Canagliflozin Attenuates PromarkerD Diabetic Kidney Disease Risk Prediction Scores
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. PromarkerD Scores
2.3. Statistical Analysis PromarkerD Scores
3. Results
3.1. Baseline Participant Characteristics
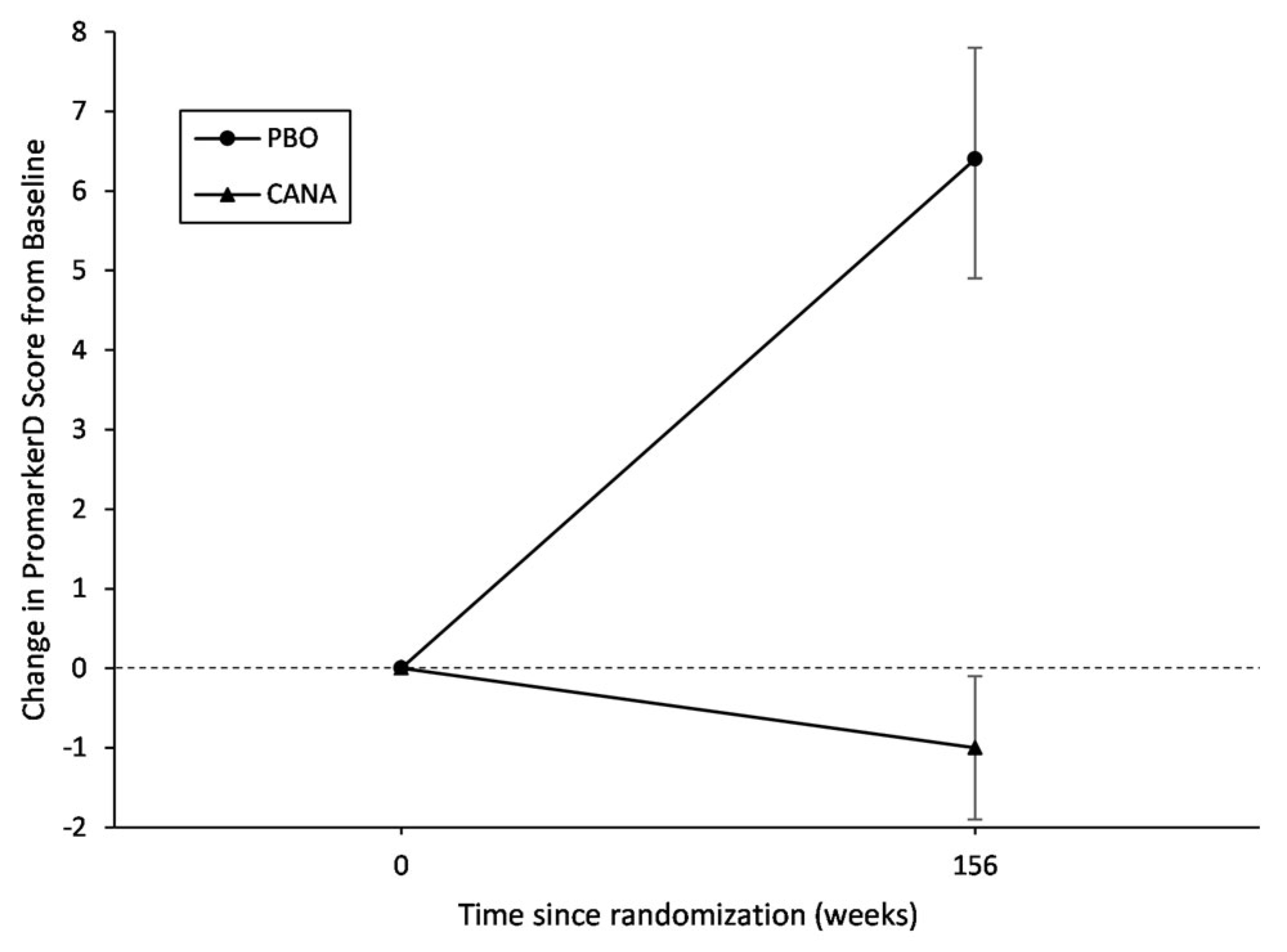
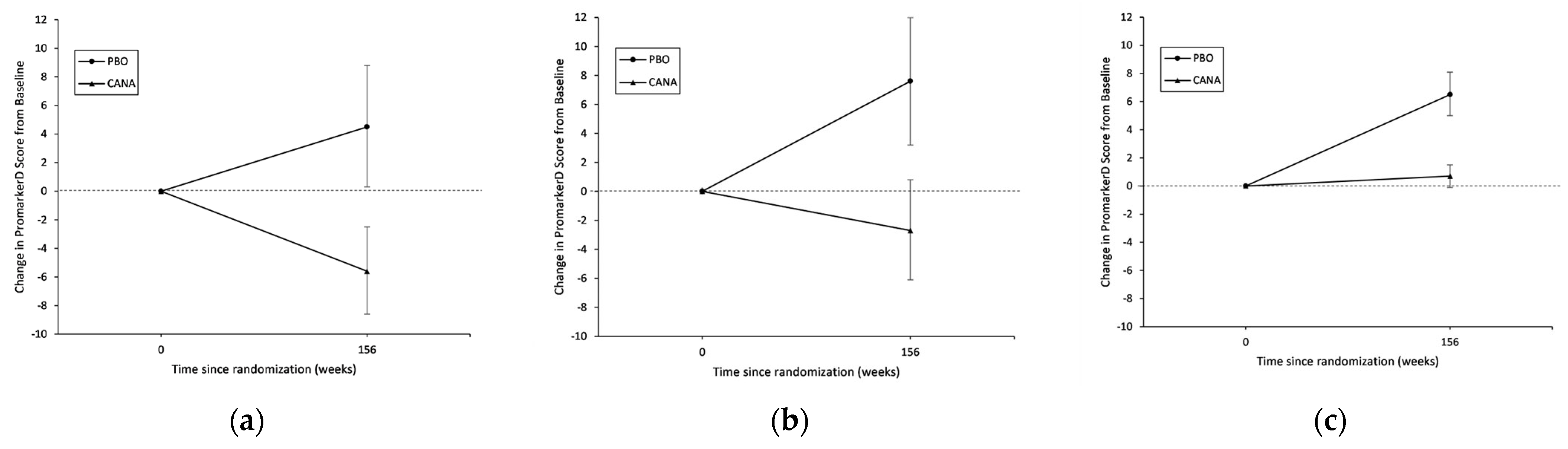
3.2. Effect of Canagliflozin on Change in PromarkerD Score
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Koye, D.N.; Magliano, D.J.; Reid, C.M.; Jepson, C.; Feldman, H.I.; Herman, W.H.; Shaw, J.E. Risk of Progression of Nonalbuminuric CKD to End-Stage Kidney Disease in People With Diabetes: The CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2018, 72, 653–661. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. United States Renal Data System 2022 Annual Data Report. Available online: https://usrds-adr.niddk.nih.gov/2022 (accessed on 15 December 2022).
- Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Kerschbaum, J.; Rudnicki, M.; Dzien, A.; Dzien-Bischinger, C.; Winner, H.; Heerspink, H.L.; Rosivall, L.; Wiecek, A.; Mark, P.B.; Eder, S.; et al. Intra-individual variability of eGFR trajectories in early diabetic kidney disease and lack of performance of prognostic biomarkers. Sci. Rep. 2020, 10, 19743. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.E.; Davis, W.A.; Ito, J.; Winfield, K.; Stoll, T.; Bringans, S.D.; Lipscombe, R.J.; Davis, T.M.E. Identification of Novel Circulating Biomarkers Predicting Rapid Decline in Renal Function in Type 2 Diabetes: The Fremantle Diabetes Study Phase II. Diabetes Care 2017, 40, 1548–1555. [Google Scholar] [CrossRef]
- Peters, K.E.; Davis, W.A.; Ito, J.; Bringans, S.D.; Lipscombe, R.J.; Davis, T.M.E. Validation of a protein biomarker test for predicting renal decline in type 2 diabetes: The Fremantle Diabetes Study Phase II. J. Diabetes Complicat. 2019, 33, 107406. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.E.; Xu, J.; Bringans, S.D.; Davis, W.A.; Davis, T.M.E.; Hansen, M.K.; Lipscombe, R.J. PromarkerD Predicts Renal Function Decline in Type 2 Diabetes in the Canagliflozin Cardiovascular Assessment Study (CANVAS). J. Clin. Med. 2020, 9, 3212. [Google Scholar] [CrossRef]
