R.; de Souza, A.L.C.; Santos, L.M.M.; Fonseca, S.F.; dos Santos, J.M.; Ribeiro, V.G.C.; Leite, H.R.; Figueiredo, P.H.S.; Fernandes, J.S.C.; et al. Acute whole-body vibration exercise promotes favorable handgrip neuromuscular modifications in rheumatoid arthritis: A cross-over randomized clinical. BioMed Res. Int. 2021, 2, 9774980. [Google Scholar] [CrossRef]
- Sousa-Gonçalves, C.R.; Tringali, G.; Tamini, S.; De Micheli, R.; Soranna, D.; Taiar, R.; Sá-Caputo, D.; Moreira-Marconi, E.; Paineiras-Domingos, L.; Bernardo-Filho, M.; et al. Acute effects of whole-body vibration alone or in combination with maximal voluntary contractions on cardiorespiratory, musculoskeletal, and neuromotor fitness in obese male adolescents. Dose-Response 2019, 17, 1559325819890492. [Google Scholar] [CrossRef]
- Ahmadi, A.; Eftekhari, M.H.; Mazloom, Z.; Masoompour, M.; Fararooei, M.; Eskandari, M.H.; Mehrabi, S.; Bedeltavana, A.; Famouri, M.; Zare, M.; et al. Fortified whey beverage for improving muscle mass in chronic obstructive pulmonary disease: A single-blind, randomized clinical trial. Resp. Res. 2020, 21, 216. [Google Scholar] [CrossRef]
- Strandkvist, V.; Andersson, M.; Backman, H.; Larsson, A.; Stridsman, C.; Lindberg, A. Hand grip strength is associated with fatigue among men with COPD: Epidemiological data from northern Sweden. Physiother. Theory Pract. 2020, 36, 408–416. [Google Scholar] [CrossRef]
- Cheung, C.-L.; Nguyen, U.-S.D.T.; Au, E.; Tan, K.C.B.; Kung, A.W.C. Association of handgrip strength with chronic diseases and multimorbidity. Age 2013, 35, 929–941. [Google Scholar] [CrossRef]
- Tsuburai, T.; Komase, Y.; Tsuruoka, H.; Oyama, B.; Muraoka, H.; Hida, N.; Kobayashi, T.; Matsushima, S. The relationship between peak inspiratory flow and hand grip strength measurement in men with mild chronic obstructive pulmonary disease. BMC Pulm. Med. 2022, 22, 65. [Google Scholar] [CrossRef]
- Turan, Z.; Özyemişçi Taşkıran, Ö.; Erden, Z.; Köktürk, N.; Kaymak Karataş, G. Does hand grip strength decrease in chronic obstructive pulmonary disease exacerbation? A cross-sectional study. Turk. J. Med. Sci. 2019, 49, 802–808. [Google Scholar] [CrossRef]
- Pfeifer, C.E.; Ross, L.M.; Weber, S.R.; Sui, X.; Blair, S.N. Are flexibility and muscle-strengthening activities associated with functional limitation? Sports Med. Health Sci. 2022, 4, 95–100. [Google Scholar] [CrossRef]
- Cardinale, M.; Bosco, C. The use of vibration as an exercise session. Exerc. Sport Sci. Rev. 2003, 31, 3–7. [Google Scholar] [CrossRef]
- Di Giminiani, R.; Manno, R.; Scrimaglio, R.; Sementilli, G.; Tihanyi, J. Effects of individualized whole-body vibration on muscle flexibility and mechanical power. J. Sports Med. Phys. Fit. 2010, 50, 139–151. [Google Scholar]
- Fowler, B.D.; Palombo, K.T.M.; Feland, J.B.; Blotter, J.D. Effects of whole-body vibration on flexibility and stiffness: A literature review. Int. J. Exerc. Sci. 2019, 12, 735–747. [Google Scholar]
- Kerti, M.; Balogh, Z.; Kelemen, K.; Varga, J.T. The relationship between exercise capacity and different functional markers in pulmonary rehabilitation for COPD. Int. J. Chron. Obstruct. Pulm. Dis. 2018, 13, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Ritzmann, R.; Kramer, A.; Gruber, M.; Gollhofer, A.; Taube, W. EMG activity during whole body vibration: Motion artifacts or stretch reflexes? Eur. J. Appl. Physiol. 2010, 110, 143–151. [Google Scholar] [CrossRef]
- Cochrane, D.J.; Stannard, S.R.; Firth, E.C.; Rittweger, J. Acute whole-body vibration elicits post-activation potentiation. Eur. J. Appl. Physiol. 2010, 108, 311–319. [Google Scholar] [CrossRef]
- Cardim, A.B.; Marinho, P.E.; Nascimento, J.F.; Fuzari, H.K.; Dornelas de Andrade, A. Does whole-body vibration improve the functional exercise capacity of subjects with COPD? A meta-analysis. Respir. Care 2016, 61, 1552–1559. [Google Scholar] [CrossRef]
- Torres-Nunes, L.; da Costa-Borges, P.P.; Paineiras-Domingos, L.L.; Bachur, J.A.; Coelho-Oliveira, A.C.; da Cunha de Sá-Caputo, D.; Bernardo-Filho, M. Effects of the whole-body vibration exercise on sleep disorders, body temperature, body composition, tone, and clinical parameters in a child with down syndrome who underwent total atrioventricular septal defect surgery: A case-report. Children 2023, 10, 213. [Google Scholar] [CrossRef]
- Lage, V.K.S.; Lacerda, A.C.R.; Neves, C.D.C.; Chaves, M.G.A.; Soares, A.A.; Lima, L.P.; Martins, J.B.; Matos, M.A.; Vieira, L.M.; Teixeira, A.L.; et al. Acute effects of whole-body vibration on inflammatory markers in people with chronic obstructive pulmonary disease: A pilot study. Rehabil. Res. Pract. 2018, 2, 5480214. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Song, J.K.; Hong, S.C.; Choi, J.W.; Jeon, H.J.; Shin, D.H.; Ji, E.H.; Choi, E.-H.; Lee, J.; Kim, A.; et al. Intradialytic exercise improves physical function and reduces intradialytic hypotension and depression in hemodialysis patients. Korean J. Intern. Med. 2019, 34, 588–598. [Google Scholar] [CrossRef]
- Puhan, M.A.; Gimeno-Santos, E.; Cates, C.J.; Troosters, T. Pulmonary rehabilsitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2009, 21, CD005305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).