- Peters, K.; Bringans, S.; Davis, W.; Lipscombe, R.; Davis, T. A Comparison of PromarkerD to Standard-of-Care Tests for Predicting Renal Decline in Type 2 Diabetes. In Proceedings of the American Society of Nephrology Kidney Week, Virtual Conference, 4–7 November 2021; p. 260. [Google Scholar]
- de Boer, I.H.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: Evidence-based advances in monitoring and treatment. Kidney Int. 2020, 98, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Bringans, S.; Ito, J.; Casey, T.; Thomas, S.; Peters, K.; Crossett, B.; Coleman, O.; Ebhardt, H.A.; Pennington, S.R.; Lipscombe, R. A robust multiplex immunoaffinity mass spectrometry assay (PromarkerD) for clinical prediction of diabetic kidney disease. Clin. Proteom. 2020, 17, 37. [Google Scholar] [CrossRef]
- Bringans, S.; Peters, K.; Casey, T.; Ito, J.; Lipscombe, R. The New and the Old: Platform Cross-Validation of Immunoaffinity MASS Spectrometry versus ELISA for PromarkerD, a Predictive Test for Diabetic Kidney Disease. Proteomes 2020, 8, 31. [Google Scholar] [CrossRef]
- Oshima, M.; Neal, B.; Toyama, T.; Ohkuma, T.; Li, Q.; de Zeeuw, D.; Heerspink, H.J.L.; Mahaffey, K.W.; Fulcher, G.; Canovatchel, W.; et al. Different eGFR Decline Thresholds and Renal Effects of Canagliflozin: Data from the CANVAS Program. J. Am. Soc. Nephrol. 2020, 31, 2446–2456. [Google Scholar] [CrossRef]
- Perkovic, V.; de Zeeuw, D.; Mahaffey, K.W.; Fulcher, G.; Erondu, N.; Shaw, W.; Barrett, T.D.; Weidner-Wells, M.; Deng, H.; Matthews, D.R.; et al. Canagliflozin and renal outcomes in type 2 diabetes: Results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018, 6, 691–704. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F. Apolipoprotein L1 and apolipoprotein A-IV and their association with kidney function. Curr. Opin. Lipidol. 2017, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Luo, Y.; Lai, X. The comprehensive role of apoptosis inhibitor of macrophage(AIM) in pathological conditions. Clin. Exp. Immunol. 2022, uxac095. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Yamazaki, T.; Sugisawa, R.; Gershwin, M.E.; Arai, S. AIM associated with the IgM pentamer: Attackers on stand-by at aircraft carrier. Cell. Mol. Immunol. 2018, 15, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chi, K.; Wu, D.; Hong, Q. Insulin-Like Growth Factor Binding Proteins in Kidney Disease. Front. Pharmacol. 2021, 12, 807119. [Google Scholar] [CrossRef] [PubMed]
- Fusfeld, L.; Murphy, J.T.; Yoon, Y.; Kam, L.Y.; Peters, K.E.; Lin Tan, P.; Shanik, M.; Turchin, A. Evaluation of the clinical utility of the PromarkerD in-vitro test in predicting diabetic kidney disease and rapid renal decline through a conjoint analysis. PLoS ONE 2022, 17, e0271740. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | PBO | CANA | Total |
|---|---|---|---|
| Number of samples (%) | 629 (31.3%) | 1379 (68.7%) | 2008 |
| Age (years) | 61.3 ± 7.2 | 61.8 ± 7.4 | 61.7 ± 7.4 |
| Female sex, n (%) | 200 (31.8) | 432 (31.3) | 632 (31.5) |
| BMI (kg/m2) | 32.2 ± 5.7 | 32.5 ± 6.1 | 32.4 ± 5.9 |
| Diabetes duration (years) * | 12.0 [8.0–16.6] | 12.1 [8.0–17.1] | 12.0 [8.0–17.0] |
| Fasting plasma glucose (mmol/L) * | 9.0 [7.5–10.9] | 9.1 [7.6–11.0] | 9.1 [7.6–10.9] |
| HbA1c (%) * | 8.0 [7.5–8.8] | 8.0 [7.5–8.7] | 8.0 [7.5–8.7] |
| Serum total cholesterol (mmol/L) | 4.3 ± 1.2 | 4.3 ± 1.1 | 4.3 ± 1.1 |
| Serum HDL-cholesterol (mmol/L) | 1.21 ± 0.31 | 1.19 ± 0.32 | 1.20 ± 0.32 |
| Serum triglycerides (mmol/L) † | 1.7 (1.0–2.8) | 1.7 (1.0–2.8) | 1.7 (1.0–2.8) |
| Systolic blood pressure (mmHg) | 136 ± 16 | 136 ± 15 | 136 ± 15 |
| Diastolic blood pressure (mmHg) | 78 ± 9 | 78 ± 9 | 78 ± 9 |
| Diuretic use, n (%) | 281 (44.7) | 593 (43.0) | 874 (43.5) |
| History of heart failure, n (%) | 81 (12.9) | 157 (11.4) | 238 (11.9) |
| Urine albumin to creatinine ratio (mg/g) * | 10.9 [6.3–32.1] | 11.1 [6.3–30.0] | 11.1 [6.3–30.9] |
| eGFR (mL/min/1.73 m2) | 82.2 ± 16.0 | 82.4 ± 15.5 | 82.3 ± 15.6 |
| PromarkerD score (%) *,¥ | 3.7 [0.7–14.3] | 3.7 [0.7–14.3] | 3.9 [0.7–14.5] |
| PromarkerD risk category, n (%) ¥ | |||
| Low | 421 (66.9) | 919 (66.6) | 1340 (66.7) |
| Moderate | 99 (15.7) | 188 (13.6) | 287 (14.3) |
| High | 109 (17.3) | 272 (19.7) | 381 (19.0) |
| Differences in the Means * | ||||||
|---|---|---|---|---|---|---|
| N | Mean | 95% CI | Δ PromarkerD | 95% CI | p-Value | |
| All Participants (n = 1975) | ||||||
| Placebo | ||||||
| Baseline | 629 | 9.6 | 8.6, 10.6 | ref | ||
| Year 3 | 629 | 16.0 | 14.3, 17.7 | 6.4 | 4.9, 7.8 | <0.001 |
| Canagliflozin | ||||||
| Baseline | 1346 | 18.1 | 16.9, 19.3 | ref | ||
| Year 3 | 1346 | 17.1 | 15.9, 18.3 | −1.0 | −1.9, −0.1 | 0.039 |
| PromarkerD High-Risk (n = 377) | ||||||
| Placebo | ||||||
| Baseline | 109 | 33.2 | 31.2, 35.2 | ref | ||
| Year 3 | 109 | 37.7 | 33.4, 42.1 | 4.5 | 0.3, 8.8 | 0.035 |
| Canagliflozin | ||||||
| Baseline | 268 | 48.5 | 45.8, 51.1 | ref | ||
| Year 3 | 268 | 42.9 | 39.6, 46.2 | −5.6 | −8.6, −2.5 | <0.001 |
| PromarkerD Moderate-Risk (n = 281) | ||||||
| Placebo | ||||||
| Baseline | 99 | 14.2 | 13.7, 14.8 | ref | ||
| Year 3 | 99 | 21.8 | 17.4, 26.3 | 7.6 | 3.2, 12.0 | <0.001 |
| Canagliflozin | ||||||
| Baseline | 182 | 27.6 | 25.1, 30.1 | ref | ||
| Year 3 | 182 | 24.9 | 21.9, 28.0 | −2.7 | −6.1, 0.8 | 0.14 |
| PromarkerD Low-Risk (n = 1317) | ||||||
| Placebo | ||||||
| Baseline | 421 | 2.4 | 2.2, 2.7 | ref | ||
| Year 3 | 421 | 9.0 | 7.4, 10.6 | 6.5 | 5.0, 8.1 | <0.001 |
| Canagliflozin | ||||||
| Baseline | 896 | 7.1 | 6.4, 7.9 | ref | ||
| Year 3 | 896 | 7.8 | 7.0, 8.7 | 0.7 | −0.1, 1.5 | 0.091 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, K.E.; Bringans, S.D.; O’Neill, R.S.; Lumbantobing, T.S.C.; Lui, J.K.C.; Davis, T.M.E.; Hansen, M.K.; Lipscombe, R.J. Canagliflozin Attenuates PromarkerD Diabetic Kidney Disease Risk Prediction Scores. J. Clin. Med. 2023, 12, 3247. https://doi.org/10.3390/jcm12093247
Peters KE, Bringans SD, O’Neill RS, Lumbantobing TSC, Lui JKC, Davis TME, Hansen MK, Lipscombe RJ. Canagliflozin Attenuates PromarkerD Diabetic Kidney Disease Risk Prediction Scores. Journal of Clinical Medicine. 2023; 12(9):3247. https://doi.org/10.3390/jcm12093247
Chicago/Turabian StylePeters, Kirsten E., Scott D. Bringans, Ronan S. O’Neill, Tasha S. C. Lumbantobing, James K. C. Lui, Timothy M. E. Davis, Michael K. Hansen, and Richard J. Lipscombe. 2023. "Canagliflozin Attenuates PromarkerD Diabetic Kidney Disease Risk Prediction Scores" Journal of Clinical Medicine 12, no. 9: 3247. https://doi.org/10.3390/jcm12093247
APA StylePeters, K. E., Bringans, S. D., O’Neill, R. S., Lumbantobing, T. S. C., Lui, J. K. C., Davis, T. M. E., Hansen, M. K., & Lipscombe, R. J. (2023). Canagliflozin Attenuates PromarkerD Diabetic Kidney Disease Risk Prediction Scores. Journal of Clinical Medicine, 12(9), 3247. https://doi.org/10.3390/jcm12093247









